Electrostatic Self-Assembly of CdS Quantum Dots with Co9S8 Hollow Nanotubes for Enhanced Visible Light Photocatalytic H2 Production
Abstract
:1. Introduction
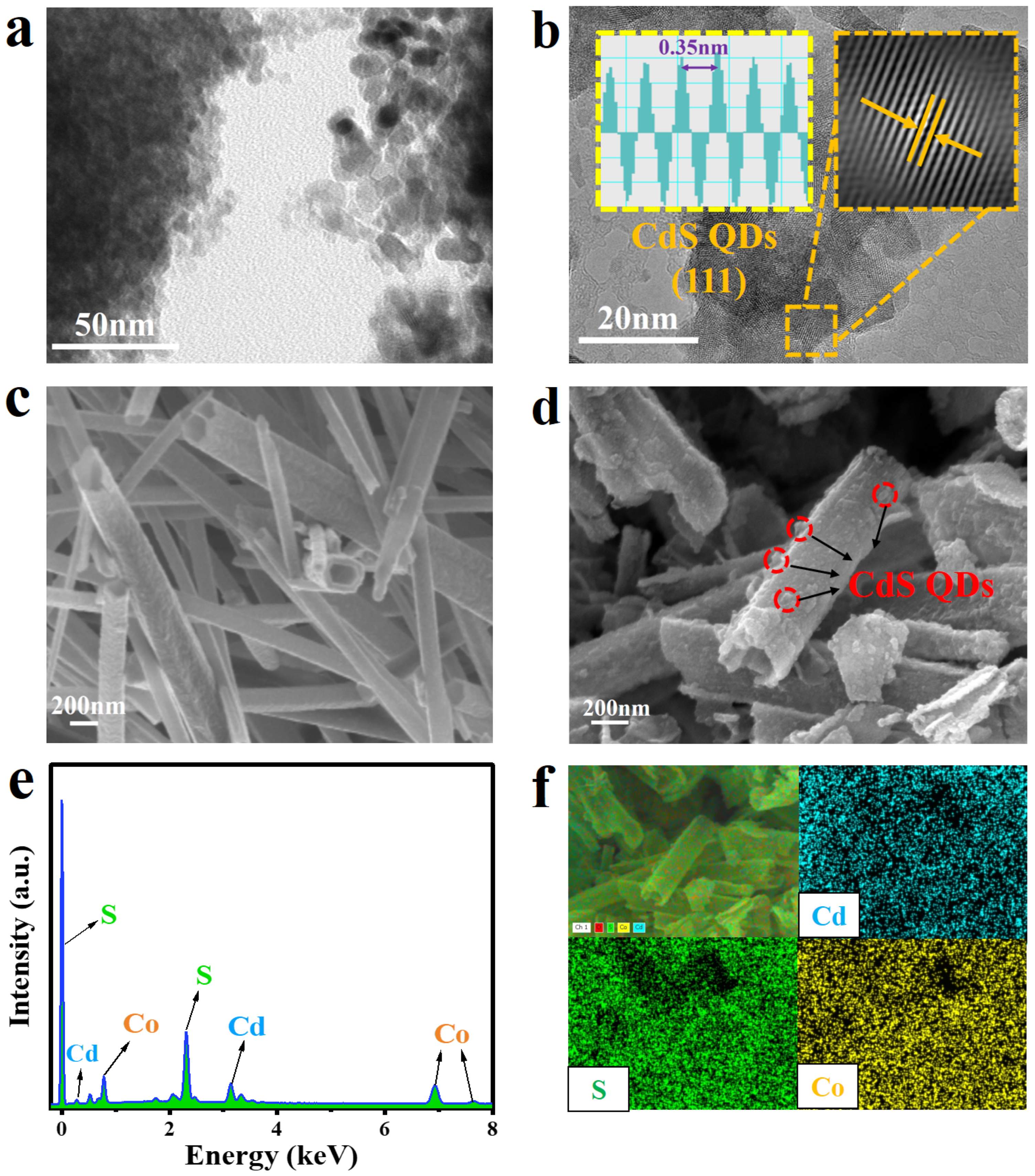
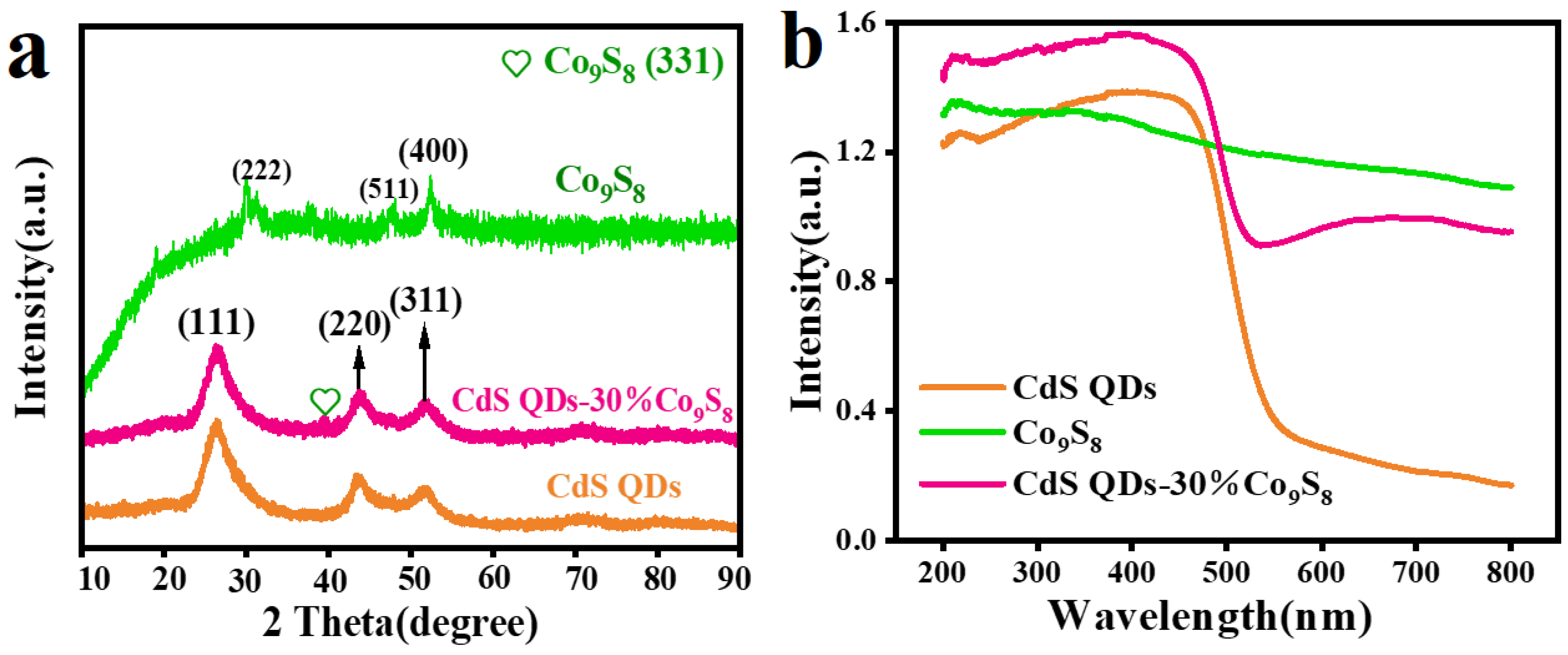
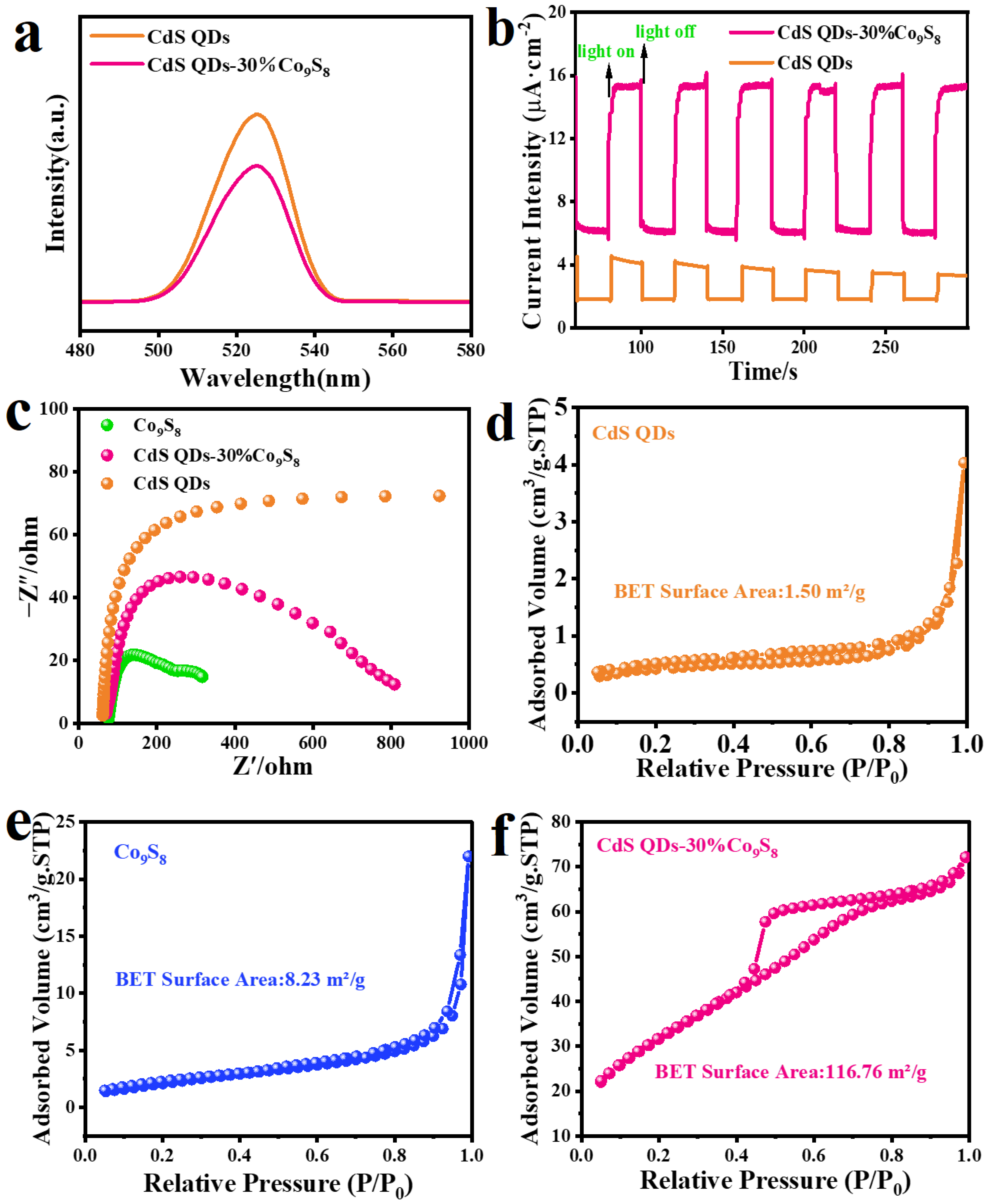
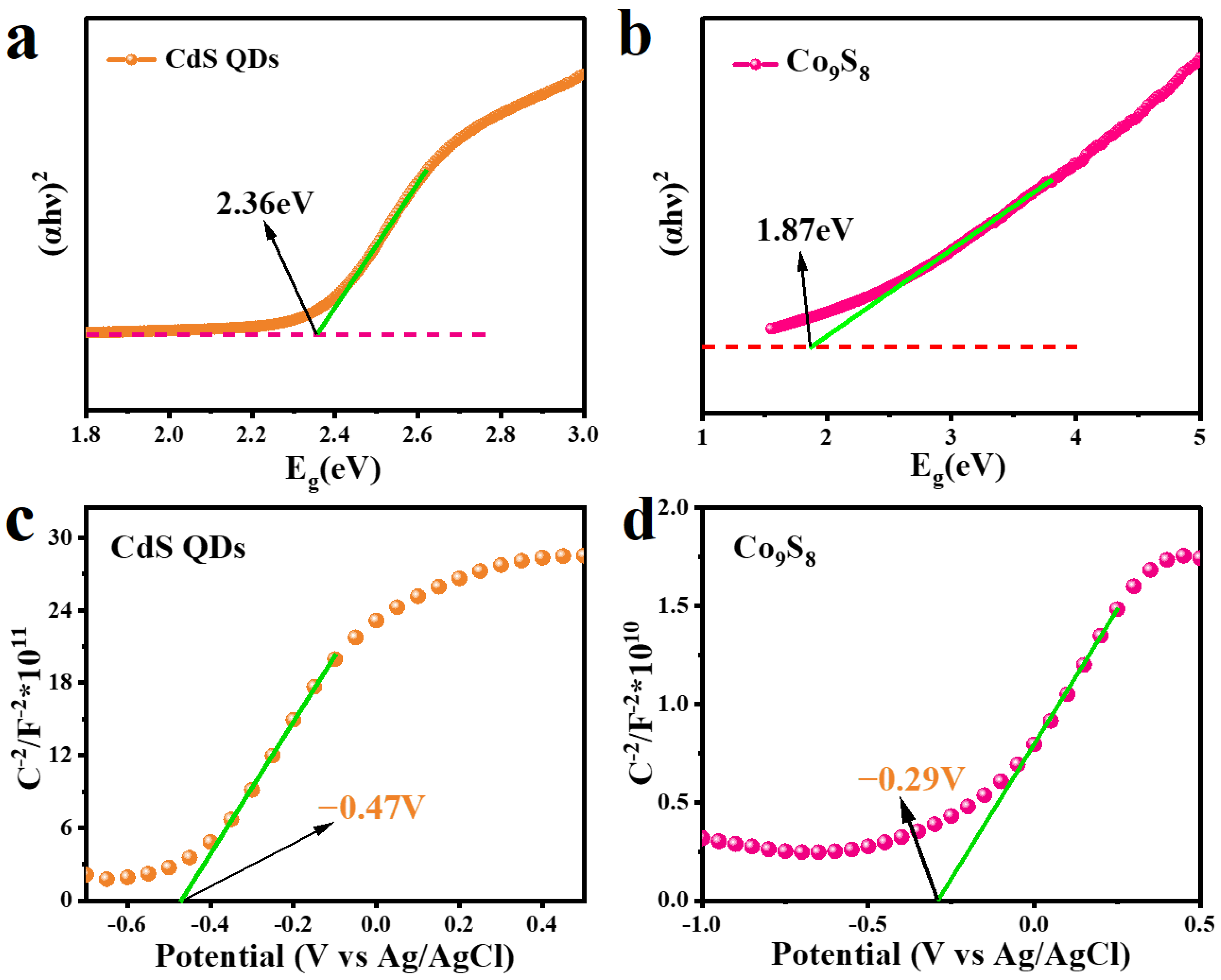
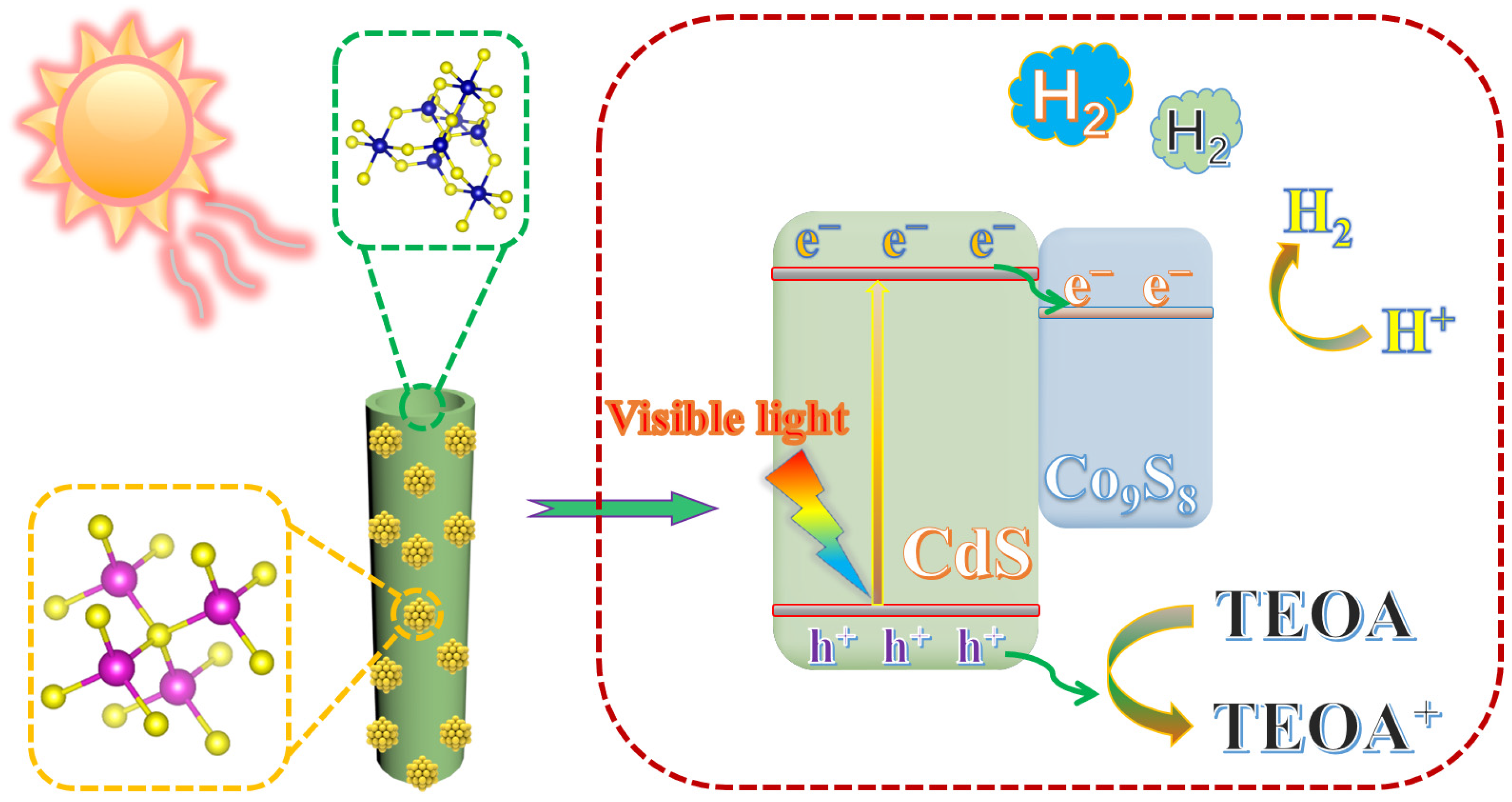
2. Results and Discussion
3. Experimental Section
3.1. Materials
3.2. Preparation of CdS QDs
3.3. Preparation of Co9S8 Nanotubes
3.4. Positive Electrochemical Treatment of Co9S8 Nanotubes
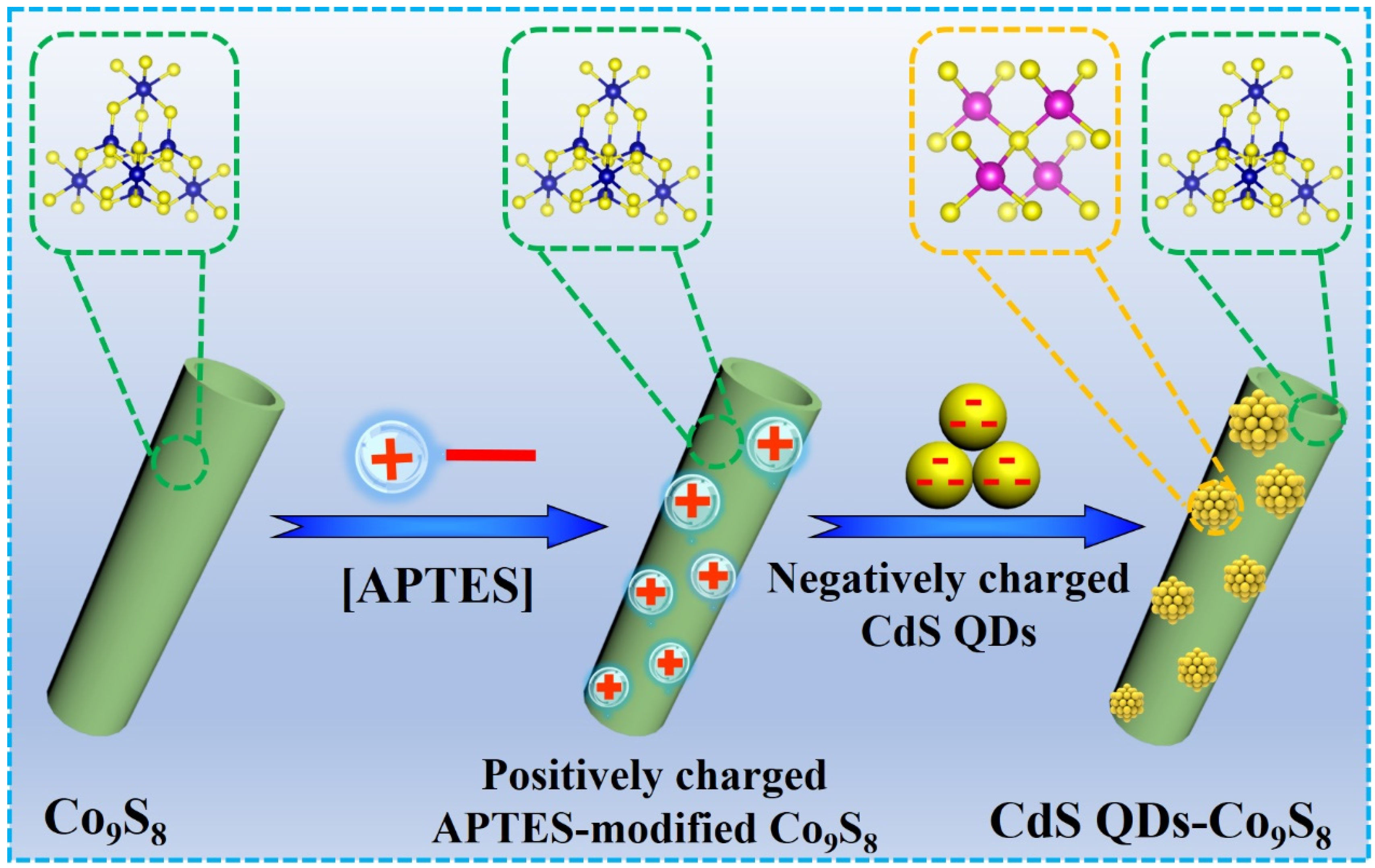
3.5. Electrostatic Assembly of CdS QDs-Co9S8
3.6. Activity Evaluation of Photocatalytic H2 Evolution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, K.-Q.; Li, Y.-H.; Zhang, F.; Qi, M.-Y.; Chen, X.; Tang, Z.-R.; Yamada, Y.M.A.; Anpo, M.; Conte, M.; Xu, Y.-J. Rationally designed transition metal hydroxide nanosheet arrays on graphene for artificial CO2 reduction. Nat. Commun. 2020, 11, 5181. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Zuo, C.; Liu, M.; Tai, X. A review on Cu2O-Based composites in photocatalysis: Synthesis, modification, and applications. Molecules 2023, 28, 5576. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Wu, P.; Li, L.; Yu, F.; Ma, J. Adsorption/desorption behavior of ciprofloxacin on aged biodegradable plastic PLA under different exposure conditions. J. Environ. Chem. Eng. 2023, 11, 109256. [Google Scholar] [CrossRef]
- Li, X.-X.; Liu, X.-C.; Liu, C.; Zeng, J.-M.; Qi, X.-P. Co3O4/stainless steel catalyst with synergistic effect of oxygen vacancies and phosphorus doping for overall water splitting. Tungsten 2022, 5, 100–108. [Google Scholar] [CrossRef]
- Luo, H.; Gao, H.; Zhang, X.; Yang, F.; Liu, C.; Xu, K.; Guo, D. Caterpillar-like 3D graphene nanoscrolls@CNTs hybrids decorated with Co-doped MoSe2 nanosheets for electrocatalytic hydrogen evolution. J. Mater. Sci. Technol. 2023, 136, 43–53. [Google Scholar] [CrossRef]
- Xiao, Y.; Jiang, Y.; Zhou, E.; Zhang, W.; Liu, Y.; Zhang, J.; Wu, X.; Qi, Q.; Liu, Z. In-suit fabricating an efficient electronic transport channels via S-scheme polyaniline/Cd0.5Zn0.5S heterojunction for rapid removal of tetracycline hydrochloride and hydrogen production. J. Mater. Sci. Technol. 2023, 153, 205–218. [Google Scholar] [CrossRef]
- Wei, Y.; Hao, J.-G.; Zhang, J.-L.; Huang, W.-Y.; Ouyang, S.-b.; Yang, K.; Lu, K.-Q. Integrating Co(OH)2 nanosheet arrays on graphene for efficient noble-metal-free EY-sensitized photocatalytic H2 evolution. Dalton Trans. 2023, 52, 13923–13929. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Z.; Yan, Y.; Wei, Y.; Wang, J.; Shen, Y.; Yang, K.; Weng, B.; Lu, K. Rational photodeposition of cobalt phosphate on flower-like ZnIn2S4 for efficient photocatalytic hydrogen evolution. Molecules 2024, 29, 465. [Google Scholar] [CrossRef]
- Wu, K.; Shang, Y.; Li, H.; Wu, P.; Li, S.; Ye, H.; Jian, F.; Zhu, J.; Yang, D.; Li, B.; et al. Synthesis and hydrogen production performance of MoP/a-TiO2/Co-ZnIn2S4 flower-like composite photocatalysts. Molecules 2023, 28, 4350. [Google Scholar] [CrossRef]
- Yuan, H.; Mei, J.-H.; Gong, Y.-N.; Zhong, D.-C.; Lu, T.-B. Cobalt-based heterogeneous catalysts for photocatalytic carbon dioxide reduction. Tungsten 2023, 6, 410–421. [Google Scholar] [CrossRef]
- Yan, Y.Q.; Wu, Y.Z.; Wu, Y.H.; Weng, Z.L.; Liu, S.J.; Liu, Z.G.; Lu, K.Q.; Han, B. Recent Advances of CeO2-Based Composite Materials for Photocatalytic Applications. ChemSusChem 2024, 17, e202301778. [Google Scholar] [CrossRef]
- Camara, F.; Gavaggio, T.; Dautreppe, B.; Chauvin, J.; Pécaut, J.; Aldakov, D.; Collomb, M.-N.; Fortage, J. Electrochemical properties of a rhodium(III) mono-terpyridyl complex and use as a catalyst for light-driven hydrogen evolution in water. Molecules 2022, 27, 6614. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Wang, Z.; Cheng, F.; Zhang, P.; Mai, W.; Tong, Y. Ceria and ceria-based nanostructured materials for photoenergy applications. Nano Energy 2017, 34, 313–337. [Google Scholar] [CrossRef]
- Wang, J.-H.; Yang, S.-W.; Ma, F.-B.; Zhao, Y.-K.; Zhao, S.-N.; Xiong, Z.-Y.; Cai, D.; Shen, H.-D.; Zhu, K.; Zhang, Q.-Y.; et al. RuCo alloy nanoparticles embedded within N-doped porous two-dimensional carbon nanosheets: A high-performance hydrogen evolution reaction catalyst. Tungsten 2023, 6, 114–123. [Google Scholar] [CrossRef]
- Gao, R.; Bai, J.; Shen, R.; Hao, L.; Huang, C.; Wang, L.; Liang, G.; Zhang, P.; Li, X. 2D/2D covalent organic framework/CdS Z-scheme heterojunction for enhanced photocatalytic H2 evolution: Insights into interfacial charge transfer mechanism. J. Mater. Sci. Technol. 2023, 137, 223–231. [Google Scholar] [CrossRef]
- Lu, K.-Q.; Chen, Y.; Xin, X.; Xu, Y.-J. Rational utilization of highly conductive, commercial Elicarb graphene to advance the graphene-semiconductor composite photocatalysis. Appl. Catal. B Environ. 2018, 224, 424–432. [Google Scholar] [CrossRef]
- Mehdi Sabzehmeidani, M.; Karimi, H.; Ghaedi, M. CeO2 nanofibers-CdS nanostructures n–n junction with enhanced visible-light photocatalytic activity. Arab. J Chem. 2020, 13, 7583–7597. [Google Scholar] [CrossRef]
- Yue, D.; Qian, X.; Kan, M.; Ren, M.; Zhu, Y.; Jiang, L.; Zhao, Y. Sulfurated [NiFe]-based layered double hydroxides nanoparticles as efficient co-catalysts for photocatalytic hydrogen evolution using CdTe/CdS quantum dots. Appl. Catal. B 2017, 209, 155–160. [Google Scholar] [CrossRef]
- Zhang, Z.; Rogers, C.R.; Weiss, E.A. Energy transfer from CdS QDs to a photogenerated Pd complex enhances the rate and selectivity of a Pd-photocatalyzed heck reaction. J. Am. Chem. Soc. 2019, 142, 495–501. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, W.; Tang, Y.; Guo, Y.; Geng, Z.; Liu, L.; Tan, X.; Wang, H.; Yu, T.; Ye, J. Unravelling unsaturated edge S in amorphous NiSx for boosting photocatalytic H2 evolution of metastable phase CdS confined inside hydrophilic beads. Appl. Catal. B Environ. 2022, 305, 121055. [Google Scholar] [CrossRef]
- Li, Y.-H.; Qi, M.-Y.; Li, J.-Y.; Tang, Z.-R.; Xu, Y.-J. Noble metal free CdS@CuS-NixP hybrid with modulated charge transfer for enhanced photocatalytic performance. Appl. Catal. B Environ. 2019, 257, 117934. [Google Scholar] [CrossRef]
- Ma, X.; Lei, Z.; Wang, C.; Fu, Z.; Hu, X.; Fan, J.; Liu, E. Fabrication of P-doped Co9S8/g-C3N4 heterojunction for excellent photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2021, 46, 36781–36791. [Google Scholar] [CrossRef]
- Larimi, A.; Rahimi, M.; Khorasheh, F. Carbonaceous supports decorated with Pt–TiO2 nanoparticles using electrostatic self-assembly method as a highly visible-light active photocatalyst for CO2 photoreduction. Renew. Energ. 2020, 145, 1862–1869. [Google Scholar] [CrossRef]
- Chen, Z.; Wan, S.; Cheng, B.; Wang, W.; Xiang, Y.; Yu, J.; Cao, S. Efficient overall photosynthesis of H2O2 by the BTz@Mn0.2Cd0.8S S-scheme heterojunction. Sci. China Chem. 2024, 67, 1953–1960. [Google Scholar] [CrossRef]
- Kandi, D.; Martha, S.; Thirumurugan, A.; Parida, K.M. Modification of BiOI microplates with CdS QDs for enhancing stability, Optical property, Electronic behavior toward rhodamine B decolorization, and photocatalytic hydrogen evolution. J. Phys. Chem. C 2017, 121, 4834–4849. [Google Scholar] [CrossRef]
- Feng, C.; Chen, Z.; Jing, J.; Sun, M.; Han, J.; Fang, K.; Li, W. Synergistic effect of hierarchical structure and Z-scheme heterojunction constructed by CdS nanoparticles and nanoflower-structured Co9S8 with significantly enhanced photocatalytic hydrogen production performance. J. Photochem. Photobiol. A Chem. 2021, 409, 113160. [Google Scholar] [CrossRef]
- Liu, Z.-G.; Wei, Y.; Xie, L.; Chen, H.-Q.; Wang, J.; Yang, K.; Zou, L.-X.; Deng, T.; Lu, K.-Q. Decorating CdS with cobaltous hydroxide and graphene dual cocatalyst for photocatalytic hydrogen production coupled selective benzyl alcohol oxidation. Mol. Catal. 2024, 553, 113738. [Google Scholar] [CrossRef]
- Jiang, X.; Fan, D.; Yao, X.; Dong, Z.; Li, X.; Ma, S.; Liu, J.; Zhang, D.; Li, H.; Pu, X.; et al. Highly efficient flower-like ZnIn2S4/CoFe2O4 photocatalyst with p-n type heterojunction for enhanced hydrogen evolution under visible light irradiation. J. Colloid Interface Sci. 2023, 641, 26–35. [Google Scholar] [CrossRef]
- Ma, M.-Y.; Yu, H.-Z.; Deng, L.-M.; Wang, L.-Q.; Liu, S.-Y.; Pan, H.; Ren, J.-W.; Maximov, M.Y.; Hu, F.; Peng, S.-J. Interfacial engineering of heterostructured carbon-supported molybdenum cobalt sulfides for efficient overall water splitting. Tungsten 2023, 5, 589–597. [Google Scholar] [CrossRef]
- Nagoor Meeran, M.; Haridharan, N.; Shkir, M.; Algarni, H.; Reddy Minnam Reddy, V. Rationally designed 1D CdS/TiO2@Ti3C2 multi-components nanocomposites for enhanced visible light photocatalytic hydrogen production. Chem. Phys. Lett. 2022, 809, 140150. [Google Scholar] [CrossRef]
- Pan, J.; Ou, W.; Li, S.; Chen, Y.; Li, H.; Liu, Y.; Wang, J.; Song, C.; Zheng, Y.; Li, C. Photocatalytic hydrogen production enhancement of Z-Scheme CdS quantum dots/Ni2P/Black Ti3+–TiO2 nanotubes with dual-functional Ni2P nanosheets. Int. J. Hydrogen Energy 2020, 45, 33478–33490. [Google Scholar] [CrossRef]
- Zhang, N.; Wu, X.; Lv, K.; Chu, Y.; Wang, G.; Zhang, D. Synthesis and highly efficient photocatalysis applications of CdS QDs and Au NPs Co-modified KTaO3 perovskite cubes. Phys. Chem. Chem. Phys. 2023, 25, 14028–14037. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Bian, Y.; Liu, Y.; Zhu, A.; Wu, H.; Cui, H.; Chu, D.; Pan, J. Construction of Z-Scheme system for enhanced photocatalytic H2 evolution based on CdS quantum dots/CeO2 nanorods heterojunction. ACS Sustain. Chem. Eng. 2018, 6, 2552–2562. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, X.; Zhao, Y.; Zhang, P.; Chen, J.; Jiang, J.; Xie, T. The photogenerated charge characteristics in Ni@NiO/CdS hybrids for increased photocatalytic H2 generation. RSC Adv. 2019, 9, 39604–39610. [Google Scholar] [CrossRef]
- Zhang, F.; Zhuang, H.-Q.; Zhang, W.; Yin, J.; Cao, F.-H.; Pan, Y.-X. Noble-metal-free CuS/CdS photocatalyst for efficient visible-light-driven photocatalytic H2 production from water. Catal. Today 2019, 330, 203–208. [Google Scholar] [CrossRef]
- Fang, M.; Yang, Z.; Guo, Y.; Xia, X.; Pan, S. Piezoelectric effect achieves efficient carriers’ spatial separation and enhanced photocatalytic H2 evolution of UiO-66-NH2@CdS by transforming charge transfer mechanism. Sep. Purif. Technol. 2024, 328, 125069. [Google Scholar] [CrossRef]
- Ahmad, I.; Shukrullah, S.; Naz, M.Y.; Bhatti, H.N. A Cu medium designed Z-scheme ZnO–Cu–CdS heterojunction photocatalyst for stable and excellent H2 evolution, methylene blue degradation, and CO2 reduction. Dalton Trans. 2023, 52, 6343–6359. [Google Scholar] [CrossRef]
- Lu, C.; Du, S.; Zhao, Y.; Wang, Q.; Ren, K.; Li, C.; Dou, W. Efficient visible-light photocatalytic H2 evolution with heterostructured Ag2S modified CdS nanowires. RSC Adv. 2021, 11, 28211–28222. [Google Scholar] [CrossRef]
- Wang, F.; Yu, Z.; Shi, K.; Li, X.; Lu, K.; Huang, W.; Yu, C.; Yang, K. One-pot synthesis of N-doped NiO for enhanced photocatalytic CO2 reduction with efficient charge transfer. Molecules 2023, 28, 2435. [Google Scholar] [CrossRef]
- Tan, C.-L.; Qi, M.-Y.; Tang, Z.-R.; Xu, Y.-J. Cocatalyst decorated ZnIn2S4 composites for cooperative alcohol conversion and H2 evolution. Appl. Catal. B Environ. 2021, 298, 120541. [Google Scholar] [CrossRef]
- Han, C.; Zeng, Z.; Zhang, X.; Liang, Y.; Kundu, B.K.; Yuan, L.; Tan, C.-L.; Zhang, Y.; Xu, Y.-J. All-in-One: Plasmonic Janus Heterostructures for Efficient Cooperative Photoredox Catalysis. Angew. Chem. Int. Ed. Engl. 2024, e202408527. [Google Scholar] [CrossRef]
- Chen, J.; Ren, Y.; Fu, Y.; Si, Y.; Huang, J.; Zhou, J.; Liu, M.; Duan, L.; Li, N. Integration of Co single atoms and Ni clusters on defect-rich ZrO2 for strong photothermal coupling boosts photocatalytic CO2 reduction. ACS Nano 2024, 18, 13035–13048. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Xu, X.; Luo, R.; Yuan, S.; Zhou, J.; Lei, J. Postsynthetic annulation of three-dimensional covalent organic frameworks for boosting CO2 photoreduction. J. Am. Chem. Soc. 2023, 145, 15473–15481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Luo, Y.-G.; Chen, Y.-H.; Zhan, J.-Y.; Wan, H.; Liu, Z.-J. Photothermal conversion boosted photocatalytic CO2 reduction over S-scheme CeO2@Cu-TCPP in situ experiments and DFT calculation. ACS Sustain. Chem. Eng. 2023, 11, 4813–4824. [Google Scholar] [CrossRef]
- Wang, A.; Zheng, Z.; Wang, H.; Chen, Y.; Luo, C.; Liang, D.; Hu, B.; Qiu, R.; Yan, K. 3D hierarchical H2-reduced Mn-doped CeO2 microflowers assembled from nanotubes as a high-performance Fenton-like photocatalyst for tetracycline antibiotics degradation. Appl. Catal. B Environ. 2020, 277, 119171. [Google Scholar] [CrossRef]
- Zhu, C.; He, Q.; Wang, W.; Du, F.; Yang, F.; Chen, C.; Wang, C.; Wang, S.; Duan, X. S-scheme photocatalysis induced by ZnIn2S4 nanoribbons-anchored hierarchical CeO2 hollow spheres for boosted hydrogen evolution. J. Colloid Interface Sci. 2022, 620, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Lu, D.; Zhang, B.; Kondamareddy, K.K.; Zeng, Y.; Zhang, Y.; Wang, J.; Zhou, M.; Neena, D.; Hao, H.; et al. Interfacial optimization of CeO2 nanoparticles loaded two-dimensional graphite carbon nitride toward synergistic enhancement of visible-light-driven photoelectric and photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2022, 47, 2313–2326. [Google Scholar] [CrossRef]
- Hu, M.; Wu, C.; Feng, S.; Hua, J. A high crystalline perylene-based hydrogen-bonded organic framework for enhanced photocatalytic H2O2 evolution. Molecules 2023, 28, 6850. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, X.; Zhang, K.; Ma, Y.; Wang, H.; Li, H.; Huang, H.; Sun, X.; Ma, T. Construction of a 2D/2D crystalline porous materials based S-scheme heterojunction for efficient photocatalytic H2 production. Adv. Energy Mater. 2024, 14, 2303638. [Google Scholar] [CrossRef]
- Kong, D.; Fan, H.; Yin, D.; Zhang, D.; Pu, X.; Yao, S.; Su, C. AgFeO2 nanoparticle/ZnIn2S4 microsphere p–n heterojunctions with hierarchical nanostructures for efficient visible-light-driven H2 evolution. ACS Sustain. Chem. Eng. 2021, 9, 2673–2683. [Google Scholar] [CrossRef]
- Li, Q.; Lu, Q.; Guo, E.; Wei, M.; Pang, Y. Hierarchical Co9S8/ZnIn2S4 nanoflower enables enhanced hydrogen evolution photocatalysis. Energy Fuels 2022, 36, 4541–4548. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Y.; Liu, X.; Huo, P.; Yan, Y.; Wang, L.; Liao, G.; Liu, C. Interface engineering of Co9S8/CdIn2S4 ohmic junction for efficient photocatalytic H2 evolution under visible light. J. Colloid Interface Sci. 2021, 600, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Construction of Hierarchical Hollow Co9S8/ZnIn2S4 Tubular Heterostructures for Highly Efficient Solar Energy Conversion and Environmental Remediation. Angew. Chem. Int. Ed. Engl. 2020, 59, 8255–8261. [Google Scholar] [CrossRef] [PubMed]



| Photocatalysts | Light Sources | Sacrificial Agents | H2 (μmol·g−1·h−1) | Reference |
|---|---|---|---|---|
| CdS QDs-30% Co9S8 | 300 W Xe lamp (λ ≥ 420 nm) | TEOA | 9642.7 | this work |
| CdS/TiO2@Ti3C2 | 300 W Xe lamp (λ ≥ 420 nm) | TEOA | 3115.0 | [30] |
| CdS QDs/Ni2P/B-TiO2 | 300 W Xe arc lamp | Na2S/Na2SO3 | 3303.9 | [31] |
| CdS/Au/KTaO3 | Xe lamp (λ ≥ 420 nm) | Na2S/Na2SO3 | 2892.0 | [32] |
| CdS QDs/CeO2 | 300 W Xe lamp (λ ≥ 300 nm) | Na2S/Na2SO3 | 101.1 | [33] |
| Ni@NiO/CdS | 500 W Xe lamp | TEOA | 4380.0 | [34] |
| CuS/CdS | 300 W Xe lamp (λ ≥ 420 nm) | lactic acid (10 vol%) | 5617.0 | [35] |
| UiO-66-NH2@CdS | 300 W Xe lamp (λ ≥ 420 nm) | Na2S/Na2SO3 | 2028.5 | [36] |
| ZnO-Cu-CdS | 300 W Xe lamp (λ ≥ 420 nm) | glycerol | 4655.0 | [37] |
| Ag2S-CdS | 300 W Xe lamp (λ ≥ 420 nm) | lactic acids (2 vol%) | 777.3 | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Wu, Y.; Lu, C.; Wei, Y.; Wang, J.; Weng, B.; Huang, W.-Y.; Zhang, J.-L.; Yang, K.; Lu, K. Electrostatic Self-Assembly of CdS Quantum Dots with Co9S8 Hollow Nanotubes for Enhanced Visible Light Photocatalytic H2 Production. Molecules 2024, 29, 3530. https://doi.org/10.3390/molecules29153530
Yan Y, Wu Y, Lu C, Wei Y, Wang J, Weng B, Huang W-Y, Zhang J-L, Yang K, Lu K. Electrostatic Self-Assembly of CdS Quantum Dots with Co9S8 Hollow Nanotubes for Enhanced Visible Light Photocatalytic H2 Production. Molecules. 2024; 29(15):3530. https://doi.org/10.3390/molecules29153530
Chicago/Turabian StyleYan, Yuqing, Yonghui Wu, Chenggen Lu, Yu Wei, Jun Wang, Bo Weng, Wei-Ya Huang, Jia-Lin Zhang, Kai Yang, and Kangqiang Lu. 2024. "Electrostatic Self-Assembly of CdS Quantum Dots with Co9S8 Hollow Nanotubes for Enhanced Visible Light Photocatalytic H2 Production" Molecules 29, no. 15: 3530. https://doi.org/10.3390/molecules29153530









