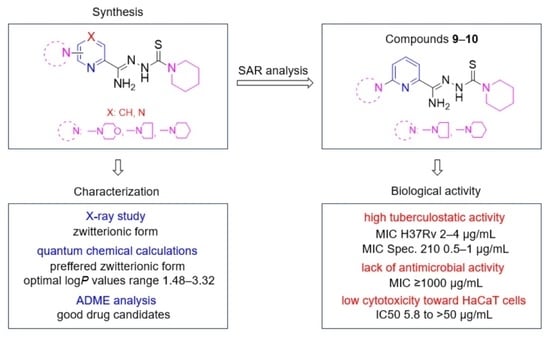
Synthesis and Biological Activity of Piperidinothiosemicarbazones Derived from Aminoazinecarbonitriles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Activities
2.2.1. Tuberculostatic Activity Assay
2.2.2. Antimicrobial Activity Assay
2.2.3. Cytotoxic Activity Assay
2.3. X-ray Study
2.4. ADME Analysis
2.5. Structure–Activity Relationship
3. Materials and Methods
3.1. Chemistry
3.1.1. Procedure for the Preparation of Nitriles 1–7
Method A (1, 2, 4, 6, 7)
Method B (3, 5)
3.1.2. Procedure for the Preparation of Piperidinothiosemicarbazones 8–14, DMK-20, and DMK-16
Method A (8)
Method B (9, DMK-16)
Method C (10,14, DMK-20)
Method D (11)
Method E (12,13)
3.2. Biological Activities
3.2.1. Tuberculostatic Activity Assay
3.2.2. Antimicrobial Activity Assay
3.2.3. Cytotoxic Activity Assay
3.3. X-ray Study
3.4. ADME Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Z.; Zeng, X.; Tsui, S.K.-W. Investigating function roles of hypothetical proteins encoded by the Mycobacterium tuberculosis H37Rv genome. BMC Genom. 2019, 20, 394. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2022. Available online: https://www.who.int/publications/i/item/9789240061729 (accessed on 21 April 2023).
- Dukes, M.N.G. Index of side effects. In Side Effects of Drugs Annual; Aronson, J.K., Ed.; Elsevier Science Ltd.: Amsterdam, The Netherlands, 1993; Volume 16, pp. 583–622. [Google Scholar]
- Onakpoya, I.J. Chapter 23–Drugs used in the treatment of tuberculosis and leprosy. In Side Effects of Drugs Annual; Ray, S.D., Ed.; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2022; Volume 44, pp. 311–331. [Google Scholar]
- UNAIDS. Global HIV & AIDS Statistics–Fact Sheet. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 21 April 2022).
- GBD 2019 Tuberculosis Collaborators. Global, regional, and national sex differences in the global burden of tuberculosis by HIV status, 1990–2019: Results from the Global Burden of Disease Study. Lancet Infect. Dis. 2019, 22, 222–241. [Google Scholar]
- Sharan, R.; Bucşan, A.N.; Ganatra, S.; Paiardini, M.; Mohan, M.; Mehra, S.; Khader, S.A.; Kaushal, D. Chronic Immune Activation in TB/HIV Co-infection. Trends Microbiol. 2020, 28, 619–632. [Google Scholar] [CrossRef]
- Jackson, S.; Kabir, Z.; Comiskey, C. Effects of migration on tuberculosis epidemiological indicators in low and medium tuberculosis incidence countries: A systematic review. J. Clin. Tuberc. Other Mycobact. Dis. 2021, 23, 100225. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, P.; Hajikhani, B.; Sameni, F.; Noorisepehr, N.; Zare, F.; Bostanshirin, N.; Yazdani, S.; Goudarzi, M.; Sayyari, S.; Dadashi, M. COVID-19 and tuberculosis coinfection: An overview of casereports/case series and meta-analysis of prevalence studies. Heliyon 2023, 9, e13637. [Google Scholar] [CrossRef]
- CDC 2022 Special Report. COVID-19 US Impact on Antimibicrobial Resistance. Available online: https://www.cdc.gov/drugresistance/pdf/covid19-impact-report-508.pdf (accessed on 21 April 2023).
- CDC Report. Antibiotic Resistance Threats in the United States 2019. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed on 21 April 2023).
- Keerthana, G.; Karthick, V.; Dey, H.; Kausar, T.; Udhaya, K.S.; Thirumal, K.D.; Zayed, H.; Priya Doss, C.G. Chapter Three-Elucidating the mechanism of antimicrobial resistance in Mycobacterium tuberculosis using gene interaction networks. In Advances in Protein Chemistry and Structural Biology; Doncy, R., Ed.; Academic Press: Cambridge, MA, USA, 2023; Volume 134, pp. 53–74. [Google Scholar]
- Perveen, S.; Kumari, D.; Singh, K.; Sharma, R. Tuberculosis drug discovery: Progression and future interventions in the wake of emerging resistance. Eur. J. Med. Chem. 2022, 229, 114066. [Google Scholar] [CrossRef]
- Black, T.A.; Buchwald, U.K. The pipeline of new molecules and regimens against drug-resistant tuberculosis. J. Clin. Tuberc. Other Mycobact. Dis. 2021, 25, 100285. [Google Scholar] [CrossRef]
- Krug, S.; Parveen, S.; Bishai, W.R. Host-Directed Therapies: Modulating Inflammation to Treat Tuberculosis. Front Immunol. 2021, 12, 660916. [Google Scholar] [CrossRef]
- Bourguignon, T.; Godinez-Leon, J.S.; Gref, R. Nanosized Drug Delivery Systems to Fight Tuberculosis. Pharmaceutics 2023, 15, 393. [Google Scholar] [CrossRef]
- Islam, B.; Islam, I.; Nath, N.; Emran, T.B.; Rahman, R.; Sharma, R.; Matin, M.M. Recent Advances in Pyridine Scaffold: Focus on Chemistry, Synthesis, and Antibacterial Activities. Biomed. Res. Int. 2023, 2023, 9967591. [Google Scholar] [CrossRef]
- Hassan, N.W.; Saudi, M.N.; Abdel-Ghany, Y.S.; Ismail, A.; Elzahhar, P.A.; Sriram, D.; Nassra, R.; Abdel-Aziz, M.M.; El-Hawash, S.A. Novel pyrazine based anti-tubercular agents: Design, synthesis, biological evaluation and in silico studies. Bioorg. Chem. 2020, 96, 103610. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.; Lv, Z.; Zhang, G.; Xu, Z. Recent updates on 1,2,3-triazole-containing hybrids with in vivo therapeutic potential against cancers: A mini-review. Eur. J. Med. Chem. 2023, 251, 115254. [Google Scholar] [CrossRef]
- Gao, J.; Hou, H.; Gao, F. Current scenario of quinolone hybrids with potential antibacterial activity against ESKAPE pathogens. Eur. J. Med. Chem. 2023, 247, 115026. [Google Scholar] [CrossRef]
- Hu, Y.-Q.; Zhang, S.; Zhao, F.; Gao, C.; Feng, L.-S.; Lv, Z.-S.; Xu, Z.; Wu, X. Isoniazid derivatives and their anti-tubercular activity. Eur. J. Med. Chem. 2017, 133, 255–267. [Google Scholar] [CrossRef]
- Gobis, K.; Foks, H.; Kędzia, A.; Wierzbowska, M.; Zwolska, Z. Synthesis and antibacterial activity of novel pyridine and pyrazine derivatives obtained from amidoximes. J. Heterocyclic Chem. 2009, 46, 1271–1279. [Google Scholar] [CrossRef]
- Krause, M.; Foks, H.; Augustynowicz-Kopeć, E.; Napiórkowska, A.; Szczesio, M.; Gobis, K. Synthesis and tuberculostatic activity evaluation of novel benzazoles with alkyl, cycloalkyl or pyridine moiety. Molecules 2018, 23, 985. [Google Scholar] [CrossRef]
- Krause, M.; Foks, H.; Ziembicka, D.; Augustynowicz-Kopeć, E.; Głogowska, A.; Korona-Głowniak, I.; Bojanowski, K.; Siluk, D.; Gobis, K. 4-substituted picolinohydrazonamides as a new class of potential antitubercular agents. Eur. J. Med. Chem. 2020, 190, 112106. [Google Scholar] [CrossRef]
- Abdullah, A.H.; Zahra, J.A.; El-Abadelah, M.M.; Sabri, S.S.; Khanfar, M.A.; Matar, S.A.; Voelter, W. Synthesis and antibacterial activity of N1-(carbazol-3-yl)amidrazones incorporating piperazines and related congeners. Z. Naturforsch. 2016, 71, 857–867. [Google Scholar] [CrossRef]
- Hkiri, S.; Hafidh, A.; Cavalier, J.-F.; Touil, S.; Samarat, A. Design, synthesis, antimicrobial evaluation, and molecular docking studies of novel symmetrical 2,5-difunctionalized 1,3,4-oxadiazoles. J. Heterocycl. Chem. 2019, 57, 1044–1054. [Google Scholar] [CrossRef]
- Wei, Z.-Y.; Chi, K.-Q.; Yu, Z.-K.; Liu, H.-Y.; Sun, L.-P.; Zheng, C.-J.; Piao, H.-R. Synthesis and biological evaluation of chalcone derivatives containing aminoguanidine or acylhydrazone moieties. Bioorg. Med. Chem. Lett. 2016, 26, 5920–5925. [Google Scholar] [CrossRef]
- Song, M.; Wang, S.; Wang, Z.; Fu, Z.; Zhou, S.; Cheng, H.; Liang, Z.; Deng, X. Synthesis, antimicrobial and cytotoxic activities, and molecular docking studies of N-arylsulfonylindoles containing an aminoguanidine, a semicarbazide, and a thiosemicarbazide moiety. Eur. J. Med. Chem. 2019, 166, 108–118. [Google Scholar] [CrossRef]
- Cerqueira, F.; Maia, M.; Gabriel, C.; Medeiros, R.; Cravo, S.; Ribeiro, A.; Dantas, D.; Dias, A.; Saraiva, L.; Raimundo, L.; et al. Mechanism of Antifungal Activity by 5-Aminoimidazole-4-Carbohydrazonamide Derivatives against Candida albicans and Candida krusei. Antibiotics 2021, 10, 183. [Google Scholar] [CrossRef]
- Ziembicka, D.; Gobis, K.; Szczesio, M.; Olczak, A.; Augustynowicz-Kopeć, E.; Głogowska, A.; Korona-Głowniak, I.; Bojanowski, K. Synthesis and structure-activity relationship of 2,6-disubstituted thiosemicarbazone derivatives of pyridine as potential antituberculosis agents. Materials 2023, 16, 448. [Google Scholar] [CrossRef]
- Gobis, K.; Foks, H.; Zwolska, Z.; Augustynowicz-Kopec, E. Synthesis of 2-aminoaryl-5-substituted-1,3,4-thiadiazoles in a thermal 1,3-dipolar cycloaddition reaction. Phosphorus Sulfur Silicon Relat. Elem. 2005, 180, 2653–2666. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.-L. Patterns in hydrogen bonding: Functionality and graph set analysis in crystals. Angew. Chem. Int. Ed. Engl. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendolski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemis-try libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of drug absorption using multivariate statistics. J. Med. Chem. 2000, 3, 3867–3877. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bio-availability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple selection criteria f or drug-like chemical matter. J. Med. Chem. 2001, 44, 1841–1946. [Google Scholar] [CrossRef]
- Soledade, M.; Santos, C.S.; Matos, A.M.; Reis, M.; Martins, F. Lipophilicity assessment of some isoniazid derivatives active against Mycobacterium tuberculosis. Colloids Surf. A Physicochem. Eng. Asp. 2020, 599, 124820. [Google Scholar]
- Gacki, M.; Kafarska, K.; Pietrzak, A.; Korona-Głowniak, I.; Wolf, W.M. Quasi-Isostructural Co(II) and Ni(II) Complexes with Mefenamato Ligand: Synthesis, Characterization, and Biological Activity. Molecules 2020, 25, 3099. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. C Struct. Chem. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-Egg to Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef]







| Compd. | MIC [µg/mL] | |
|---|---|---|
| H37Rv | Spec. 210 | |
| 8 | 16 | 4 |
| 9 | 4 | 1 |
| 10 | 2 | 0.5 |
| 11 | >512 | >512 |
| 12 | 256 | 256 |
| 13 | 4 | 4 |
| DMK-20 | 6.25 | 6.25 |
| DMK-16 | 6.25 | 6.25 |
| 14 | 2 | 4 |
| INH | 0.125 | 8 |
| Chemicals Microorganism | 9 | 10 | 13 | 14 | CIP | VAN | FCZ |
|---|---|---|---|---|---|---|---|
| MIC [µg/mL] | |||||||
| Gram-positive bacteria | |||||||
| B. cereus ATCC 10876 | >1000 | >1000 | >1000 | 0.12 | 0.12 | 0.98 | - |
| B. subtilis ATCC 6633 | >1000 | >1000 | - | 0.12 | 0.03 | 0.24 | - |
| S. epidermidis ATCC 12228 | >1000 | >1000 | 15.6 | 0.06 | 0.49 | 0.98 | - |
| S. aureus ATCC 25923 | >1000 | >1000 | 1000 | 0.06 | 0.49 | 0.98 | - |
| M. luteus ATCC 10240 | >1000 | >1000 | 62.5 | 0.06 | 0.98 | 0.12 | - |
| Gram-negative bacteria | |||||||
| P. aeruginosa ATCC 9027 | >1000 | >1000 | >1000 | 500 | - | 0.49 | - |
| P. mirabilis ATCC 12453 | >1000 | >1000 | >1000 | 250 | - | 0.03 | - |
| E. coli ATCC 25922 | >1000 | >1000 | >1000 | 500 | - | 0.004 | - |
| K. pneumoniae ATCC 13883 | >1000 | >1000 | >1000 | 1000 | - | 0.06 | - |
| Yeasts | |||||||
| C. parapsilosis ATCC 22019 | 1000 | >1000 | 1000 | 250 | - | - | 1.95 |
| C. albicans ATCC 102231 | 1000 | >1000 | >1000 | 250 | - | - | 0.98 |
| Compd. | IC50-HaCaT [µg/mL] | SI IC50-HaCaT/MIC-MT | ||
|---|---|---|---|---|
| MTT | SULF | MTT | SULF | |
| 9 | >50 | >50 | >12.5 | >12.5 |
| 10 | 5.80 | >50 | 2.90 | >25 |
| Crystal Data | |
|---|---|
| Chemical formula | C7.50H11.50N3.50O0.50S0.50 |
| Mr | 174.73 |
| Crystal system, space group | Monoclinic, P21/c |
| Temperature (K) | 100 |
| a, b, c (Å) | 9.7274 (1), 14.3441 (2), 12.6387 (2) |
| β (°) | 96.100 (1) |
| V (Å3) | 1753.50 (4) |
| Z | 8 |
| Radiation type | Cu Kα |
| Data collection | |
| No. of measured, independent, and observed [I > 2σ(I)] reflections | 32,919, 3637, 3482 |
| Rint | 0.043 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.031, 0.078, 1.03 |
| No. of reflections | 3637 |
| No. of parameters | 244 |
| ∆max, ∆min (e Å−3) | 0.28, −0.24 |
| D–H⋯A | D–H | H⋯A | D⋯A | D–H⋯A |
|---|---|---|---|---|
| N3–H3⋯S1 | 0.88 | 2.34 | 2.8330 (10) | 116 |
| Compd. | MIC [μg/mL] | logP |
|---|---|---|
| 8 | 16 | 2.19 |
| 9 | 4 | 2.91 |
| 10 | 2 | 3.32 |
| 11 | >512 | 0.85 |
| 12 | 256 | 1.57 |
| 13 | 4 | 1.99 |
| DMK-20 | 6.25 | 1.48 |
| DMK-16 | 6.25 | 2.19 |
| 14 | 2 | 2.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziembicka, D.; Gobis, K.; Szczesio, M.; Augustynowicz-Kopeć, E.; Głogowska, A.; Korona-Głowniak, I.; Bojanowski, K. Synthesis and Biological Activity of Piperidinothiosemicarbazones Derived from Aminoazinecarbonitriles. Pharmaceuticals 2023, 16, 1267. https://doi.org/10.3390/ph16091267
Ziembicka D, Gobis K, Szczesio M, Augustynowicz-Kopeć E, Głogowska A, Korona-Głowniak I, Bojanowski K. Synthesis and Biological Activity of Piperidinothiosemicarbazones Derived from Aminoazinecarbonitriles. Pharmaceuticals. 2023; 16(9):1267. https://doi.org/10.3390/ph16091267
Chicago/Turabian StyleZiembicka, Dagmara, Katarzyna Gobis, Małgorzata Szczesio, Ewa Augustynowicz-Kopeć, Agnieszka Głogowska, Izabela Korona-Głowniak, and Krzysztof Bojanowski. 2023. "Synthesis and Biological Activity of Piperidinothiosemicarbazones Derived from Aminoazinecarbonitriles" Pharmaceuticals 16, no. 9: 1267. https://doi.org/10.3390/ph16091267