Wound Modulations in Glaucoma Surgery: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Initial Search (Figure 2)

2.2. Preliminary Screening
2.3. Eligibility Assessment
3. Results
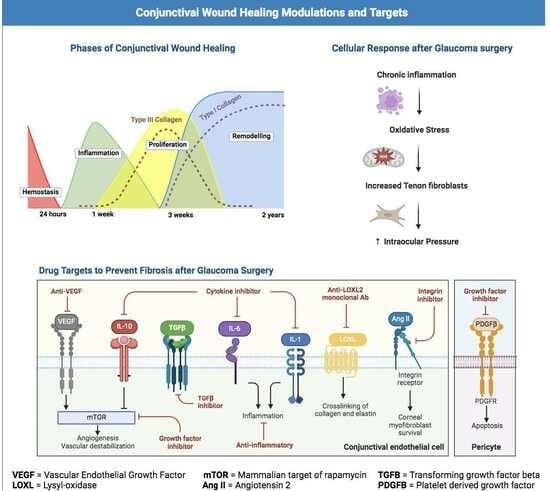
3.1. Overview of the Wound Healing Process
3.1.1. Hemostasis
3.1.2. Inflammation
3.1.3. Proliferation
3.1.4. Remodeling
3.2. Fibrosis
3.3. Wound Healing in Trabeculectomy
3.4. Wound Healing after Glaucoma Drainage Devices (GDDs) and Bleb-Forming MIGS
3.5. Current Glaucoma Wound Healing Agents
3.6. Landmark 5-FU and MMC Studies
| Author/Year/Country | Results |
| Kitazawa Y. et al., 1991. Japan [92] | Thirty-two patients undergoing trabeculectomy were assigned to receive either MMC (seventeen eyes) or 5-FU (fifteen eyes). The mean preoperative IOPs (mmHg) were 28.7 ± 7.9 (MMC) and 32.7 ± 10.0 (5-FU). At the final post-op visit, the mean postoperative IOPs were 8.6 ± 3.8 (MMC) and 12.3 ± 4.2 (5-FU). The incidence of corneal complications was lower in the MMC group (12%) compared to the 5-FU group (53%). |
| Katz GJ et al., 1995. USA [93] | In a high-risk filtration study, 20 patients received MMC and 9 received 5-FU. The mean preoperative IOP’s (mmHg, MMC vs. 5-FU) were 32.6 ± 10.5 and 31.5 ± 9.8, respectively (p = 0.78). At 32 months, the postoperative IOP’s were, similarly, 9.0 ± 4.9 vs. 16.3 ± 4.8 (p = 0.0003). The MMC group required fewer medications for IOP control (0.5 vs. 1.6) (p = 0.01). |
| Lamping et al., 1995. USA [94] | A total of 74 pseudophakic patients with glaucoma underwent trabeculectomy, and received either 5-FU (40 eyes) or MMC (40 eyes). Preoperative IOP’s (mmHg, MMC vs. 5-FU) were 30.6 vs. 31.5, respectively. At 12 months post-op, the IOP’s were, similary, 12.8 vs. 14.8 mmHg (p = 0.001). The MMC-treated eyes required fewer IOP-lowering medications (0.6) compared to 5-FU-treated eyes (1.05) (p = 0.03). |
| Zadok D et al., 1995. Israel [95] | This trabeculectomy study compared postoperative subconjunctival injections of 5-FU (19 eyes) with single intraoperative application of subconjunctival MMC (20 eyes). At 6 months, IOPs averaged 10.9 mmHg (MMC-treated eyes) vs. 14.2 mmHg (5-FU-treated eyes) (p = 0.14). The MMC-treated group was on fewer medications (0.3 vs. 1.1, p < 0.001). |
| Cohen et al., 1996. USA [96] | In a combined cataract and trabeculectomy study, 72 eyes were randomized to MMC (0.5 mg/mL) vs. a placebo. At 6 months, significantly fewer medications were required for the MMC group (0.5 vs. 1.2; p = 0.002). Similarly, at 12 months, the MMC group had significantly reduced mean IOP (7.65 mmHg vs. 3.84 mmHg; p = 0.001). However, the MMC group showed large filtering blebs and more frequent wound leaks. |
| Costa et al., 1996. Brazil [97] | A total of 28 eyes with advanced POAG were given either MMC (0.2 mg/mL) or saline solution intraoperatively for 3 min. Mean IOPs were significantly lower in the MMC group compared to the controls at the final post-op visit (p = 0.001). The IOP (mmHg) was ≤15 in 85.7% (MMC) vs. 28.6% (control, p = 0.002). Choroidal effusions (35.7% vs. 14.3%, p = 0.0065) and shallow AC (35.7% vs. 7.1%) were more common in the MMC group. |
| Carlson et al., 1997. USA [98] | In a combined phacoemulsification and trabeculectomy procedure, 29 patients received either MMC [0.5 mg/mL] or a placebo. Pre-op IOPs (mmHg) were 18.4 ± 2.7 (MMC) vs. 19.1 ± 4.0 (placebo). At 8 months, MMC-treated eyes had a lower average IOP (12.3 ± 1.6) compared to the placebo-treated eyes (15.2 ± 1.5). At 12 months, IOPs averaged 12.6 ± 1.0 (MMC) and 16.2 ± 1.5 (placebo). On average, the MMC group had lower post-op IOP levels than the placebo group (p = 0.04). |
| Singh et al., 1997. USA [99] | A total of 101 eyes of black Ghanian patients with POAG were treated with either 5-FU and MMC after trabeculectomy. The 5-FU group (50.0 mg/mL for 5 min) had 57 patients, and the MMC group (0.5 mg/mL for 3.5 min) had 44 patients. Overall mean pre-op IOP (mmHg) was 30.1. Patients receiving MMC (IOP = 14.7) had a lower mean postoperative IOP than those receiving 5-FU (IOP = 16.7; p = 0.05). |
| Singh et al., 1997. USA [100] | In a black West African population, 81 eyes were divided to receive MMC or 5-FU during trabeculectomy. A total of 37 received 5-FU (50 mg/mL for 5 min) and 44 received MMC (0.4 mg/mL for 2 min). Pre-op IOP (mmHg) was 30.7 (MMC) vs. 32 (5-FU). The mean post-op IOP was 13.7 (MMC) vs. 16.3 (5-FU, p = 0.05). |
| Andreanos et al., 1997. Greece [101] | The study assessed MMC in 46 patients (26 M + 20 F) undergoing a repeat trabeculectomy. Patients were randomly assigned to MMC (24) vs. control group (22). Pre-op IOPs (mmHg) ranged from 27 to 38. Post-op complications were higher in the MMC group, including choroidal effusion (8.3% vs. 0%) and shallow AC (29.2% vs. 13.6%). Mean IOP (≤20 mmHg after 18 months) was 83.3% in the MMC group compared to 63.6% in the control group. |
| Singh et al., 2000. USA [102] | In this trabeculectomy study, 54 eyes received MMC (0.4 mg/mL for 2 min) and 54 eyes received 5-FU (50 mg/mL for 5 min). At 3 years post-op, there was no statistically significant difference between the two groups for mean preoperative IOP, or post-op interventions/complications. |
| DeBry et al., 2002. USA [68] | In this trabeculectomy study involving 239 eyes, a Kaplan–Meier analysis suggested 5-year probabilities of developing endophthalmitis (7.5%), bleb leaks (17.9%), and blebitis (6.3%). Trabeculectomy with MMC was associated with significant morbidity, and the risk of complications reached 23% at 5 years. |
| WuDunn et al., 2002. USA [103] | A total of 115 eyes underwent trabeculectomy [57 eyes (5-FU) and 58 eyes (MMC)]. The mean preoperative IOP (mmHg) was 24.3 (5-FU) vs. 21.9 (MMC), with no statistical significance (p = 0.09). At 12 months, 94% of 5-FU eyes and 89% of MMC eyes reached the target IOP of 21 mmHg (p = 0.49). |
| Sisto et al., 2007. Italy [104] | A total of 40 eyes with neovascular glaucoma were divided to receive post-op 5-FU (18) vs. intraoperative MMC (22) after filtration surgery. Pre-op IOPs (mmHg) were 40.4 ± 10.3 (5-FU) and 42 ± 11.3 (MMC), respectively. The mean follow-up period was 35.8 (5-FU) and 18.6 (MMC) months. Although the mean IOP significantly decreased in both groups [from 40 to 14.7 (5-FU) group (p < 0.0001); vs. 42 to 29.9 (MMC) group (p = 0.0006)], the difference between the two groups was not significant. |
| Mostafaei et al., 2011. Iran [105] | A total of 40 patients with high-risk open angle glaucoma received either MMC or 5-FU. Mean preoperative IOPs (mmHg) were 30.6 (5-FU) and 31.2 (MMC), respectively. At 6 months, the mean IOPs postoperatively for 5-FU (13.6) and MMC (11.4) were similar. The relative success of 5-FU vs. MMC was 0.93 [95% CI: 0.8–1.1]. |
| Fendi et al., 2013. Brazil [106] | A meta-analysis of 5 randomized controlled clinical trials comprising 416 patients comparing MMC against 5-FU was carried out. Pre-op IOP was ≥21 mmHg in both groups. Lower IOPs (mean difference 2.17 mmHg) and higher success rates were observed in the MMC arm (92%) than in the 5-FU arm (84.2%, p = 0.01). |
3.7. Experimental Wound Healing Agents
3.7.1. Nanoparticles
3.7.2. Targeting mRNAs
3.7.3. Infliximab
3.8. Future Directions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Friedman, D.S.; Wolfs, R.C.; O’Colmain, B.J.; Klein, B.E.; Taylor, H.R.; West, S.; Leske, M.C.; Mitchell, P.; Congdon, N.; Kempen, J. Eye Diseases Prevalence Research Group. Prevalence of open-angle glaucoma among adults in the United States. Arch. Ophthalmol. 2004, 122, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Murgoitio-Esandi, J.; Xu, B.X.; Song, B.J.; Zhou, Q.; Oberai, A.A. A Mechanistic Model of Aqueous Humor Flow to Study Effects of Angle Closure on Intraocular Pressure. Trans. Vis. Sci. Technol. 2023, 12, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; McLaren, J.W.; Overby, D.R. Unconventional aqueous humor outflow: A review. Exp. Eye Res. 2017, 158, 94–111. [Google Scholar] [CrossRef] [PubMed]
- Al-Humimat, G.; Marashdeh, I.; Daradkeh, D.; Kooner, K.S. Investigational Rho Kinase Inhibitors for the Treatment of Glaucoma. J. Exp. Pharmacol. 2021, 13, 197–212. [Google Scholar] [CrossRef] [PubMed]
- SooHoo, J.R.; Seibold, L.K.; Radcliffe, N.M.; Kahook, M.Y. Minimally invasive glaucoma surgery: Current implants and future innovations. Can. J. Ophthalmol. 2014, 49, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Wagner, I.V.; Stewart, M.W.; Dorairaj, S.K. Updates on the Diagnosis and Management of Glaucoma. Mayo Clin. Proc. Innov. Qual. Outcomes 2022, 6, 618–635. [Google Scholar] [CrossRef]
- Vinod, K.; Gedde, S.J.; Feuer, W.J.; Panarelli, J.F.; Chang, T.C.; Chen, P.P.; Parrish, R.K., 2nd. Practice Preferences for Glaucoma Surgery: A Survey of the American Glaucoma Society. J. Glaucoma 2017, 26, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Gedde, S.J.; Schiffman, J.C.; Feuer, W.J.; Herndon, L.W.; Brandt, J.D.; Budenz, D.L. Tube versus Trabeculectomy Study Group. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am. J. Ophthalmol. 2012, 153, 789–803.e2. [Google Scholar] [CrossRef]
- Masoumpour, M.B.; Nowroozzadeh, M.H.; Razeghinejad, M.R. Current and Future Techniques in Wound Healing Modulation after Glaucoma Filtering Surgeries. Open Ophthalmol. J. 2016, 10, 68–85. [Google Scholar] [CrossRef]
- Mehta, A.; De Paola, L.; Pana, T.A.; Carter, B.; Soiza, R.L.; Kafri, M.W.; Potter, J.F.; Mamas, M.A.; Myint, P.K. The relationship between nutritional status at the time of stroke on adverse outcomes: A systematic review and meta-analysis of prospective cohort studies. Nutr. Rev. 2022, 80, 2275–2287. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Sangkuhl, K.; Shuldiner, A.R.; Klein, T.E.; Altman, R.B. Platelet aggregation pathway. Pharmacogenet Genom. 2011, 21, 516–521. [Google Scholar] [CrossRef]
- Chaudhary, P.K.; Kim, S.; Kim, S. An Insight into Recent Advances on Platelet Function in Health and Disease. Int. J. Mol. Sci. 2022, 23, 6022–6031. [Google Scholar] [CrossRef]
- Tahery, M.M.; Lee, D.A. Pharmacologic control of wound healing in glaucoma filtration surgery. J. Ocul. Pharmacol. 1989, 5, 155–179. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Chapple, I.L.C.; Hirschfeld, J.; Kantarci, A.; Wilensky, A.; Shapira, L. The role of the host—Neutrophil biology. Periodontology 2000 2023, 1–47. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef] [PubMed]
- Thiruvoth, F.M.; Mohapatra, D.P.; Kumar, D.; Chittoria, S.R.K.; Nandhagopal, V. Current concepts in the physiology of adult wound healing. Plast. Aesthetic Res. 2015, 2, 250–256. [Google Scholar] [CrossRef]
- Schultz, G.S.; Chin, G.A.; Moldawer, L.; Diegelmann, R.F. Principles of Wound Healing. Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists; Fitridge, R., Thompson, M., Eds.; Springer International Publishing: Cham, Switzerland, 2011; pp. 423–450. [Google Scholar] [CrossRef]
- Koivisto, L.; Heino, J.; Häkkinen, L.; Larjava, H. Integrins in Wound Healing. Adv. Wound Care (New Rochelle) 2014, 3, 762–783. [Google Scholar] [CrossRef]
- Alhajj, M.; Goyal, A. Physiology, Granulation Tissue. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554402/ (accessed on 14 November 2023).
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- Henderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: From mechanisms to medicines. Nature 2020, 587, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Khaw, P.T.; Bouremel, Y.; Brocchini, S.; Henein, C. The control of conjunctival fibrosis as a paradigm for the prevention of ocular fibrosis-related blindness. “Fibrosis has many friends”. Eye 2020, 34, 2163–2174. [Google Scholar] [CrossRef] [PubMed]
- Zhavoronkov, A.; Izumchenko, E.; Kanherkar, R.R.; Teka, M.; Cantor, C.; Manaye, K.; Sidransky, D.; West, M.D.; Makarev, E.; Csoka, A.B. Pro-fibrotic pathway activation in trabecular meshwork and lamina cribrosa is the main driving force of glaucoma. Cell Cycle 2016, 15, 1643–1652, Erratum in Cell Cycle 2016, 15, 2087. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, L.A.; Wynn, T.A.; Fisher, A.J. Cytokine mediated tissue fibrosis. Biochim. Biophys. Acta 2013, 1832, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, O.; Kitano-Izutani, A.; Tomoyose, K.; Reinach, P. Pathobiology of wound healing after glaucoma filtration surgery. BMC Ophthalmol. 2015, 15 (Suppl. S1), 157. [Google Scholar] [CrossRef] [PubMed]
- Macleod, T.; Berekmeri, A.; Bridgewood, C.; Stacey, M.; McGonagle, D.; Wittmann, M. The Immunological Impact of IL-1 Family Cytokines on the Epidermal Barrier. Front. Immunol. 2021, 23, 808012. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, X.F. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 2009, 19, 71–88. [Google Scholar] [CrossRef]
- Francois, B.; Jeannet, R.; Daix, T.; Walton, A.H.; Shotwell, M.S.; Unsinger, J.; Monneret, G.; Rimmelé, T.; Blood, T.; Morre, M.; et al. Interleukin-7 restores lymphocytes in septic shock: The IRIS-7 randomized clinical trial. JCI Insight 2018, 3, e98960. [Google Scholar] [CrossRef]
- Huang, Y.H.; Shi, M.N.; Zheng, W.D.; Zhang, L.J.; Chen, Z.X.; Wang, X.Z. Therapeutic effect of interleukin-10 on CCl4-induced hepatic fibrosis in rats. World J. Gastroenterol. 2006, 12, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wong, K.; Ouyang, W.; Rutz, S. Targeting IL-10 Family Cytokines for the Treatment of Human Diseases. Cold Spring Harb. Perspect. Biol. 2019, 11, a028548. [Google Scholar] [CrossRef] [PubMed]
- Steen, E.H.; Wang, X.; Balaji, S.; Butte, M.J.; Bollyky, P.L.; Keswani, S.G. The Role of the Anti-Inflammatory Cytokine Interleukin-10 in Tissue Fibrosis. Adv. Wound Care (New Rochelle) 2020, 9, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Feng, D.; Wang, H.; Hong, F.; Bertola, A.; Wang, F.S.; Gao, B. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology 2012, 56, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Arshad, T.; Mansur, F.; Palek, R.; Manzoor, S.; Liska, V. A Double Edged Sword Role of Interleukin-22 in Wound Healing and Tissue Regeneration. Front. Immunol. 2020, 11, 2148. [Google Scholar] [CrossRef]
- Tang, K.Y.; Lickliter, J.; Huang, Z.H.; Xian, Z.S.; Chen, H.Y.; Huang, C.; Xiao, C.; Wang, Y.P.; Tan, Y.; Xu, L.F.; et al. Safety, pharmacokinetics, and biomarkers of F-652, a recombinant human interleukin-22 dimer, in healthy subjects. Cell. Mol. Immunol. 2019, 16, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Keane, P.A.; Sadda, S.R. Development of Anti-VEGF Therapies for Intraocular Use: A Guide for Clinicians. J. Ophthalmol. 2012, 2012, 483034. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, L.; Li, H.; Eriksson, U. The PDGF family: Four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. 2004, 15, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.S.; Falkenham, A.; Myers, T.; Légaré, J.F. Connective tissue growth factor expression after angiotensin II exposure is dependent on transforming growth factor-β signaling via the canonical Smad-dependent pathway in hypertensive induced myocardial fibrosis. J. Renin Angiotensin Aldosterone Syst. 2018, 19, 1470320318759358. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Yang, N.; Cao, D.F.; Yin, X.X.; Zhou, H.H.; Mao, X.Y. Lysyl oxidases: Emerging biomarkers and therapeutic targets for various diseases. Biomed. Pharmacother. 2020, 131, 110791. [Google Scholar] [CrossRef] [PubMed]
- Schmandke, A.; Schmandke, A.; Strittmatter, S.M. ROCK and Rho: Biochemistry and neuronal functions of Rho-associated protein kinases. Neuroscientist 2007, 13, 454–469. [Google Scholar] [CrossRef] [PubMed]
- Trombetta-Esilva, J.; Bradshaw, A.D. The Function of SPARC as a Mediator of Fibrosis. Open Rheumatol. J. 2012, 6, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhang, Y.; Fu, S.; Lu, Z.; Ye, W.; Xiao, Y. Angiotensin II as a morphogenic cytokine stimulating fibrogenesis of human tenon’s capsule fibroblasts. Investig. Ophthalmol. Vis. Sci. 2015, 56, 855–864. [Google Scholar] [CrossRef]
- Zhong, T.; Zhang, W.; Guo, H.; Pan, X.; Chen, X.; He, Q.; Yang, B.; Ding, L. The regulatory and modulatory roles of TRP family channels in malignant tumors and relevant therapeutic strategies. Acta Pharm. Sin. B 2022, 12, 1761–1780. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Siddiqui, S.S.; Shen, X.; Malik, A.B.; Pulido, J.S.; Kumar, N.M.; Yue, B.Y. RNA interference targeting transforming growth factor-beta type II receptor suppresses ocular inflammation and fibrosis. Mol. Vis. 2004, 10, 703–711. [Google Scholar] [PubMed]
- Walkden, A.; Au, L.; Fenerty, C. Trabeculectomy Training: Review of Current Teaching Strategies. Adv. Med. Educ. Pract. 2020, 11, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Fan Gaskin, J.C.; Nguyen, D.Q.; Soon Ang, G.; O’Connor, J.; Crowston, J.G. Wound Healing Modulation in Glaucoma Filtration Surgery-Conventional Practices and New Perspectives: The Role of Antifibrotic Agents (Part I). J. Curr. Glaucoma Pract. 2014, 8, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Minckler, D.S.; Francis, B.A.; Hodapp, E.A.; Jampel, H.D.; Lin, S.C.; Samples, J.R.; Smith, S.D.; Singh, K. Aqueous shunts in glaucoma: A report by the American Academy of Ophthalmology. Ophthalmology 2008, 115, 1089–1098. [Google Scholar] [CrossRef]
- Schlunck, G.; Meyer-ter-Vehn, T.; Klink, T.; Grehn, F. Conjunctival fibrosis following filtering glaucoma surgery. Exp. Eye Res. 2016, 142, 76–82. [Google Scholar] [CrossRef]
- Epstein, E. Fibrosing response to aqueous. Its relation to glaucoma. Br. J. Ophthalmol. 1959, 43, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.; Iserovich, P. Pro-inflammatory cytokines in glaucomatous aqueous and encysted Molteno implant blebs and their relationship to pressure. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4851–4855. [Google Scholar] [CrossRef] [PubMed]
- Fuller, J.R.; Bevin, T.H.; Molteno, A.C.; Vote, B.J.; Herbison, P. Anti-inflammatory fibrosis suppression in threatened trabeculectomy bleb failure produces good long term control of intraocular pressure without risk of sight threatening complications. Br. J. Ophthalmol. 2002, 86, 1352–1354. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Corticosteroid effects on cell signalling. Eur. Respir. J. 2006, 27, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Chang-Lin, J.E.; Attar, M.; Acheampong, A.A.; Robinson, M.R.; Whitcup, S.M.; Kuppermann, B.D.; Welty, D. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Investig. Ophthalmol. Vis. Sci. 2011, 52, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.D.; Hammel, N.; Fenerty, C.; Karaconji, T. Glaucoma Drainage Devices. In Surgical Management of Childhood Glaucoma; Grajewski, A., Bitrian, E., Papadopoulos, M., Freedman, S., Eds.; Springer: Cham, Switzerland, 2018; pp. 99–127. [Google Scholar] [CrossRef]
- Pinchuk, L.; Riss, I.; Batlle, J.F.; Kato, Y.P.; Martin, J.B.; Arrieta, E.; Palmberg, P.; Parrish, R.K., 2nd.; Weber, B.A.; Kwon, Y.; et al. The development of a micro-shunt made from poly(styrene-block-isobutylene-block-styrene) to treat glaucoma. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Blasco, B.; García-Feijóo, J.; Perucho-Gonzalez, L.; Güemes-Villahoz, N.; Morales-Fernandez, L.; Mendez-Hernández, C.D.; Martinez de la Casa, J.M.; Konstas, A.G. Evaluation of a Novel Ab Εxterno MicroShunt for the Treatment of Glaucoma. Adv. Ther. 2022, 39, 3916–3932. [Google Scholar] [CrossRef]
- Beckers, H.J.M.; Aptel, F.; Webers, C.A.B.; Bluwol, E.; Martínez-de-la-Casa, J.M.; García-Feijoó, J.; Lachkar, Y.; Méndez-Hernández, C.D.; Riss, I.; Shao, H.; et al. Safety and Effectiveness of the PRESERFLO® MicroShunt in Primary Open-Angle Glaucoma: Results from a 2-Year Multicenter Study. Ophthalmol. Glaucoma 2022, 5, 195–209. [Google Scholar] [CrossRef]
- Perkins, T.W.; Cardakli, U.F.; Eisele, J.R.; Kaufman, P.L.; Heatley, G.A. Adjunctive mitomycin C in Molteno implant surgery. Ophthalmology 1995, 102, 91–97. [Google Scholar] [CrossRef]
- Perkins, T.W.; Gangnon, R.; Ladd, W.; Kaufman, P.L.; Libby, C.M. Molteno implant with mitomycin C: Intermediate-term results. J. Glaucoma 1998, 7, 86–92. [Google Scholar] [CrossRef]
- Lee, D.; Shin, D.H.; Birt, C.M.; Kim, C.; Kupin, T.H.; Olivier, M.M.; Khatana, A.K.; Reed, S.Y. The effect of adjunctive mitomycin C in Molteno implant surgery. Ophthalmology 1997, 104, 2126–2135. [Google Scholar] [CrossRef] [PubMed]
- Cantor, L.; Burgoyne, J.; Sanders, S.; Bhavnani, V.; Hoop, J.; Brizendine, E. The effect of mitomycin C on Molteno implant surgery: A 1-year randomized, masked, prospective study. J. Glaucoma 1998, 7, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Al-Mobarak, F.; Khan, A.O. Two-year survival of Ahmed valve implantation in the first 2 years of life with and without intraoperative mitomycin-C. Ophthalmology 2009, 116, 1862–1865. [Google Scholar] [CrossRef] [PubMed]
- Do, A.T.; Parikh, H.; Panarelli, J.F. Subconjunctival microinvasive glaucoma surgeries: An update on the Xen gel stent and the PreserFlo MicroShunt. Curr. Opin. Ophthalmol. 2020, 31, 132–138. [Google Scholar] [CrossRef] [PubMed]
- DeBry, P.W.; Perkins, T.W.; Heatley, G.; Kaufman, P.; Brumback, L.C. Incidence of late-onset bleb-related complications following trabeculectomy with mitomycin. Arch. Ophthalmol. 2002, 120, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Yap, Z.L.; Seet, L.F.; Chu, S.W.; Toh, L.Z.; Ibrahim, F.I.; Wong, T.T. Effect of valproic acid on functional bleb morphology in a rabbit model of minimally invasive surgery. Br. J. Ophthalmol. 2022, 106, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Seet, L.F.; Yap, Z.L.; Chu, S.W.L.; Toh, L.Z.; Ibrahim, F.I.; Teng, X.; Wong, T.T. Effects of Valproic Acid and Mitomycin C Combination Therapy in a Rabbit Model of Minimally Invasive Glaucoma Surgery. Transl. Vis. Sci. Technol. 2022, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Ayyala, R.S.; Duarte, J.L.; Sahiner, N. Glaucoma drainage devices: State of the art. Expert. Rev. Med. Devices 2006, 3, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.A. Ab interno approach to the subconjunctival space using a collagen glaucoma stent. J. Cataract. Refract. Surg. 2014, 40, 1301–1306. [Google Scholar] [CrossRef]
- Shute, T.S.; Dietrich, U.M.; Baker, J.F.; Carmichael, K.P.; Wustenberg, W.; Ahmed, I.I.; Sheybani, A. Biocompatibility of a Novel Microfistula Implant in Nonprimate Mammals for the Surgical Treatment of Glaucoma. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3594–3600. [Google Scholar] [CrossRef]
- Acosta, A.C.; Espana, E.M.; Yamamoto, H.; Davis, S.; Pinchuk, L.; Weber, B.A.; Orozco, M.; Dubovy, S.; Fantes, F.; Parel, J.M. A newly designed glaucoma drainage implant made of poly(styrene-b-isobutylene-b-styrene): Biocompatibility and function in normal rabbit eyes. Arch. Ophthalmol. 2006, 124, 1742–1749. [Google Scholar] [CrossRef] [PubMed]
- van Mechelen, R.; Wolters, J.E.; Herfs, M.; Bertens, C.J.F.; Gijbels, M.; Pinchuk, L.; Gorgels, T.; Beckers, H.J.M. Wound Healing Response After Bleb-Forming Glaucoma Surgery With a SIBS Microshunt in Rabbits. Trans. Vis. Sci. Technol. 2022, 11, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.G.; Sinha, N.R.; Mohan, R.R.; Webel, A.D. Novel Therapies for the Prevention of Fibrosis in Glaucoma Filtration Surgery. Biomedicines 2023, 11, 657. [Google Scholar] [CrossRef] [PubMed]
- Cabourne, E.; Clarke, J.C.; Schlottmann, P.G.; Evans, J.R. Mitomycin C versus 5-Fluorouracil for wound healing in glaucoma surgery. Cochrane Database Syst. Rev. 2015, 2015, CD006259. [Google Scholar] [CrossRef] [PubMed]
- Jampel, H.D. Effect of brief exposure to mitomycin C on viability and proliferation of cultured human Tenon’s capsule fibroblasts. Ophthalmology 1992, 99, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
- Bass, P.D.; Gubler, D.A.; Judd, T.C.; Williams, R.M. Mitomycinoid alkaloids: Mechanism of action, biosynthesis, total syntheses, and synthetic approaches. Chem. Rev. 2013, 113, 6816–6863. [Google Scholar] [CrossRef] [PubMed]
- Adegbehingbe, B.O.; Oluwatoyin, H.O. Intra-operative 5-FU in Glaucoma Surgery: A Nigerian Teaching Hospital Experience. Middle East. Afr. J. Ophthalmol. 2008, 15, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Horsley, M.B.; Kahook, M.Y. Anti-VEGF therapy for glaucoma. Curr. Opin. Ophthalmol. 2010, 21, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Araujo, S.V.; Spaeth, G.L.; Roth, S.M.; Starita, R.J. A ten-year follow-up on a prospective, randomized trial of postoperative corticosteroids after trabeculectomy. Ophthalmology 1995, 102, 1753–1759. [Google Scholar] [CrossRef]
- Barnes, P.J. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br J Pharmacol. 2006, 148, 245–254. [Google Scholar] [CrossRef]
- Lama, P.J.; Fechtner, R.D. Antifibrotics and wound healing in glaucoma surgery. Surv. Ophthalmol. 2003, 48, 314–346. [Google Scholar] [CrossRef] [PubMed]
- Broadway, D.C.; Grierson, I.; Stürmer, J.; Hitchings, R.A. Reversal of topical antiglaucoma medication effects on the conjunctiva. Arch. Ophthalmol. 1996, 114, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.C.; Parapuram, S.K.; Tripathi, B.J.; Zhong, Y.; Chalam, K.V. Corticosteroids and glaucoma risk. Drugs Aging 1999, 15, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, H.N.; Mills, D.W.; Brecker, B. Steroid-induced elevation of intraocular pressure. Arch. Ophthalmol. 1963, 70, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Whitescarver, T.D.; Hobbs, S.D.; Wade, C.I.; Winegar, J.W.; Colyer, M.H.; Reddy, A.; Drayna, P.M.; Justin, G.A. A History of Anti-VEGF Inhibitors in the Ophthalmic Literature: A Bibliographic Review. J. Vitr. Dis. 2020, 5, 304–312. [Google Scholar] [CrossRef]
- Lopilly Park, H.Y.; Kim, J.H.; Ahn, M.D.; Park, C.K. Level of vascular endothelial growth factor in tenon tissue and results of glaucoma surgery. Arch. Ophthalmol. 2012, 130, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, A.A. Trabeculectomy with or without Intraoperative Sub-conjunctival Injection of Bevacizumab in Treating Refractory Glaucoma. J. Clin. Exp. Ophthalmol. 2011, 2, 2. [Google Scholar] [CrossRef]
- Muhsen, S.; Compan, J.; Lai, T.; Kranemann, C.; Birt, C. Postoperative adjunctive bevacizumab versus placebo in primary trabeculectomy surgery for glaucoma. Int. J. Ophthalmol. 2019, 12, 1567–1574. [Google Scholar] [CrossRef]
- Kitazawa, Y.; Kawase, K.; Matsushita, H.; Minobe, M. Trabeculectomy with mitomycin. A comparative study with fluorouracil. Arch. Ophthalmol. 1991, 109, 1693–1698. [Google Scholar] [CrossRef]
- Katz, G.J.; Higginbotham, E.J.; Lichter, P.R.; Skuta, G.L.; Musch, D.C.; Bergstrom, T.J. Mitomycin C versus 5-fluorouracil in high-risk glaucoma filtering surgery. Extended follow-up. Ophthalmology 1995, 102, 1263–1299. [Google Scholar] [CrossRef]
- Lamping, K.A.; Belkin, J.K. 5-Fluorouracil and mitomycin C in pseudophakic patients. Ophthalmology 1995, 102, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Zadok, D.; Zadok, J.; Turetz, J.; Krakowski, D.; Nemet, P. Intraoperative mitomycin versus postoperative 5-fluorouracil in primary glaucoma filtering surgery. Ann. Ophthalmol. Glaucoma 1995, 27, 336–340. [Google Scholar]
- Cohen, J.S.; Greff, L.J.; Novack, G.D.; Wind, B.E. A placebo controlled, double-masked evaluation of mitomycin C in combined glaucoma and cataract procedures. Ophthalmology 1996, 103, 1934–1942. [Google Scholar] [CrossRef]
- Costa, V.P.; Comegno, P.E.; Vasconcelos, J.P.; Malta, R.F.; Jose, N.K. Low Dose mitomycin C trabeculectomy in patients with advanced glaucoma. J. Glaucoma 1996, 5, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.W.; Alward, W.L.; Barad, J.P.; Zimmerman, M.B.; Carney, B.L. A randomized study of mitomycin augmentation in combined phacoemulsification and trabeculectomy. Ophthalmology 1997, 104, 719–724. [Google Scholar] [CrossRef]
- Singh, K.; Byrd, S.; Egbert, P.R.; Budenz, D. Risk of hypotony after primary trabeculectomy with antifibrotic agents in a black west African population. J. Glaucoma 1998, 7, 82–85. [Google Scholar] [CrossRef]
- Singh, K.; Egbert, P.R.; Byrd, S.; Budenz, D.L.; Williams, A.S.; Decker, J.H. Trabeculectomy with intraoperative 5-fluorouracil vs mitomycin C. Am. J. Ophthalmol. 1997, 123, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Andreanos, D.; Georgopoulos, G.T.; Vergados, J.; Papaconstantinou, D.; Liokis, N.; Theodossiadis, P. Clinical evaluation of the effect of mitomycin-C in re-operation for primary open angle glaucoma. Eur. J. Ophthalmol. 1997, 7, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Mehta, K.; Shaikh, N.M.; Tsai, J.C.; Moster, M.R.; Budenz, D.L. Trabeculectomy with intraoperative mitomycin C versus 5-fluorouracil. Prospective randomized clinical trial. Ophthalmology 2000, 107, 2305–2309. [Google Scholar] [CrossRef]
- WuDunn, D.; Cantor, L.B.; Palanca-Capistrano, A.M.; Hoop, J.; Alvi, N.P.; Finley, C. A prospective randomized trial comparing intraoperative 5-fluorouracil vs mitomycin C in primary trabeculectomy. Am. J. Ophthalmol. 2002, 134, 521–528. [Google Scholar] [CrossRef]
- Sisto, D.; Vetrugno, M.; Trabucco, T.; Cantatore, F.; Ruggeri, G.; Sborgia, C. The role of antimetabolites in filtration surgery for neovascular glaucoma: Intermediate-term follow-up. Acta Ophthalmol. Scand. 2007, 85, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Mostafaei, A. Augmenting trabeculectomy in glaucoma with subconjunctival mitomycin C versus subconjunctival 5- fluorouracil: A randomized clinical trial. Clin. Ophthalmol. 2011, 5, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Fendi, L.; Arruda, G.; Scott, I.; Paula, J. Mitomycin C versus 5-fluorouracil as an adjunctive treatment for trabeculectomy: A meta-analysis of randomized clinical trials. Clin. Exp. Ophthalmol. 2013, 41, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, C.K.; Upadhyay, N.; Verma, A.; Jain, A.; Charyulu, R.N.; Jain, S. Nanomedicine and Tissue Engineering, Nanotechnology Applications for Tissue Engineering; William Andrew Publishing: Norwich, NY, USA, 2015; pp. 1–19. ISBN 9780323328890. [Google Scholar] [CrossRef]
- Zarbin, M.A.; Montemagno, C.; Leary, J.F.; Ritch, R. Nanotechnology in ophthalmology. Can. J. Ophthalmol. 2010, 45, 457–476. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Kim, S.H.; Khang, D.; Lee, J.Y. Potential Therapeutic Usage of Nanomedicine for Glaucoma Treatment. Int. J. Nanomed. 2020, 15, 5745–5765. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wang, J.; Wang, T.; Zhong, J.; Bao, Y.; Hao, H. Recent Progress on nanostructures for drug delivery applications. J. Nanomater. 2016, 2016, 5762431. [Google Scholar] [CrossRef]
- Bao, H.; Jiang, K.; Meng, K.; Liu, W.; Liu, P.; Du, Y.; Wang, D. TGF-β2 induces proliferation and inhibits apoptosis of human Tenon capsule fibroblast by miR-26 and its targeting of CTGF. Biomed. Pharmacother. 2018, 104, 558–565. [Google Scholar] [CrossRef]
- Tong, J.; Fu, Y.; Xu, X.; Fan, S.; Sun, H.; Liang, Y.; Xu, K.; Yuan, Z.; Ge, Y. TGF-Β1 Stimulates Human Tenon’s Capsule Fibroblast Proliferation by MiR-200b and Its Targeting of P27/Kip1 and RND3. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2747–2756. [Google Scholar] [CrossRef]
- Drewry, M.D.; Challa, P.; Kuchtey, J.G.; Navarro, I.; Helwa, I.; Hu, Y.; Mu, H.; Stamer, W.D.; Kuchtey, R.W.; Liu, Y. Differentially Expressed MicroRNAs in the Aqueous Humor of Patients with Exfoliation Glaucoma or Primary Open-Angle Glaucoma. Hum. Mol. Genet. 2018, 27, 1263–1275. [Google Scholar] [CrossRef]
- Yu, S.; Tam, A.L.C.; Campbell, R.; Renwick, N. Emerging Evidence of Noncoding RNAs in Bleb Scarring after Glaucoma Filtration Surgery. Cells 2022, 11, 1301. [Google Scholar] [CrossRef]
- Wang, W.-H.; Deng, A.-J.; He, S.-G. A Key Role of MicroRNA-26a in the Scar Formation after Glaucoma Filtration Surgery. Artif. Cells Nanomed. Biotechnol. 2018, 46, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Ran, W.; Zhu, D.; Feng, Q. TGF-Β2 Stimulates Tenon’s Capsule Fibroblast Proliferation in Patients with Glaucoma via Suppression of MiR-29b Expression Regulated by Nrf2. Int. J. Clin. Exp. Pathol. 2015, 8, 4799–4806. [Google Scholar] [PubMed]
- Deng, M.; Hou, S.-Y.; Tong, B.-D.; Yin, J.-Y.; Xiong, W. The Smad2/3/4 Complex Binds MiR-139 Promoter to Modulate TGFβ-Induced Proliferation and Activation of Human Tenon’s Capsule Fibroblasts through the Wnt Pathway. J. Cell. Physiol. 2019, 234, 13342–13352. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Sun, Y.-B.; Hao, J.-L.; Lu, C.-W.; Bi, M.-C.; Song, E. Neuroprotective Effects of Overexpressed MicroRNA-200a on Activation of Glaucoma-Related Retinal Glial Cells and Apoptosis of Ganglion Cells via Downregulating FGF7-Mediated MAPK Signaling Pathway. Cell Signal. 2019, 54, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Chen, F.; Du, W.; Zhu, J.; Xie, Z. TGF-Β1 Induces Human Tenon’s Fibroblasts Fibrosis via MiR-200b and Its Suppression of PTEN Signaling. Curr. Eye Res. 2019, 44, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Fu, Y.; Tong, J.; Fan, S.; Xu, K.; Sun, H.; Liang, Y.; Yan, C.; Yuan, Z.; Ge, Y. MicroRNA-216b/Beclin 1 Axis Regulates Autophagy and Apoptosis in Human Tenon’s Capsule Fibroblasts upon Hydroxycamptothecin Exposure. Exp. Eye Res. 2014, 123, 43–55. [Google Scholar] [CrossRef]
- Zhu, H.; Dai, L.; Li, X.; Zhang, Z.; Liu, Y.; Quan, F.; Zhang, P.; Yu, L. Role of the Long Noncoding RNA H19 in TGF-Β1-Induced Tenon’s Capsule Fibroblast Proliferation and Extracellular Matrix Deposition. Exp. Cell Res. 2020, 387, 111802. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, F.; Pan, Z.; Luo, H.; Liu, K.; Duan, X. LncRNA NR_003923 Promotes Cell Proliferation, Migration, Fibrosis, and Autophagy via the MiR-760/MiR-215-3p/IL22RA1 Axis in Human Tenon’s Capsule Fibroblasts. Cell Death Dis. 2019, 10, 594. [Google Scholar] [CrossRef]
- Sui, H.; Fan, S.; Liu, W.; Li, Y.; Zhang, X.; Du, Y.; Bao, H. LINC00028 Regulates the Development of TGFβ1-Treated Human Tenon Capsule Fibroblasts by Targeting MiR-204-5p. Biochem. Biophys. Res. Commun. 2020, 525, 197–203. [Google Scholar] [CrossRef]
- You, K.; Gu, H.; Yuan, Z.; Xu, X. Tumor Necrosis Factor Alpha Signaling and Organogenesis. Front. Cell Dev. Biol. 2021, 9, 727075. [Google Scholar] [CrossRef]
- Collotta, D.; Colletta, S.; Carlucci, V.; Fruttero, C.; Fea, A.M.; Collino, M. Pharmacological Approaches to Modulate the Scarring Process after Glaucoma Surgery. Pharmaceuticals 2023, 16, 898. [Google Scholar] [CrossRef] [PubMed]
- Ebert, E.C. Infliximab and the TNF-alpha system. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G612–G620. [Google Scholar] [CrossRef] [PubMed]









| Parameter | Description |
|---|---|
| Population | Patients with glaucoma regardless of the site. |
| Intervention | Incisional/filtration glaucoma procedures, with or without antifibrotic agents. |
| Comparison | Results of patients who underwent glaucoma surgery with and without antifibrotic agents. |
| Outcomes | Quality of IOP control, postoperative complications, visual acuity. |
| Study Design | Randomized or nonrandomized controlled (or uncontrolled). |
| Antifibrotic Targets | Mechanism of Action | Applications |
|---|---|---|
| IL-1 [29] | IL-1 controls integrin expression in leukocytes and endothelial cells. | 1-methyl hydrazino analogs are an excellent IL-1 blocker and reduce inflammation. |
| IL-6 [28,30] | IL-6 stimulates B-cell differentiation, T-cell activation, and immunoglobulin production. | Tocilizumab is an anti- IL-6 receptor antibody, which, in a rheumatoid arthritis clinical study, reduced inflammation and fibrosis. |
| IL-7 [31,32] | IL-7 is a profibrotic growth factor and activates signaling that suppresses fibroblast-driven ECM expression. | In a septic shock trial, IL-7 application restored CD4+ and CD8 cell count. |
| IL-10 [33,34,35] | IL-10 is an anti-inflammatory cytokine which reduces production of inflammatory cytokine mRNA. | In a mice study, IL-10 increased the number of neutrophils and monocytes. |
| IL-22 [36,37,38] | IL-22, a pro-inflammatory cytokine, upregulates acute phase proteins. | In a hepatitis clinical trial, IL-22 protected against epithelial cell injury and reduced inflammation. |
| Anti-VEGF [39] | VEGF is a potent mediator of angiogenesis, vasculogenesis and vascular endothelial cell permeability. | Anti-VEGF therapies inhibit vascular endothelial growth factor, thus preventing angiogenesis and the disruption of the blood–retinal barrier. |
| Platelet-derived growth factor (PDGF) [40] | The PDGF family consists of disulphide-linked dimers and induces proliferation of macrophages and fibroblasts migration into a wound site. | ARC126 and ARC127 are PDGFβ inhibitors, and they reduced both epiretinal membrane formation and retinal detachment. |
| Connective tissue growth factor (CTGF) [41] | CTGF is a fibrogenic cytokine upregulated by TGF-β causes persistent fibrosis through CTGF. | Targeting either CTGF or TGF-β signaling may reduce scar tissue formation. |
| Matrix metalloproteinases (MMPs) [28,42] | MMPs are a group of proteolytic enzymes which degrade most extracellular matrix proteins during wound remodeling. | Administration of GM6001, an MMP inhibitor, reduced scar formation after glaucoma surgery in rabbits. |
| Lysyl oxidase (LOX) and lysyl oxidase-like proteins (LOXL) [28,43] | Lysyl oxidase (LOX) and lysyl oxidase-like (LOXL) are ECM enzymes which crosslink collagen and elastin, leading to fibrosis. | Anti LOXL2 monoclonal antibody (GS-607601) reduced inflammation and fibrosis after glaucoma surgery in rabbits. |
| Rho kinase inhibitors [28,44] | ROCK 1 and 2 are downstream components of Rho-GTPase Rho mediated signaling and play an important role in cytoskeletal organization controlling cellular morphology migration and motility. Rac1 is a low-molecular-weight Rho GTPase. | In a lab experiment, inhibiting Rac1 with NSC23766 or siRNA achieved reduction in conjunctival tissue fibrosis and collagen matrix contraction. |
| Secreted protein acidic and rich in cysteine (SPARC) inhibitors [28,45] | SPARC is a 43 kDa collagen-binding matricellular glycoprotein that modulates cellular interactions with the surrounding ECM. SPARC contributes to ECM organization and cell migration. | In an in vitro experiment, SPARC knockdown resulted in TGFβ2-driven upregulation of Type I collagen, and fibronectin expression was suppressed. Reducing SPARC expression may suppress subconjunctival fibrosis. |
| Angiotensin II [28,46] | Angiotensin II is an effector molecule and causes ocular fibrosis. Activation of NF-κB by angiotensin II leads to the survival of corneal myofibroblasts, and, consequently. fibrosis. | In lab experiments, angiotensin-converting enzyme inhibitors (ACE II s) and angiotensin receptor (AT2) antagonists effectively suppressed vascular damage. |
| Transient receptor potential (TRP) channel antagonists [28,47] | The TRP channels are activated by multiple endogenous and external stimuli and mediate several wound healing functions. Their receptor-induced responses include cell proliferation and migration, along with immune cell activation, tissue infiltration, and fibrosis. | In an alkali-burn mouse wound healing model, treatment with a TRPV1 antagonist effectively suppressed fibrosis. Additionally, in vitro experiments using ocular fibroblasts demonstrated that the TRPV1 antagonist inhibited the transdifferentiation of myofibroblasts. |
| Transforming growth factor-β (TGF-β) inhibitor [28,48] | TGF-β plays a significant role as an effective mediator in the development of scar tissue in the eye. | In lab experiments, tranilast suppressed TGF-β activation and resulted in the suppression of collagen production. In vitro experiments using siRNA to suppress the TGF-β type II receptor gene demonstrated both suppression of fibronectin production and inhibition of cell migration. |
| Agent | Mechanism of Action | Administration |
|---|---|---|
| Mitomycin C (MMC) [79] | An alkaloid, produced by Streptomyces caespitosus; works by inhibiting DNA-dependent RNA synthesis and triggering apoptosis. | Either via MMC-soaked sponge or subconjunctival injection postoperatively. |
| 5-fluorouracil (5-FU) [80] | A pyrimidine analog, interferes with ribosomal RNA synthesis; diminishes episcleral scar formation by inducing apoptosis of fibroblasts in Tenon’s capsule. | Similar to MMC. |
| Corticosteroids [84] | Reduce the expression of cytokines, such as TNF-alpha, IL-1, IL-2, IL-10, and IL-12, which decrease the number of tissue macrophages and blood monocytes during the inflammatory phase of wound healing. | Topical, subconjunctival injection, or oral perioperatively. |
| Bevacizumab [89] | Selectively binds to and blocks circulating VEGF to reduce micro-angiogenesis, thereby limiting the blood supply to scarred granulation tissue during the proliferative phase of wound healing. | Subconjunctival injection postoperatively. |
| Noncoding RNAs | Authors, Year, Country | Summary | Pro/Anti-Fibrotic Role |
|---|---|---|---|
| miR-26a | Wang et al., 2018, China [115] | miR-26a is significantly downregulated in filtering tract scars and is inversely correlated with connective tissue growth factor (CTGF) mRNA levels. | Anti-fibrotic |
| miR-29b | Ran et al., 2015, China [116] | TGF-β2 stimulates the proliferation of human tenon fibroblasts (HTF) by suppressing miR-29b expression, which is regulated by Nrf2. | Anti-fibrotic |
| miR-139 | Deng et al., 2019, China [117] | Overexpression of miR-139 effectively counteracted the TGFβ1-induced increase in collagen I and α-smooth muscle actin levels, as well as HTF proliferation. | Anti-fibrotic |
| miR-200a | Peng et al., 2019, China [118] | miR-200a is reduced, while FGF7 is increased in glaucoma. miR-200a has a protective function on the glaucomatous optic nerve injury through its effect by suppressing the MAPK signaling pathway mediated by FGF7. | Anti-fibrotic |
| miR-200b | Tong et al., 2019, China [119] | The induction of fibrosis in HTFs occurs through TGF-β1-mediated miR-200b by suppressing the PTEN gene signaling pathway. | Pro-fibrotic |
| miR216b | Xu et al., 2014, China [120] | miR-216b directly targeted and decreased the expression of Beclin 1, a pro-apoptotic molecule. In HTFs treated with hydroxycamptothecin, miR-216b regulates both autophagy and apoptosis by modulating Beclin 1. | Pro-fibrotic |
| Lnc H19 | Zhu et al., 2020, China [121] | TGF-β induced the expression of H19 in HTFs, and suppressing H19 inhibited the effects of TGF-β. The findings suggest that H19 modulates β-catenin expression via miR-200a in TGF-β-treated HTFs. Therefore, suppressing H19 may result in attenuation of scar after glaucoma surgery. | Pro-fibrotic |
| Lnc NR003923 | Zhao et al., 2019, China [122] | Inhibiting NR003923 expression in HTFs resulted in the suppression of cell migration, proliferation, fibrosis, and autophagy induced by TGF-β. | Pro-fibrotic |
| LINC00028 | Sui et al., 2020, China [123] | In HTFs treated with TGFβ1, the decrease in LINC00028 expression inhibits migration, proliferation, invasion, epithelial-mesenchymal transition, fibrosis, and autophagy. | Pro-fibrotic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dave, B.; Patel, M.; Suresh, S.; Ginjupalli, M.; Surya, A.; Albdour, M.; Kooner, K.S. Wound Modulations in Glaucoma Surgery: A Systematic Review. Bioengineering 2024, 11, 446. https://doi.org/10.3390/bioengineering11050446
Dave B, Patel M, Suresh S, Ginjupalli M, Surya A, Albdour M, Kooner KS. Wound Modulations in Glaucoma Surgery: A Systematic Review. Bioengineering. 2024; 11(5):446. https://doi.org/10.3390/bioengineering11050446
Chicago/Turabian StyleDave, Bhoomi, Monica Patel, Sruthi Suresh, Mahija Ginjupalli, Arvind Surya, Mohannad Albdour, and Karanjit S. Kooner. 2024. "Wound Modulations in Glaucoma Surgery: A Systematic Review" Bioengineering 11, no. 5: 446. https://doi.org/10.3390/bioengineering11050446