1. Introduction
Herba Epimedii, the dried aerial parts of
Epimedium brevicornu Maxim,
Epimedium sagittatum Maxim,
Epimedium pubescens Maxim and
Epimedium koreanum Nakai, have been traditionally used as tonic, aphrodisiac and antirheumatic medical herbs for cardiovascular, bone loss and impotence diseases in East Asian countries for many years. The major active constituents of Epimedii are flavonoids, of which more than 141 flavonoid compounds, including flavonols, flavones, chalcones, flavanones, and flavonol glycosides, have been isolated [
1,
2]. Icariin (
Figure 1), one of the most abundant flavonoids of Herba Epimedii, has numerous pharmacological and biological activities, including prevention of osteoporosis, erectile impotence, and anti-cancer and anti-depression properties [
3,
4,
5].
Figure 1.
Chemical structure of icariin.
Figure 1.
Chemical structure of icariin.
In the clinic,
Epimedium fried with suet oil was usually used, which could enhance the kidney invigorating and “Yang” strengthening roles of
Epimedium [
6]. “Frying” treatment consists of two parts: “heat” and “suet oil”. The role the “heat” factor during processing has been clarified before: heating causes
Epimedium to produce more absorbable bioactive flavonoids [
7]. However, the mechanism of action of how suet oil increases the effects has not been elucidated.
Sodium deoxycholate (DOC), used in the preparation of self-assembled nanomicelles, is one of the bile acid salts, inherent to the human body. It is a natural biosurfactant, which can promote the preparation of micelles, vesicles and other drug carriers. Bile salts can work with lipids such as phospholipids, glycerides, fatty acids, as well as other surface-active agent to prepare mixed micelles. These mixed micelles can promote a drug’s absorption as the carrier [
8].
Suet oil is a kind of fatty oil from Capra hircus linnaeus or Ovis aries linnaeus, containing unsaturated fatty acids, linoleic acid and saturated fatty acids and so on. Its properties are similar to that of a surfactant, which is helpful for the preparation of nanomicelles. Our experiments found that suet oil can work with DOC to prepare mixed micelles, as the carrier of icariin under simulated gastrointestinal tract conditions. This triggered us to explore how suet oil plays its role in the formulation of nanomicelles, and to explain the relationship between self-assembled nanomicelles with the kidney invigorating and “Yang” strengthening mechanism of Epimedium fried with suet oil. We selected icariin as the model flavonoid, and prepared icariin self-assembled nanomicelles with and without suet oil in vitro, and characterized them by Malvern Instruments size determination and TEM. The differences between self-assembled nanomicelles with and without suet oil on the transport of icariin across Caco-2 cell monolayers and its absorption in rat intestine were also evaluated and compared. Thus, the action mechanism of suet oil was preliminarily clarified.
3. Experimental
3.1. Materials
Cloned Caco-2 TC7 cells were a kind gift from Dr. Moniqué Rousset of INSERM U178, (Villejuit, France). Icariin (purity > 98%) was purchased from National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China), and sodium deoxycholate was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Hanks’ balanced salt solution (HBSS; powder form) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Suet oil was provided by Hu Qiao Pharmaceutical Co., Ltd. (Bozhou, China). Milli-Q water (Millipore, Bedford, MA, USA) was used in the study. Acetonitrile was of chromatographic grade (Merck Company Inc., Whitehouse Station, NJ, USA). All other reagents were of analytical grade.
3.2. Preparation of the Self-Assembled Nanomicelles
3.2.1. The Preparation of the icariin Self-Assembled Nanomicelles with Suet Oil
To prepare the icariin self-assembled nanomicelles with suet oil, icariin (0.676 mg) was mixed with suet oil (0.1352 mg) and the mixture was put into HBSS buffer (50 mL, pH 7.4) containing DOC (0.676 mg). Then, the solution was sonicated for 10 min at 100% power. The icariin self-assembled nanomicelles with suet oil were abbreviated as icariin + DOC + suet oil.
3.2.2. The Preparation of the Icariin Self-assembled Nanomicelles without Suet Oil
To prepare the icariin self-assembled nanomicelles without suet oil, icariin (0.676 mg) was dissolved in HBSS buffer (50 mL, pH 7.4) containing DOC (0.676 mg) and the solution was sonicated for 10 min at 100% power. The icariin self-assembled nanomicelles without suet oil were abbreviated as icariin + DOC.
3.3. Characterization of the Self-Assembled Nanomicelles
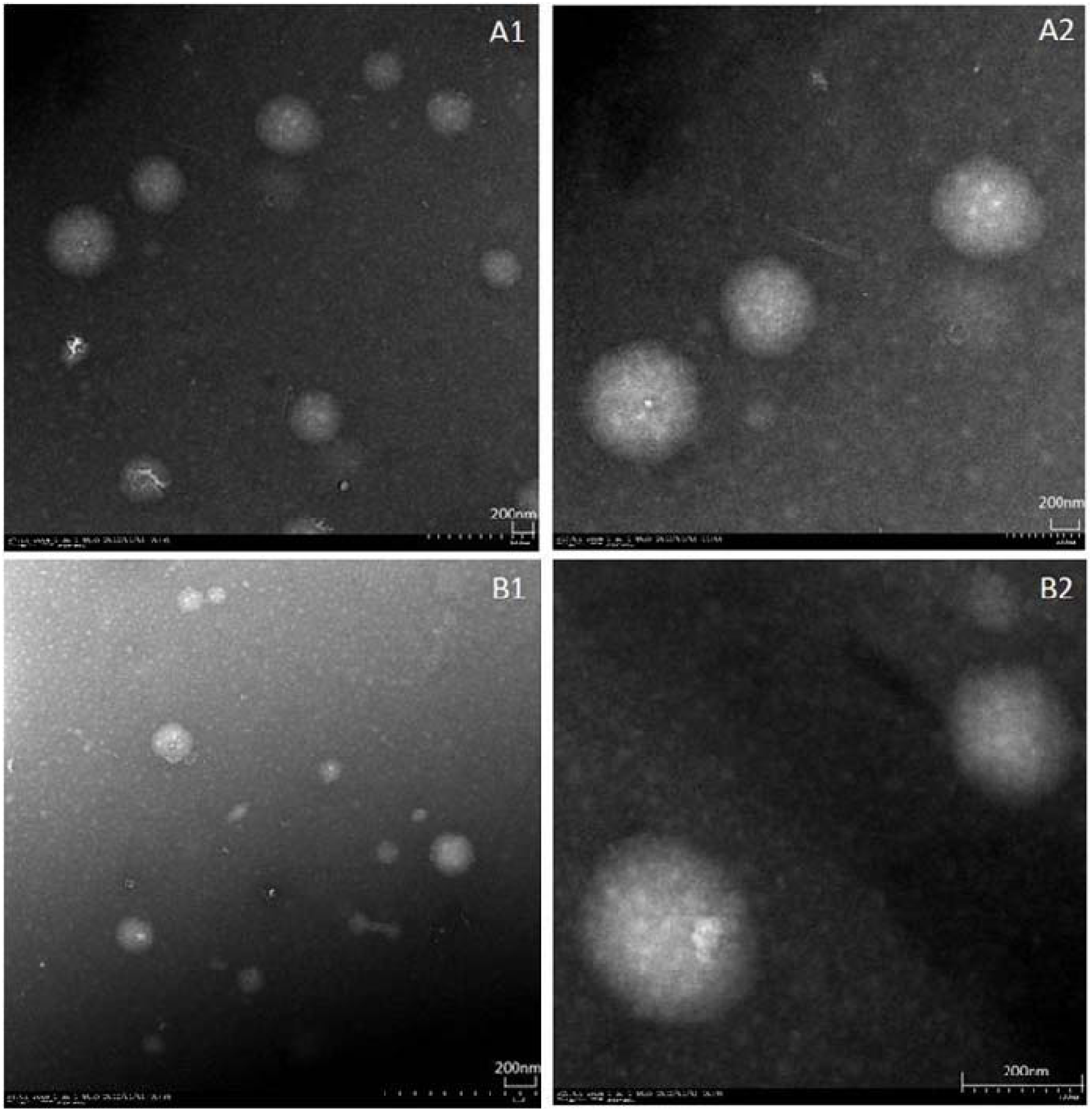
3.3.1. Particle Size, Size Distribution and Surface Morphology
The size and size distribution of self-assembled nanomicelles were determined by dynamic light scattering (DLS) on a Zetasizer Nano-ZS serie MAL1021404 (Malvern Instruments S.A., Worcestershire, UK). The morphological evaluation was performed by transmission electron microscopy (TEM, JEM-1200EX, JEOL Technics Co., Ltd., Akishima, Japan).
3.3.2. The Encapsulation Efficiency of Self-Assembled Nanomicelles
The self-assembled nanomicelles were obtained by ultracentrifugation at 30,000 rpm for 15 min. Then a HPLC assay was used to measure the amount of icariin in the supernatant. The drug entrapment efficiency (E.E.) was expressed as percentage of the icariin difference between the initial amount of icariin and the amount in the supernatant relative to the total amount used for the self-assembled micelle preparation.
The content of icariin in the supernatant was determined by the HPLC method as described below. Approximately 1 mL of the supernatant was dissolved in methanol, and the final volume was 5 mL, then a 20 μL aliquot of the resulting solution was injected into a HPLC system.
3.4. Cell Experiments
3.4.1. Cell Culture
The Caco-2 TC7 cell line was a kind gift from the laboratories of Dr. Moniqué Rousset of INSERM U178 (Villejuit, France) and was nominally similar to wild-type Caco-2 cells [
9], but it is more stable during passage since it is a cloned cell line. The culture conditions for growing Caco-2 cells have been described previously [
10,
11,
12]. For the transport assay, cells were seeded on top of Transwell inserts, consisting of 3 μm porous polycarbonate, in 6-well Transwell plates, which have a surface area of 4.2 cm
2. The seeding density (100,000 cells/cm
2), growth media (Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum), and quality control criteria were all carried out according to previously published reports [
10,
11,
12]. The Caco-2 TC7 cells were maintained at 37 °C at 90% humidity and 5% CO
2. The culture media was changed every 48 h. The monolayers, whose ecology and appearance were well developed and the transepithelial electrical resistance (TEER) values were greater than 250 Ω × cm
2, were ready for experiments between 21 and 23 days [
13] after seeding.
3.4.2. Transport Experiments in the Caco-2 Cell Culture Model
Experiments in triplicate were performed in pH 7.4 HBSS. The protocol for carrying out cell culture experiments was described before [
10,
11,
12]. In short, the cell monolayers were washed three times with 37 °C HBSS. The transepithelial electrical resistance (TEER) values of cell monolayers were measured, which were more than 250 Ω × cm
2, meeting the requirement of experiment [
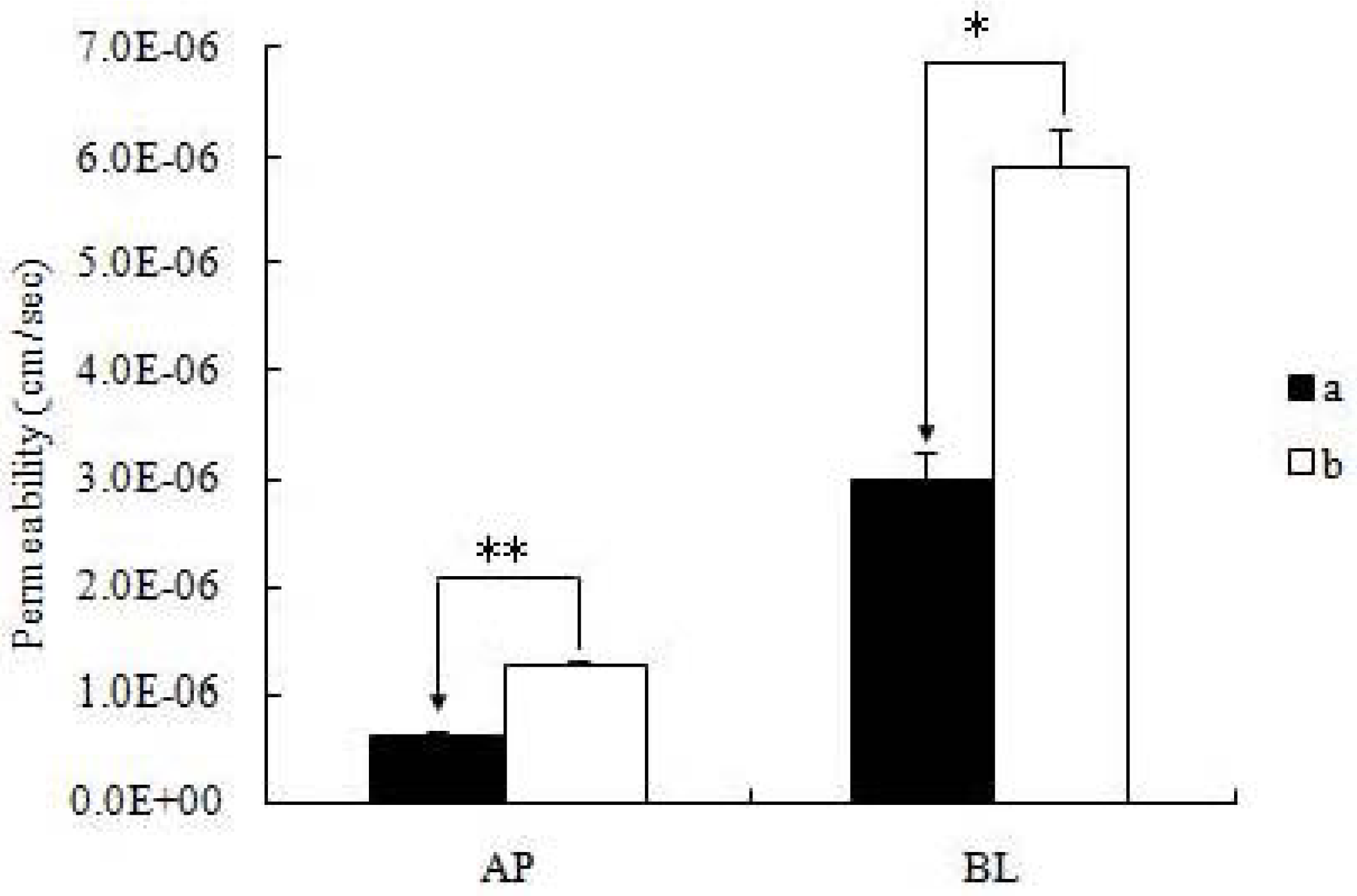
14]. The monolayers were incubated with the pH 7.4 blank HBSS for 1 h. Then the incubation medium was aspirated. Afterward, a solution containing the icariin was loaded on to the apical or basolateral side. The amount of transported icariin in the receiver media was measured as a function of time using UPLC methods to follow. Donor samples (400 μL) and receiver samples (400 μL) were taken at different times (typically 1 h), followed by the addition of 400 μl of fresh donor solution to the donor side or 400 μL of blank buffer to the receiver side. When comparing the permeability of icariin + DOC and icariin + DOC + suet oil, each sample was used at the same concentration (20 μM) and the samples were taken at 0, 1, 2, 3 and 4 h after incubation. To each transport sample (400 μL), methanol (100 μL) containing 4 μM of genistein was added as an internal standard, and then the resulting mixtures were vortexed for 30 sec, centrifuged at 15,000 rpm for 15 min. The supernatant was analyzed by UPLC method (see below). At the end of the transport experiment, integrity of the monolayer was monitored by TEER value, and there was no significant change [
15].
3.4.3. Electrical Resistance
The integrity of the monolayers was determined by measuring the electrical resistance values at the end of each experiment. For this purpose, a Bosstek type instrument with two electrodes was used and the results were expressed as Ω × cm
2 [
16].
3.4.4. Cellular Accumulation Studies in the Cell Monolayers
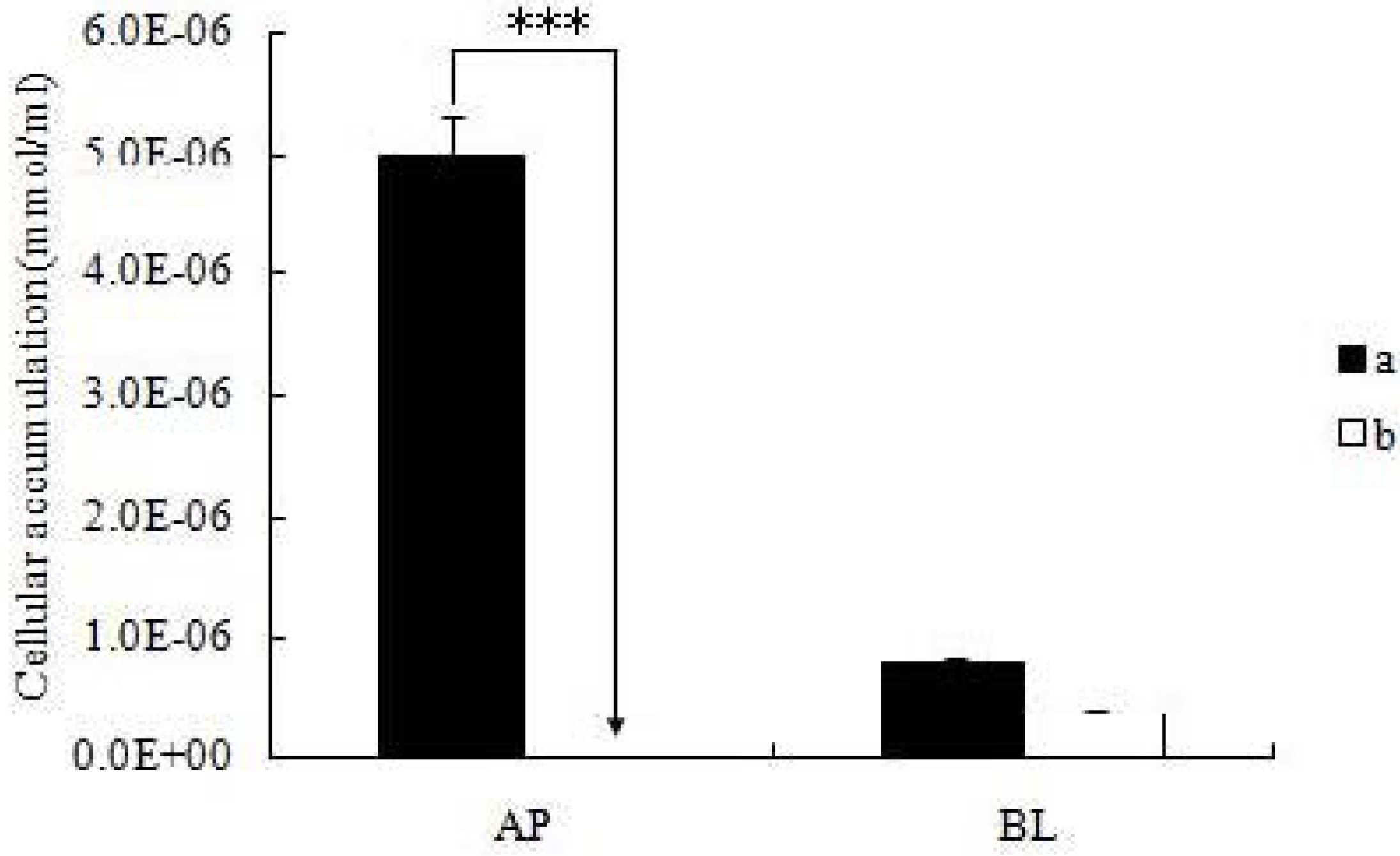
After the cell monolayers were incubated with icariin + DOC and icariin + DOC + suet oil for a predetermined period of time (4 h), respectively, the mature monolayers were gently washed three times with ice-cold saline immediately after the incubation buffer was removed. Subsequently, the monolayers were cut out together with the porous polycarbonate membranes, put in HBSS buffer (1 mL, pH 7.4), and sonicated in an ice-cold water bath for 30 min using KQ5200DE sonicator (Ultrasonic Instrument Co., Ltd., Kunshan, China) at the maximum power. Then the cellular lysates were centrifuged at 15,000 rpm for 15 min, so the supernatant can be injected into a UPLC instrument.
3.5. Animals Experiments
3.5.1. Animals
Male Sprague–Dawley rats weighing between 350 and 400 g were obtained from the SLEK Lab Animal Center of Shanghai (Shanghai, China), housed under standard conditions of temperature, humidity, and light. Food and water were provided ad libitum. The rats were fasted overnight before the day of the experiment.
3.5.2. Animal Surgery
The procedures were approved by the Animal Ethics Committee of Jiangsu Provincial Academy of Chinese Medicine. A day before the experiment, the rats were fasted overnight but were provided deionized water. After overnight fasting, rats were anesthetized. The small intestine was exposed by midline incision; the intestinal lumen was then gently flushed to remove intestinal content and each of the four segments (duodenum, upper jejunum, terminal ileum, and colon) of the intestine was cannulated with two cannulaes. The outlet of each segment was secured by ligation with silk suture. The intestine was carefully arranged and continuously monitored to avoid kinks and ensure a consistent flow after cannulation. Saline-soaked cotton was used to cover opened body cavities to prevent loss of fluids [
17].
3.5.3. Four Site Single-pass Rat Intestinal Perfusion Experiments
A single-pass perfusion method was used by perfusing the small intestine as one whole segment (from duodenum to ileum). To keep the temperature of the perfusate constant, the inlet cannulae was insulated and kept warm by a 37 °C circulating water bath. A flow rate of 0.412 mL/min was used, and the perfusate samples were collected every 30 min. The outlet concentration of drug in the perfusate was determined by UPLC.
3.6. Analytical Methods
UPLC method: The UPLC method was used to detect the icariin in the transport samples obtained from the Caco-2 model and the single-pass rat intestinal perfusion experiment. The conditions for UPLC analysis of icariin were as follows: Waters Acquity UPLC system with photodiode array detector and Empower software; column, Acquity UPLC BEH C18, 1.7 μm, 2.1 × 50 mm (Waters, Milford, MA, USA); mobile phase A, acetonitrile; mobile phase B, water containing 0.1% HCOOH; gradient, 0 to 0.5 min, 10% A, 0.5 to 1 min, 10% to 40% A, 1 to 2.5 min, 40% A, 2.5 to 3 min, 40% A to 100% A, 3 to 4 min, 10% A; flow rate, 0.4ml/min; colume temperature, 35 °C; wavelength, 270 nm; injection volume, 8 μL. The retention time for icariin is 1.395 min. The retention time for genistein as an internal standard is 1.590 min. In general, the method is selective and reproducible with day to day variability less than 2.5%. The accuracy and precision were greater than 98%. The tested linear response ranges for samples were 0.3125 to 40 μM.
HPLC method [
6]: The stationary phase, a Phenomenex® C18 (4.6 × 150mm, 5 μm) column, was used and kept at 30 °C. The mobile phase was a mixture of acetonitrile-water (75:25). The flow rate was 1.0 mL/min. Separation was monitored at 270 nm. The tested linear response ranges for samples were 0.02 to 0.64 μM.
3.7. Data Analysis
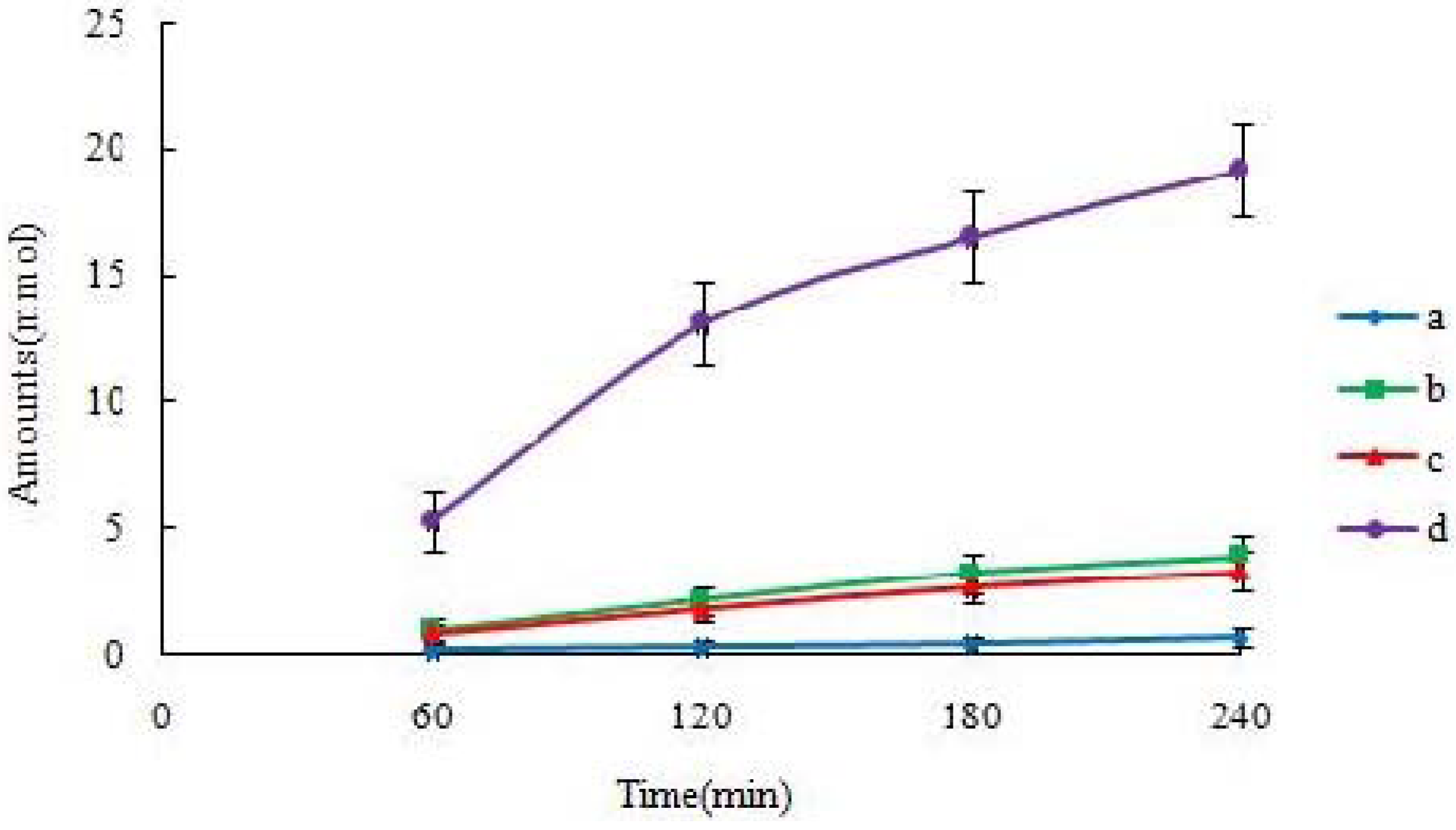
Rate of the transportation of icariin is obtained from amount transported
versus time curve using linear regression. The apparent permeability (Papp) of icariin is calculated using the following equation:
In the equation,
V is the volume of the receiver (typical volume is 2.5 mL),
S is the surface area of the cell monolayer (typical surface area is 4.2 cm
2),
C is the initial concentration,
dC/dt is the rate of concentration change in the receiver side, and
dM/dt is the rate of drug transport. The rate of drug transport is obtained by linear regression analysis (a Microsoft Excel function).
The absorption of drug was measured by the rate of its disappearance, and the permeability coefficient (
P*
eff) was determined through the rate of disappearance. The effective
P*
eff was calculated using the following equation:
In the equation,
Cm is the concentration of the permeant in the exiting perfusate;
Co is the concentration of the permeant in entering perfusate;
Gz, or Graetz number (
Gz = πDL/2
Q), is a scaling factor that incorporates flow rate (Q) and intestinal length (L), and diffusion coefficients (D) to make the permeability dimensionless. The experimental data was put into the formula, and the result could be obtained by the designed excel table. Values were indicated as mean ± S.D. for permeability in four independent rats.
3.8. Statistical Analysis
All experiments were conducted at least in triplicate. Data was presented as mean ± S.D. Student’s t test (Microsoft Excel) was used to analyze the data when there were only two groups in the experiments. A two-tailed t-test (p < 0.01) was used to identify significant differences of permeability results between the icariin + DOC group and icariin + DOC + suet oil group.
4. Conclusions
The DOC can promote the formation of micelles. Therefore, the icariin self-assembled nanomicelles without suet oil can be formed. However, the suet oil has a fatty acid composition, which have long fatty chains and surfactant properties. The self-assembled nanomicelles prepared with suet oil have smaller size than those without suet oil, and are more stable. Under the joint action of suet oil and DOC, self-assembled hybrid nanomicelles could be prepared. The formulated carrier could promote the drug’s absorption [
18,
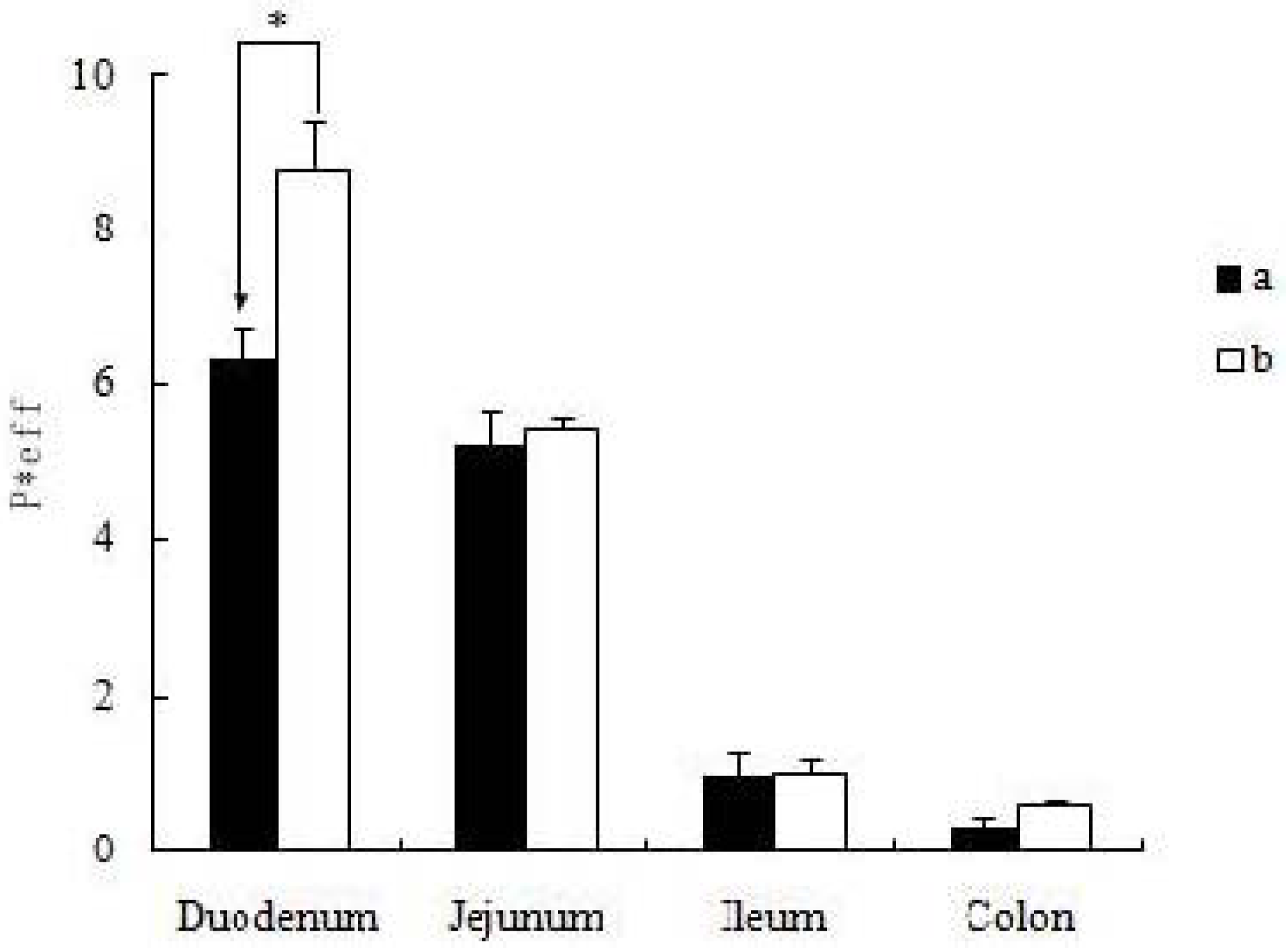
19]. The self-assembled nanomicelles with suet oil have smaller polydispersity and higher drug encapsulation efficiency than that without suet oil. In Caco-2 cell monolayers, the absorptive permeability of icariin + DOC + suet oil was 2.03 fold higher than that of icariin + DOC. In the four site single-pass rat intestinal perfusion experiment, there was statistical difference in the permeability value between icariin + DOC and icariin + DOC + suet oil in duodenum.
When the Epimedium fried with suet oil is taken orally into the body, suet oil could work together with DOC, inherent in the human body, to form mixed micelles. Icariin is one of the most important flavonoids of Epimedium, whose absorption can be promoted through the carrier. Thus, a tentative interpretation of the enhancement of activity seen with Epimedium fried with suet oil can be provided.