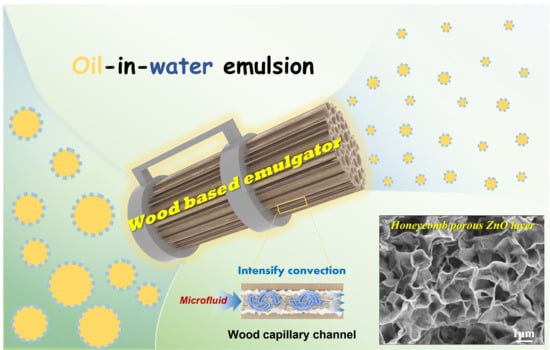
Oriented Interpenetrating Capillary Network with Surface Engineering by Porous ZnO from Wood for Membrane Emulsification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of ZnO-Wood Membrane
2.3. Characterization
2.4. Membrane Emulsification Experiment
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, Z.; Wei, Y.; Shi, A.; Zhong, J.; Rao, P.; Wang, Q.; Zhang, H. Correlation between Interfacial Layer Properties and Physical Stability of Food Emulsions: Current Trends, Challenges, Strategies, and Further Perspectives. Adv. Colloid Interface Sci. 2023, 313, 102863. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.G.; McKnight, P.J.; Tuttle, T.; Ulijn, R.V. Tripeptide Emulsifiers. Adv. Mater. 2015, 28, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Huang, G. Improving Method, Properties and Application of Polysaccharide as Emulsifier. Food Chem. 2022, 376, 131937. [Google Scholar] [CrossRef] [PubMed]
- Clark, I.C.; Fontanez, K.M.; Meltzer, R.H.; Xue, Y.; Hayford, C.; May-Zhang, A.; D’Amato, C.; Osman, A.; Zhang, J.Q.; Hettige, P.; et al. Microfluidics-Free Single-Cell Genomics with Templated Emulsification. Nat. Biotechnol. 2023, 41, 1557. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.Q.; Fu, D.F.; Chakraborty, R.; Singh, R.P.; Terry, N. Enhanced Crude Oil Depletion by Constructed Bacterial Consortium Comprising Bioemulsifier Producer and Petroleum Hydrocarbon Degraders. Bioresour. Technol. 2019, 282, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, J.; Xing, L.; Zhang, W. Applications and Effects of Ultrasound Assisted Emulsification in the Production of Food Emulsions: A Review. Trends Food Sci. Technol. 2021, 110, 493–512. [Google Scholar] [CrossRef]
- Vladisavljević, G.T. Preparation of Microemulsions and Nanoemulsions by Membrane Emulsification. Colloids Surf. A 2019, 579, 123709. [Google Scholar] [CrossRef]
- Yang, S.; Lian, Z.; Wang, M.; Liao, P.; Wu, H.; Cao, J.; Tong, X.; Tian, T.; Wang, H.; Jiang, L. Molecular Structural Modification of β-Conglycinin Using pH-Shifting with Ultrasound to Improve Emulsifying Properties and Stability. Ultrason. Sonochem. 2022, 90, 106186. [Google Scholar] [CrossRef]
- Guo, Z.; Huang, Z.; Guo, Y.; Li, B.; Yu, W.; Zhou, L.; Jiang, L.; Teng, F.; Wang, Z. Effects of High-Pressure Homogenization on Structural and Emulsifying Properties of Thermally Soluble Aggregated Kidney Bean (Phaseolus vulgaris L.) Proteins. Food Hydrocoll. 2021, 119, 106835. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, H.; Lin, X.; Li, M.; Zhao, Y.; Shang, L. Scalable Production of Biomedical Microparticles Via High-Throughput Microfluidic Step Emulsification. Small 2023, 19, 2206007. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, Q.; Liu, H.; Xiao, C.; Sun, K. A Facile and Environmental-Friendly Strategy for Preparation of Poly (Tetrafluoroethylene-Co-Hexafluoropropylene) Hollow Fiber Membrane and Its Membrane Emulsification Performance. Chem. Eng. J. 2020, 384, 123345. [Google Scholar] [CrossRef]
- Syed, U.T.; Dias, A.M.A.; de Sousa, H.C.; Crespo, J.; Brazinha, C. Greening Perfluorocarbon Based Nanoemulsions by Direct Membrane Emulsification: Comparative Studies with Ultrasound Emulsification. J. Clean. Prod. 2022, 357, 131966. [Google Scholar] [CrossRef]
- Nazir, A.; Schroën, K.; Boom, R. Premix Emulsification: A Review. J. Membr. Sci. 2010, 362, 1–11. [Google Scholar] [CrossRef]
- Wu, M.B.; Yang, H.C.; Wang, J.J.; Wu, G.P.; Xu, Z.K. Janus Membranes with Opposing Surface Wettability Enabling Oil-to-Water and Water-to-Oil Emulsification. ACS Appl. Mater. Interfaces 2017, 9, 5062–5066. [Google Scholar] [CrossRef] [PubMed]
- Nazir, A.; Vladisavljević, G.T. Droplet Breakup Mechanisms in Premix Membrane Emulsification and Related Microfluidic Channels. Adv. Colloid Interface Sci. 2021, 290, 102393. [Google Scholar] [CrossRef] [PubMed]
- Konovalova, V.; Kolesnyk, I.; Savchenko, M.; Marynin, A.; Bubela, H.; Kujawa, J.; Knozowska, K.; Kujawski, W. Preparation of Chitosan Water-in-Oil Emulsions by Stirred Cell Membrane Emulsification. Colloids Surf. A 2023, 661, 130929. [Google Scholar] [CrossRef]
- Li, J.; Xu, Z.; Zhang, J.; Jiang, H.; Chen, R. Experimental and Model Analysis of Membrane Permeance in Cross-Flow Multi-Channel Membrane Emulsification. Chem. Eng. Sci. 2024, 284, 119543. [Google Scholar] [CrossRef]
- Meng, T.; Sudarjat, H.; Am Momin, M.; Ma, J.X.; Xu, Q. Development of Uniform Fenofibrate-Loaded Biodegradable Microparticle by Membrane Emulsification. Int. J. Pharm. 2023, 650, 123675. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Z.; Liu, Q. Janus Membrane Emulsification for Facile Preparation of Hollow Microspheres. J. Membr. Sci. 2019, 592, 117384. [Google Scholar] [CrossRef]
- Vladisavljević, G.T. Preparation of Microparticles and Nanoparticles Using Membrane-Assisted Dispersion, Micromixing, and Evaporation Processes. Particuology 2024, 84, 30–44. [Google Scholar] [CrossRef]
- Guo, P.; Huang, J.; Zhao, Y.P.; Martin, C.R.; Zare, R.N.; Moses, M.A. Nanomaterial Preparation by Extrusion through Nanoporous Membranes. Small 2018, 14, 1703493. [Google Scholar] [CrossRef] [PubMed]
- Giefer, P.; Bäther, S.; Kaufmes, N.; Kieserling, H.; Heyse, A.; Wagemans, W.; Barthel, L.; Meyer, V.; Schneck, E.; Fritsching, U.; et al. Characterization of β-Lactoglobulin Adsorption on Silica Membrane Pore Surfaces and Its Impact on Membrane Emulsification Processes. J. Colloid Interface Sci. 2023, 652, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Flanc, Z.; Al-Gharabli, S.; Karadsheh, M.; Pianka, K.; Kujawski, W.; Kujawa, J. Membranes with Surficial Spherical Nanoarchitecture—Material Importance in Emulsion Formulation by Premix Membrane Emulsification. Sep. Purif. Technol. 2023, 324, 124507. [Google Scholar] [CrossRef]
- Gehrmann, S.; Bunjes, H. Influence of Membrane Material on the Production of Colloidal Emulsions by Premix Membrane Emulsification. Eur. J. Pharm. Biopharm. 2018, 126, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Liu, S.; Cao, J.; Yue, X.; Shao, J.H. A Review of Oil and Water Retention in Emulsified Meat Products: The Mechanisms of Gelation and Emulsification, the Application of Multi-Layer Hydrogels. Crit. Rev. Food Sci. Nutr. 2023, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, K.; Jun, G.; Hwang, W. Wood-Nanotechnology-Based Membrane for the Efficient Purification of Oil-in-Water Emulsions. ACS Nano 2020, 14, 17233–17240. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Su, P.; Tang, H.; Lin, Y.; Yu, Y. Hetero-Polycrystalline Membranes with Narrow and Rigid Pores for Molecular Sieving. Small 2022, 19, 2205542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, J.; Cheng, J.; Zhao, Y.; Huang, Y.; Huang, Q. A Simple and Effective Approach to Regulate and Control Pore Structure of Electrospun Ptfe Nanofiber Membrane. Sep. Purif. Technol. 2023, 326, 124848. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, Q.; Huang, Y.; Yu, S.; Xiao, C.; Hu, Q. Pore Structure Design of NFES PTFE Membrane for Membrane Emulsification. J. Membr. Sci. 2020, 611, 118365. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Liu, G.; Jin, W.; Li, X. Fungal Cell Wall-Graphene Oxide Microcomposite Membrane for Organic Solvent Nanofiltration. Adv. Funct. Mater. 2021, 31, 2100110. [Google Scholar] [CrossRef]
- Fauziah, A.R.; Yeh, L.H. Engineered Heterogenous Subnanochannel Membranes with a Tri-Continuous Pore Structure of Large Geometry Gradient for Massively Enhanced Osmotic Power Conversion from Organic Solutions. Adv. Funct. Mater. 2023, 34, 2306834. [Google Scholar] [CrossRef]
- Ouyang, W.; Zhang, S.; Wang, X. Freestanding Block Copolymer Membranes with Tunable Pore Sizes Promoted by Subnanometer Nanowires. Small 2023, 19, 2206018. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.C.; Yu, Z.L.; Qin, B.; Chen, C.; Ma, Z.Y.; Yu, S.H. Superflexible Artificial Soft Wood. Adv. Mater. 2023, 35, 2303518. [Google Scholar] [CrossRef]
- Montanari, C.; Chen, H.; Lidfeldt, M.; Gunnarsson, J.; Olsén, P.; Berglund, L.A. Sustainable Thermal Energy Batteries from Fully Bio-Based Transparent Wood. Small 2023, 19, 2301262. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Chen, C.; Zhu, S.; Zhu, M.; Dai, J.; Ray, U.; Li, Y.; Kuang, Y.; Li, Y.; Quispe, N.; et al. Processing Bulk Natural Wood into a High-Performance Structural Material. Nature 2018, 554, 224–228. [Google Scholar] [CrossRef]
- Li, L.; Cao, Q.; Wu, Y.; Zheng, Y.; Tang, H.; Ge, J.; Liang, M.; Zhou, B.; Jiang, B.; Wu, S.; et al. Wood-Derived Continuously Oriented Three-Phase Interfacial Channels for High-Performance Quasi-Solid-State Alkaline Zinc Batteries. Adv. Mater. 2023, 35, 2300132. [Google Scholar] [CrossRef]
- Liu, G.; He, Z.; Bai, Y.; Li, Y.; Wang, C.; Hu, J.; Li, X.; Luo, Y.; Chen, D. 3D Spiderweb-Like Nanowire Networks Loading Single-Atom Catalysts in Capillary Array as High-Efficiency Microreactor. Chem. Eng. J. 2023, 453, 139700. [Google Scholar] [CrossRef]
- Ram, F.; Garemark, J.; Li, Y.; Pettersson, T.; Berglund, L.A. Functionalized Wood Veneers as Vibration Sensors: Exploring Wood Piezoelectricity and Hierarchical Structure Effects. ACS Nano 2022, 16, 15805–15813. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Chen, C.; Chen, G.; Wang, C.; Liu, D.; Das, S.; Chen, G.; Li, T.; Li, J.; Liu, Y.; et al. Wood Ionic Cable. Small 2021, 17, 2008200. [Google Scholar] [CrossRef]
- Xia, Q.; Chen, C.; Yao, Y.; He, S.; Wang, X.; Li, J.; Gao, J.; Gan, W.; Jiang, B.; Cui, M.; et al. In Situ Lignin Modification toward Photonic Wood. Adv. Mater. 2021, 33, 2001588. [Google Scholar] [CrossRef]
- Liu, G.; Chen, D.; Liu, R.; Yu, Z.; Jiang, J.; Liu, Y.; Hu, J.; Chang, S. Antifouling Wood Matrix with Natural Water Transfer and Microreaction Channels for Water Treatment. ACS Sustain. Chem. Eng. 2019, 7, 6782–6791. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, S.; Tian, Z.; Duan, G.; Pan, H.; Yue, Y.; Li, S.; Jian, S.; Yang, W.; Liu, K.; et al. MOFs Meet Wood: Reusable Magnetic Hydrophilic Composites toward Efficient Water Treatment with Super-High Dye Adsorption Capacity at High Dye Concentration. Chem. Eng. J. 2022, 446, 136851. [Google Scholar] [CrossRef]
- Jiao, M.; Yao, Y.; Chen, C.; Jiang, B.; Pastel, G.; Lin, Z.; Wu, Q.; Cui, M.; He, S.; Hu, L. Highly Efficient Water Treatment Via a Wood-Based and Reusable Filter. ACS Mater. Lett. 2020, 2, 430–437. [Google Scholar] [CrossRef]
- He, R.; Neupane, M.; Zia, A.; Huang, X.; Bowers, C.; Wang, M.; Lu, J.; Yang, Y.; Dong, P. Binder-Free Wood Converted Carbon for Enhanced Water Desalination Performance. Adv. Funct. Mater. 2022, 32, 2208040. [Google Scholar] [CrossRef]
- Li, M.; Liu, G.; Wang, C.; Chang, S.; Hu, J. Removal of Plastics from Micron Size to Nanoscale Using Wood Filter. Materials 2024, 17, 1361. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Gong, A.S.; Zhu, M.; Chen, G.; Lacey, S.D.; Jiang, F.; Li, Y.; Wang, Y.; Dai, J.; Yao, Y.; et al. Mesoporous, Three-Dimensional Wood Membrane Decorated with Nanoparticles for Highly Efficient Water Treatment. ACS Nano 2017, 11, 4275–4282. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ram, F.; Chen, B.; Garemark, J.; Berglund, L.; Dai, H.; Li, Y. Scalable Hierarchical Wood/ZnO Nanohybrids for Efficient Mechanical Energy Conversion. Mater. Des. 2023, 226, 111665. [Google Scholar] [CrossRef]
- Ivanov, N.P.; Dran’kov, A.N.; Shichalin, O.O.; Lembikov, A.O.; Buravlev, I.Y.; Mayorov, V.Y.; Balanov, M.I.; Rogachev, K.A.; Kaspruk, G.D.; Pisarev, S.M.; et al. Composite Magnetic Sorbents Based on Magnetic Fe3O4 Coated by Zn and Al Layered Double Hydroxide for U(VI) Removal from Aqueous Media. J. Radioanal. Nucl. Chem. 2024, 333, 1213–1230. [Google Scholar] [CrossRef]
- Balybina, V.A.; Dran’kov, A.N.; Shichalin, O.O.; Savel’eva, N.Y.; Kokorina, N.G.; Kuular, Z.C.; Ivanov, N.P.; Krasitskaya, S.G.; Ivanets, A.I.; Papynov, E.K. Mesoporous Layered Double Hydroxides: Synthesis for High Effective Uranium Ions Sorption from Seawater and Salt Solutions on Nanocomposite Functional Materials. J. Compos. Sci. 2023, 7, 458. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Q.; Jing, W.; Zhong, Z.; Xing, W. Pore Structure and Surface Property Design of Silicon Carbide Membrane for Water-in-Oil Emulsification. J. Membr. Sci. 2022, 648, 120347. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, K.; Dong, Y.; Zhang, W.; Zhang, S.; Li, J. Bulk Superhydrophobility of Wood Via in-Situ Deposition of ZnO Rods in Wood Structure. Surf. Coat. Technol. 2020, 383, 125240. [Google Scholar] [CrossRef]
- Mei, C.Q.; Fan, Y.M.; Mei, L.; Luo, G.Q. Surface Characteristics of Modified Poplar Wood-Flour and Its Effect on the Mechanical Properties of Wood Polymer Composites. Rev. Adv. Mater. Sci. 2013, 33, 203–210. [Google Scholar]
- Hu, R.; Yang, J.; Yang, P.; Wu, Z.; Xiao, H.; Liu, Y.; Lu, M. Fabrication of ZnO@Cotton Fabric with Anti-Bacterial and Radiation Barrier Properties Using an Economical and Environmentally Friendly Method. Cellulose 2020, 27, 2901–2911. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Cen, K.; Luo, M.; Li, H.; Lu, B. Pyrolysis Polygeneration of Poplar Wood: Effect of Heating Rate and Pyrolysis Temperature. Bioresour. Technol. 2016, 218, 780–788. [Google Scholar] [CrossRef]
- Garemark, J.; Ram, F.; Liu, L.; Sapouna, I.; Cortes Ruiz, M.F.; Larsson, P.T.; Li, Y. Advancing Hydrovoltaic Energy Harvesting from Wood through Cell Wall Nanoengineering. Adv. Funct. Mater. 2022, 33, 2208933. [Google Scholar] [CrossRef]
- Dragosavac, M.M.; Sovilj, M.N.; Kosvintsev, S.R.; Holdich, R.G.; Vladisavljević, G.T. Controlled Production of Oil-in-Water Emulsions Containing Unrefined Pumpkin Seed Oil Using Stirred Cell Membrane Emulsification. J. Membr. Sci. 2008, 322, 178–188. [Google Scholar] [CrossRef]
- Holdich, R.G.; Dragosavac, M.M.; Vladisavljević, G.T.; Piacentini, E. Continuous Membrane Emulsification with Pulsed (Oscillatory) Flow. Ind. Eng. Chem. Res. 2013, 52, 507–515. [Google Scholar] [CrossRef]
- Vladisavljević, G.T.; Wang, B.; Dragosavac, M.M.; Holdich, R.G. Production of Food-Grade Multiple Emulsions with High Encapsulation Yield Using Oscillating Membrane Emulsification. Colloid. Surface. A 2014, 458, 78–84. [Google Scholar] [CrossRef]
- Fan, M.; Xu, J.; Sun, H.; Wang, S.; Zhang, X.; Wang, H.; Yin, W. Enhancement of Chaotic Mixing Performance in Laminar Flow with Reciprocating and Rotating Coupled Agitator. Chem. Eng. Sci. 2023, 280, 118988. [Google Scholar] [CrossRef]
- Enders, A.; Siller, I.G.; Urmann, K.; Hoffmann, M.R.; Bahnemann, J. 3D Printed Microfluidic Mixers-a Comparative Study on Mixing Unit Performances. Small 2019, 15, 1804326. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Liu, X.; Liu, G.; Chang, S.; Hu, J. Oriented Interpenetrating Capillary Network with Surface Engineering by Porous ZnO from Wood for Membrane Emulsification. Materials 2024, 17, 2113. https://doi.org/10.3390/ma17092113
Chen Y, Liu X, Liu G, Chang S, Hu J. Oriented Interpenetrating Capillary Network with Surface Engineering by Porous ZnO from Wood for Membrane Emulsification. Materials. 2024; 17(9):2113. https://doi.org/10.3390/ma17092113
Chicago/Turabian StyleChen, Yaodong, Xiaolin Liu, Gonggang Liu, Shanshan Chang, and Jinbo Hu. 2024. "Oriented Interpenetrating Capillary Network with Surface Engineering by Porous ZnO from Wood for Membrane Emulsification" Materials 17, no. 9: 2113. https://doi.org/10.3390/ma17092113