Molecular Mechanisms of Adipose Organ Remodelling
A special issue of Cells (ISSN 2073-4409). This special issue belongs to the section "Tissues and Organs".
Deadline for manuscript submissions: closed (31 December 2023) | Viewed by 4365
Special Issue Editors
Interests: diabetes; metabolic syndrome; nutrition; adipose organ; insulin resistance; metabolism; obesity; metabolic diseases; fat; metabolic endocrinology; breast; cancer; alveologenesis; pregnancy; lactation; postlactation
Special Issues, Collections and Topics in MDPI journals
Interests: adipose organ; cancer; heart; neurodegenerative diseases; regenerative medicine
Special Issue Information
Dear Colleagues,
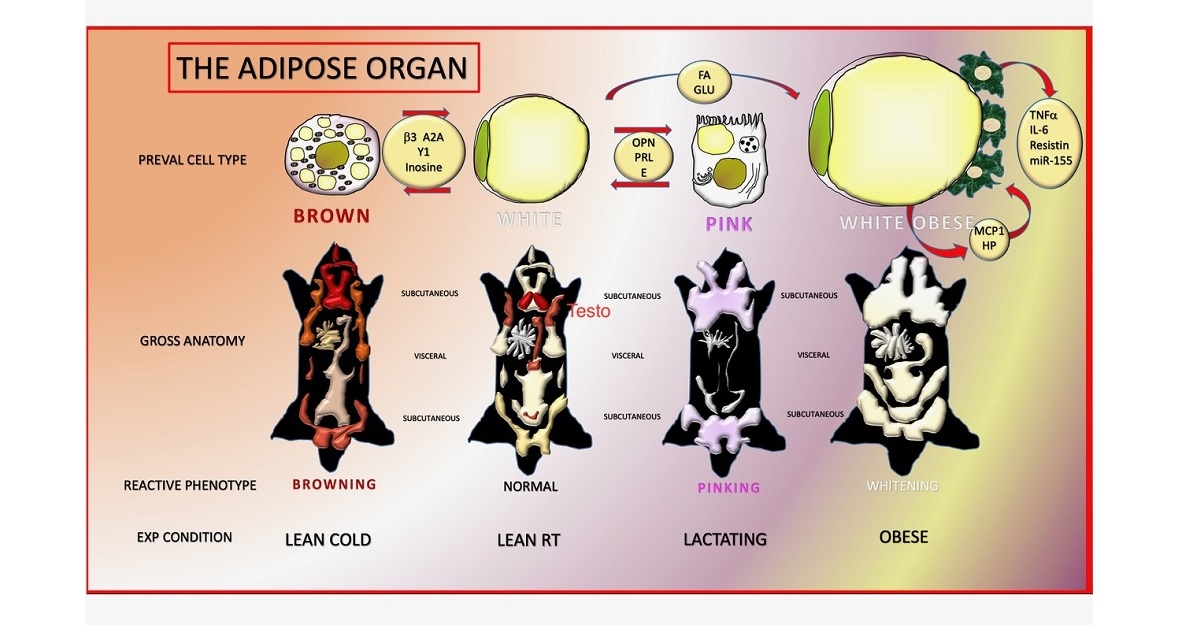
Adipose tissues are organized to form a dissectible structure called the adipose organ. White adipose tissue (WAT) is formed by unilocular spherical cells containing a lipid vacuole that occupies most of the cell volume. This cell secretes fatty acids required for the 24 hours activity of the heart and all other tissues. Furthermore, it also produces two hormones that induce nutritional behaviours (search for food and food intake) and hepatic glucogenesis. Brown adipose tissue (BAT) is formed by smaller cells rich in mitochondria with several small lipid vacuoles. BAT burns lipids in special mitochondria provided with a unique protein (UCP1) responsible for the final result of its physiology: thermogenesis. Thus, although both tissues are formed by cells fulfilling the definition of adipocytes because of their abundant cytoplasmic lipids, they have different anatomies and different physiologies, which raises the question of their eventual cooperation. Usually, different tissues contained in organs cooperate for a finalistic purpose. Cold is the physiologic stimulus for BAT, and chronic cold also induces a browning of WAT, with the increment of thermogenesis. On the other hand, during a normal diet, WAT takes care of the metabolic needs of the organism, but in the case of a chronic positive energy balance, the organism exerts all of its ability to store precious calories, considering that a fasting period is not predictable; thus, in this particular condition, BAT convert into WAT to increase the body’s storing capacity. This theory offers an explanation as to why WAT and BAT are contained in the same organ, but implies a novel basic property for cells: the ability for a physiologic reversible gene reprogramming with a change of phenotype. We observed another striking example of cell conversion in the adipose organ during pregnancy and lactation. The alveolar part of mammary glands develops only in this period, in which the adipose component of the organ gradually disappears. Electron microscopy, lineage tracing, and explants data suggest that mammary alveolar cells are derived, at least in part, from the direct conversion of adipocytes.
Thus, the adipose organ is provided with a high level of plasticity, and a deeper knowledge of the molecular mechanisms responsible for its plasticity ,main purpose of this special issue, could open novel avenues for drug targets to treat obesity and related disorders, including breast cancer.
Prof. Dr. Saverio CintiDr. Laura Graciotti
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the special issue website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- adipose organ
- white adipose tissue
- brown adipose tissue
- breast
- alveologenesis
- pregnancy
- lactation
- post-lactation
- transdifferentiation
- conversion
- plasticity
- remodelling
- molecular mechanisms
- obesity
- diabetes
- fat
- endocrinology







