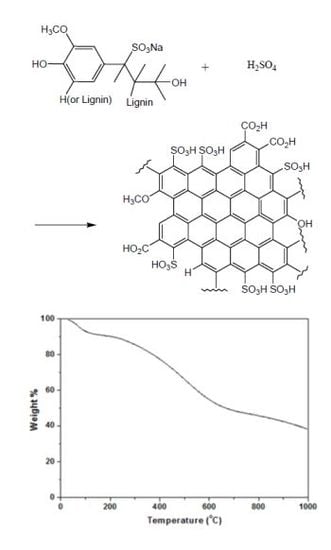
Preparation of a Sulfonated Carbonaceous Material from Lignosulfonate and Its Usefulness as an Esterification Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Material Synthesis and Acid Density, BET Surface Area

| Samples | N2 adsorption (Surface area) | E.A | T. A (mmol H+/g) | SO3H (mmol/g) | CO2H (mmol/g) | OH (mmol/g) | |
|---|---|---|---|---|---|---|---|
| Run | (Raw materials) | ||||||
| 1 | Gly(100%), Sul | 1.79 m2/g | 4N.A | 4.86 | 1.24 | 3.66 | 0 |
| 2 | 1)Lig(100%), Sul | 3.19 m2/g | CH0.72O0.53S0.017 | 4.64 | 0.96 | 3.36 | 0.32 |
| 3 | 2)Lig(100%),Sul(fum) | 2.18 m2/g | CH0.59O0.26S0.015 | 5.90 | 1.24 | 3.66 | 1.04 |
| 4 | 3)Amberlyst-15 | 45 m2/g | 4)N.A | 4.66 | 4.60 | N.A | N.A |
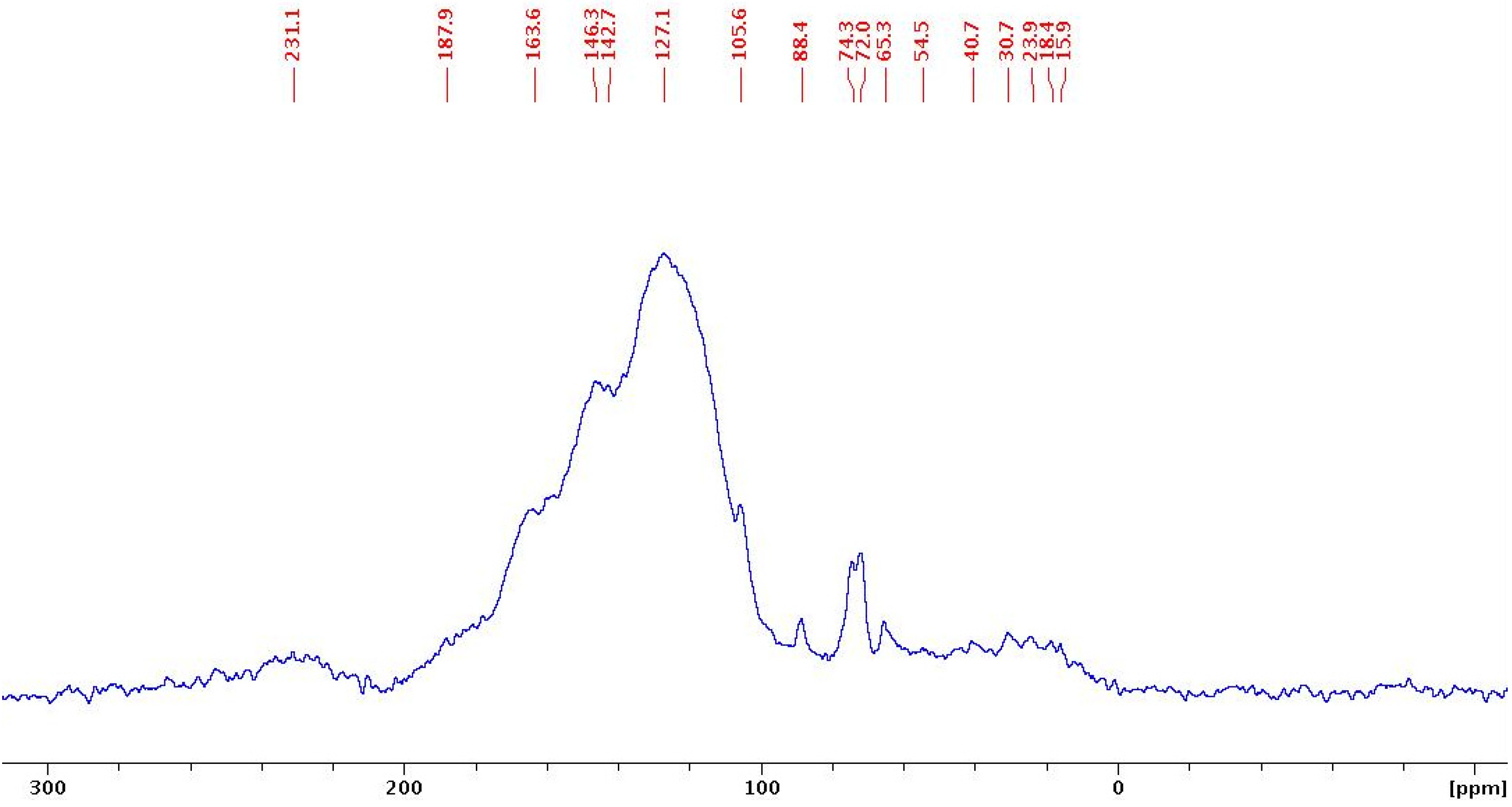
2.2. Spectroscopic Studies by IR and Solid-state 13C-NMR

2.3. Hydrolytic Stability Studies
2.4. Thermal Stability Studies by TGA

2.5. Catalytic Activity Studies

| Reaction Run | Substrate(g) | 2)Alcohol(g) 10eq. | Catalyst(g) | Temperature(°C)/Reaction Time(hr) | 7)Yields(%) |
|---|---|---|---|---|---|
| 1 | 1)CHCA(1.28) | EtOH(4.6) | c-H2SO4(0.1) | (76)/(7) | 98 |
| 2 | CHCA(1.28) | EtOH(4.6) | c-H2SO4(0.0182) | (76)/(23) | 88 |
| 3 | CHCA(1.28) | EtOH(4.6) | 3)Amberlyst-15(0.3) | (76)/(23) | 83 |
| 4 | CHCA(1.28) | EtOH(4.6) | 4)SC-I(0.3) | (76)/(23) | 85 |
| 5 | CHCA(1.28) | EtOH(4.6) | 5)SC-I(0.3) | (76)/(23) | 84 |
| 6 | CHCA(1.28) | EtOH(4.6) | 6)SC-II(0.3) | (76)/(23) | 92 |
| 7 | Levulinic Acid(2.90) | EtOH(11.5) | Amberlyst-15(0.3) | (76)/(23) | 75 |
| 8 | Levulinic Acid(2.90) | EtOH(11.5) | SC-I(0.3) | (76)/(23) | 67 |
| 9 | Levulinic Acid(2.90) | EtOH(11.5) | 5)SC-I(0.3) | (76)/(23) | 51 |

3. Experimental
3.1. Instruments and Reagents
3.2. Synthesis
3.2.1. Preparation of the Catalysts
3.2.1.1. Preparation of the Catalyst from Sodium Lignosulfonate
3.2.1.2. Sulfonated Carbon II(SC-II)
3.2.2. Determination of the Acidity
3.2.3. Synthesis of the Esters
3.2.3.1. Ethyl cyclohexanecarboxylate
3.2.3.2. Ethyl levulinate
3.2.4. Stability Test of the Sulfonated Carbons under Hydrolytic Condition
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Prager, R.H.; Yurui, Z. Preparation of carboxylate esters of polyhydric alcohols by using a sulfonated charcoal catalyst. Aust. J. Chem. 1989, 42, 1003–1005. [Google Scholar] [CrossRef]
- Patney, H.K. Sulfonated charcoal, a mild and efficient reagent for the preparation of cyclic acetals, dithioacetals and benzodioxepines. Tetrahedron Lett. 1991, 3, 413–416. [Google Scholar] [CrossRef]
- Harish, K.P. Sulfonated charcoal, a mild and efficient reagent for the tetrahydropyranylation of alcohols and phenols. Synth. Commun. 1991, 21, 2329–2333. [Google Scholar] [CrossRef]
- Toda, M.; Takagaki, A.; Okamura, M.; Kondo, J.N.; Hayashi, S.; Domen, K.; Hara, M. Biodiesel made with sugar catalyst. Nature 2005, 438, 178. [Google Scholar] [CrossRef]
- Hu, B.; Yu, S.H.; Wang, K.; Liu, L.; Xu, X.W. Functional carbonaceous materials from hydrothermal carbonization of biomass: An effective chemical process. Dalton Trans. 2008, 40, 5414–5423. [Google Scholar]
- Suganuma, S.; Nakajima, K.; Kitano, M.; Yamaguchi, D.; Kato, H.; Hayashi, S.; Hara, M. Hydrolysis of cellulose by amorphous carbon bearing SO3H, COOH, and OH groups. J. Am. Chem. Soc. 2008, 130, 12787–12793. [Google Scholar] [CrossRef]
- Yu, H.; Jin, Y.; Li, Z.; Peng, F.; Wang, H. Synthesis and characterization of sulfonated single-walled carbon nanotubes and their performance as solid acid catalyst. Solid State Chem. 2008, 181, 432–438. [Google Scholar] [CrossRef]
- Junaun, J.; Ellis, N. Glycerol etherification by tert-Butanol catalyzed by sulfonated carbon catalyst. J. Appl. Sci. 2010, 10, 2633–2637. [Google Scholar] [CrossRef]
- Vyver, S.V.D.; Peng, L.; Geboers, J.; Schepers, H.; Clippel, F.D.; Gommes, C.J.; Goderis, E.B.; Jacobs, P.A.; Sels, B.F. Sulfonated silica/carbon nanocomposites as novel catalyst for hydrolysis of cellulose to glucose. Green Chem. 2010, 12, 1560–1563. [Google Scholar] [CrossRef]
- Hu, B.; Li, C.; Zhao, S.-X.; Rong, L.-M.; Lv, S.Q.; Liang, X.; Qi, C. Highly efficient procedure for the synthesis of fructone fragrance using a novel carbon based acid. Molecules 2010, 15, 5369–5377. [Google Scholar] [CrossRef]
- Liu, X.Y.; Huang, M.; Ma, H.L.; Zhang, Z.Q.; Gao, J.M.; Zhu, Y.L.; Han, X.J.; Guo, X.Y. Preparation of a carbon-based solid acid catalyst by sulfonating activated carbon in a chemical reduction process. Molecules 2010, 15, 7188–7196. [Google Scholar] [CrossRef]
- Liu, F.; Sun, J.; Sun, Q.; Zhu, L.; Wang, L.; Meng, X.; Qi, C.; Xiao, F.S. High-temperature synthesis of magnetically active and SO3H-functionalized ordered mesoporous carbon with good catalytic performance. Catal. Today 2012, 186, 115–120. [Google Scholar] [CrossRef]
- Liu, F.; Sun, J.; Zhu, L.; Meng, X.; Qi, C.; Xiao, F.S. Sulfated graphene as an efficient solid catalyst for acid-catalyzed liquid reactions. J. Mater. Chem. 2012, 22, 5495–5502. [Google Scholar]
- Zang, X.; Zhang, Z.; Wang, F.; Wang, Y.; Song, Q.; Xu, J. Lignosulfonate-based heterogeneous sulfonic acid catalyst for hydrolyzing glycosidic bonds of polysaccharides. J. Mol. Catal. A-Chem. 2013, 377, 102–107. [Google Scholar] [CrossRef]
- Hara, M.; Yoshida, T.; Takagaki, A.; Takata, T.; Kondo, J. N.; Hayashi, S.; Domen, K. A carbon materials as a strong protonic acid. Angew. Chem. Int. Ed. 2004, 63, 2955–2958. [Google Scholar]
- Nakajima, K.; Okamura, M.; Kondo, J.N.; Domen, K.; Tatsumi, T.; Hayashi, S.; Hara, M. Amorphous carbon bearing sulfonic acid groups in mesoporous silica as a selective catalyst. Chem. Mater. 2009, 21, 186–193. [Google Scholar] [CrossRef]
- Kitano, M.; Arai, K.; Kodama, A.; Kousaka, T.; Nakajima, K.; Hayashi, S.; Hara, M. Preparation of a sulfonated porous carbon catalyst with high specific surface area. Catal Lett. 2009, 131, 242–249. [Google Scholar] [CrossRef]
- Liang, X.; Yang, J. Synthesis of a novel carbon based strong acid catalyst through hydrothermal carbonization. Catal Lett. 2009, 132, 460–463. [Google Scholar] [CrossRef]
- Boonoun, P.; Laosiripojana, N.; Muangnapoh, C.; Jongsomjit, B.; Panpranot, J.; Mekasuwandumrong, O.; Shotipruk, A. Application of sulfonated carbon-based catalyst for reactive extraction of 1,3-propanediol from model fermentation mixture. Ind. Eng. Chem. Res. 2010, 49, 12352–12357. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Zhao, Y.; Shen, J. Preparation of a novel sulfonated carbon catalyst for the etherification of isopentene with methanol to produce tert-amyl methyl ether. Catal. Comm. 2010, 11, 824–828. [Google Scholar] [CrossRef]
- Mirkhani, V.; Moghadam, M.; Tangestaninejad, S.; Mohammadpoor-Baltork, I.; Mahdavi, M. Synthesis, characterization and investigation of catalytic activity of a highly sulfonated carbon solid acid in the synthesis of dihydropyrimidinones under solvent-free conditions. J. Iran. Chem. Soc. 2011, 8, 608–615. [Google Scholar] [CrossRef]
- Daengprasert, W.; Boonoun, P.; Laosiripojana, N.; Goto, M.; Shotipruk, A. Application of sulfonated carbon-based catalyst for solvothermal conversion of cassava waste to hydroxymethylfurfural and furfural. Ind. Eng. Chem. Res. 2011, 50, 7903–7910. [Google Scholar] [CrossRef]
- Hara, M.; Domen, K. Sulfonated amophorous carbon, process for producing the same and use thereof. U.S. Patent 2008/0227996 A1, 2008. [Google Scholar]
- Yanagawa, S.; Kondo, H.; Hara, M. Sulfonic acid group-containing carbonaceous material. U.S. Patent 2010/0048835 A1, 2010. [Google Scholar]
- Anu, B.L.; Devi, P.; Gangadar, K.N.; Prasad, P.S.; Prasad, R.B.N. Novel glycerol-based heterogeneous sold acid catalysts useful for the esterification of fatty acids, a process and use thereof. U.S. Patent 2010/0286421 A1, 2010. [Google Scholar]
- Okamura, M.; Takagaki, A.; Toda, M.; Kondo, J.N.; Domen, K.; Tatsumi, T.; Hara, M.; Hayashi, S. Acid-Catalyzed reactions on flexible polycyclic aromatic carbon in amorphous carbon. Chem. Mater. 2006, 18, 3039–3045. [Google Scholar] [CrossRef]
- Fernandes, D.R.; Rocha, A.S.; Claudio, E.F.M.; Mota, J.A.; Silva, V.T.d. Levulinic acid esterification with ethanol to ethyl levulinate production over solid acid catalysts. Appl. Catal. A: General 2012, 425–426, 199–204. [Google Scholar]
- Bart, H.J.; Reidetschlager, J.; Schatka, K.; Lehmann, A. Efficient conversion of furfuryl alcohol into alkyl levulinate catalyzed by an organic-inorganic hybrid solid acid catalyst. Ind. Eng. Chem. Res. 1994, 33, 21–25. [Google Scholar] [CrossRef]
- Liu, Y.; Lotero, E.; Goodwin, J.G., Jr. Effect of carbon chain length on esterification of carboxylic acids using acid catalysis. J. Catal. 2006, 242, 278–286. [Google Scholar] [CrossRef]
- Kabza, K.G.; Chapados, B.R.; Gestwicki, J.E.; McGrat, J.L. Microwave-induced esterification using heterogeneous acid catalyst in a low dielectric constant medium. J. Org. Chem. 2000, 65, 1210–1214. [Google Scholar] [CrossRef]
- Lilja, J.; Murzin, D.Y.; Salmi, T.; Aumo, J.; Mȁki-Arvela, P.; Sundell, M. Esterification of different acids over heterogenous and homogenous catalyst and correlation with the taft equation. J. Mol. Catal. 2002, 182–183, 555–563. [Google Scholar]
- Benak, K.R.; Dominuez, L.; Economy, J.; Mangun, C.L. Sulfonation of pyropolymeric fibers derived from phenol-formaldehyde resins. Carbon 2002, 40, 2323–2332. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the sulfonated carbonaceous material made from lignosulfonate is available from the author.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, D. Preparation of a Sulfonated Carbonaceous Material from Lignosulfonate and Its Usefulness as an Esterification Catalyst. Molecules 2013, 18, 8168-8180. https://doi.org/10.3390/molecules18078168
Lee D. Preparation of a Sulfonated Carbonaceous Material from Lignosulfonate and Its Usefulness as an Esterification Catalyst. Molecules. 2013; 18(7):8168-8180. https://doi.org/10.3390/molecules18078168
Chicago/Turabian StyleLee, Duckhee. 2013. "Preparation of a Sulfonated Carbonaceous Material from Lignosulfonate and Its Usefulness as an Esterification Catalyst" Molecules 18, no. 7: 8168-8180. https://doi.org/10.3390/molecules18078168