Carbon Nanotube-Induced Pulmonary Granulomatous Disease: Twist1 and Alveolar Macrophage M1 Activation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sarcoidosis Patients Display an M1 Profile in Bronchoalveolar Lavage
2.2. Twist1 is Elevated in Sarcoidosis Alveolar Macrophages
2.3. Macrophage M1 Inducers Upregulate Twist1
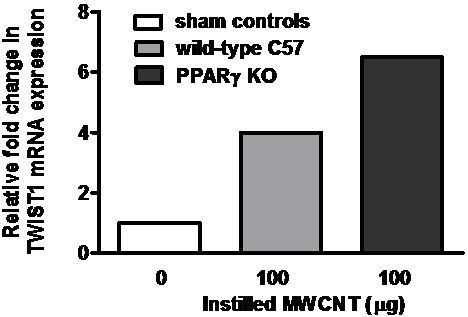
2.4. Twist1 Is Elevated in Alveolar Macrophages from MWCNT-Granuloma Bearing Mice
2.5. Discussion
3. Experimental Section
3.1. Human Study Population
3.2. Human Cell Collection and Culture
3.3. Immunocytochemistry
3.4. Microarray Analysis
3.5. Quantitative mRNA Expression
3.6. Murine MWCNT Model
3.7. Characterization of Carbon Nanotubes
3.8. Statistical Analyses
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- De Volder, M.F.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar]
- Lam, C.W.; James, J.T.; McCluskey, R.; Arepalli, S.; Hunter, R.L. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit. Rev. Toxicol 2006, 36, 189–217. [Google Scholar]
- Bonner, J.C. Carbon nanotubes as delivery systems for respiratory disease: Do the dangers outweigh the potential benefits? Expert Rev. Respir. Med 2011, 5, 779–787. [Google Scholar]
- Shvedova, A.A.; Kisin, E.R.; Mercer, R.; Murray, A.R.; Johnson, V.J.; Potapovich, A.I.; Tyurina, Y.Y.; Gorelik, O.; Arepalli, S.; Schwegler-Berry, D.; et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am. J. Physiol. Lung Cell Mol. Physiol 2005, 289, L698–L708. [Google Scholar]
- Mercer, R.R.; Hubbs, A.F.; Scabilloni, J.F.; Wang, L.; Battelli, L.A.; Friend, S.; Castranova, V.; Porter, D.W. Pulmonary fibrotic response to aspiration of multi-walled carbon nanotubes. Part. Fibre Toxicol 2011. [Google Scholar] [CrossRef]
- Wang, X.; Katwa, P.; Podila, R.; Chen, P.; Ke, P.C.; Rao, A.M.; Walters, D.M.; Wingard, C.J.; Brown, J.M. Multi-walled carbon nanotube instillation impairs pulmonary function in C57BL/6 mice. Part. Fibre Toxicol 2011. [Google Scholar] [CrossRef]
- Huizar, I.; Malur, A.; Midgette, Y.A.; Kukoly, C.; Chen, P.; Ke, P.C.; Podila, R.; Rao, A.M.; Wingard, C.J.; Dobbs, L.; et al. Novel murine model of chronic granulomatous lung inflammation elicited by carbon nanotubes. Am. J. Respir. Cell Mol. Biol 2011, 45, 858–866. [Google Scholar]
- Chen, E.S.; Moller, D.R. Sarcoidosis—Scientific progress and clinical challenges. In Nat. Rev. Rheumatol; 2011; Volume 7, pp. 457–467. [Google Scholar]
- Bresnitz, E.A.; Stolley, P.D.; Israel, H.L.; Soper, K. Possible risk factors for sarcoidosis: A case-control study. Ann. N. Y. Acad. Sci 1986, 465, 632–642. [Google Scholar]
- Prezant, D.J.; Dhala, A.; Goldstein, A.; Janus, D.; Ortiz, F.; Aldrich, T.K.; Kelly, K.J. The incidence, prevalence, and severity of sarcoidosis in New York city firefighters. Chest 1999, 116, 1183–1193. [Google Scholar]
- Izbicki, G.; Chavko, R.; Banauch, G.I.; Weiden, M.D.; Berger, K.I.; Aldrich, T.K.; Hall, C.; Kelly, K.J.; Prezant, D.J. World trade center “sarcoid-like” granulomatous pulmonary disease in New York city fire department rescue workers. Chest 2007, 131, 1414–1423. [Google Scholar]
- Wu, M.; Gordon, R.E.; Herbert, R.; Padilla, M.; Moline, J.; Mendelson, D.; Litle, V.; Travis, W.D.; Gil, J. Case report: Lung disease in World Trade Center responders exposed to dust and smoke. Carbon nanotubes found in the lungs of World Trade Center patients and dust samples. Environ. Health Perspect 2010, 118, 499–504. [Google Scholar]
- Kunkel, S.; Lukacs, N.W.; Strieter, R.M.; Chensue, S.W. Animal models of granulomatous inflammation. Semin. Respir. Infect 1998, 13, 221–228. [Google Scholar]
- Shigehara, K.; Shijubo, N.; Ohmichi, M.; Takahashi, R.; Kon, S.; Okamura, H.; Kurimoto, M.; Hiraga, Y.; Tatsuno, T.; Abe, S.; et al. IL-12 and IL-18 are increased and stimulate IFN-γ production in sarcoid lungs. J. Immunol 2001, 166, 642–649. [Google Scholar]
- Zissel, G.; Prasse, A.; Muller-Quernheim, J. Sarcoidosis—Immunopathogenetic concepts. Semin. Respir. Crit. Care Med 2007, 28, 3–14. [Google Scholar]
- Fehrenbach, H.; Zissel, G.; Goldmann, T.; Tschernig, T.; Vollmer, E.; Pabst, R.; Muller-Quernheim, J. Alveolar macrophages are the main source for tumour necrosis factor-alpha in patients with sarcoidosis. Eur. Respir. J 2003, 21, 421–428. [Google Scholar]
- Culver, D.A.; Barna, B.P.; Raychaudhuri, B.; Bonfield, T.L.; Abraham, S.; Malur, A.; Farver, C.F.; Kavuru, M.S.; Thomassen, M.J. Peroxisome proliferator-activated receptor gamma activity is deficient in alveolar macrophages in pulmonary sarcoidosis. Am. J. Respir. Cell. Mol. Biol 2004, 30, 1–5. [Google Scholar]
- Bonfield, T.L.; Farver, C.F.; Barna, B.P.; Malur, A.; Abraham, S.; Raychaudhuri, B.; Kavuru, M.S.; Thomassen, M.J. Peroxisome proliferator-activated receptor-gamma is deficient in alveolar macrophages from patients with alveolar proteinosis. Am. J. Respir. Cell Mol. Biol 2003, 29, 677–682. [Google Scholar]
- Straus, D.S.; Glass, C.K. Anti-inflammatory actions of PPAR ligands: New insights on cellular and molecular mechanisms. Trends Immunol 2007, 28, 551–558. [Google Scholar]
- Huizar, I.; Malur, A.; Patel, J.; McPeek, M.; Dobbs, L.; Wingard, C.; Barna, B.P.; Thomassen, M.J. The role of PPARγ in carbon nanotube-elicited granulomatous lung inflammation. Respir. Res 2013. [Google Scholar] [CrossRef]
- Thisse, B.; El, M.M.; Perrin-Schmitt, F. The twist gene: Isolation of a Drosophila zygotic gene necessary for the establishment of dorsoventral pattern. Nucleic Acids Res 1987, 15, 3439–3453. [Google Scholar]
- Franco, H.L.; Casasnovas, J.; Rodriguez-Medina, J.R.; Cadilla, C.L. Redundant or separate entities?—Roles of Twist1 and Twist2 as molecular switches during gene transcription. Nucleic Acids Res 2011, 39, 1177–1186. [Google Scholar]
- Sosic, D.; Richardson, J.A.; Yu, K.; Ornitz, D.M.; Olson, E.N. Twist regulates cytokine gene expression through a negative feedback loop that represses NF-γB activity. Cell 2003, 112, 169–180. [Google Scholar]
- Crouser, E.D.; Culver, D.A.; Knox, K.S.; Julian, M.W.; Shao, G.; Abraham, S.; Liyanarachchi, S.; Macre, J.E.; Wewers, M.D.; Gavrilin, M.A.; et al. Gene expression profiling identifies MMP-12 and ADAMDEC1 as potential pathogenic mediators of pulmonary sarcoidosis. Am. J. Respir. Crit. Care Med 2009, 179, 929–938. [Google Scholar]
- Palchevskiy, V.; Hashemi, N.; Weigt, S.S.; Xue, Y.Y.; Derhovanessian, A.; Keane, M.P.; Strieter, R.M.; Fishbein, M.C.; Deng, J.C.; Lynch, J.P., III; et al. Immune response CC chemokines CCL2 and CCL5 are associated with pulmonary sarcoidosis. Fibrogenesis Tissue Repair 2011. [Google Scholar] [CrossRef]
- Petrek, M.; Kolek, V.; Szotkowska, J.; du Bois, R.M. CCC chemokine expression in pulmonary sarcoidosis. Eur. Respir. J 2002, 20, 1206–1212. [Google Scholar]
- Barna, B.P.; Culver, D.A.; Abraham, S.; Malur, A.; Bonfield, T.L.; John, N.; Farver, C.F.; Drazba, J.A.; Raychaudhuri, B.; Kavuru, M.S.; et al. Depressed peroxisome proliferator-activated receptor gamma (PPARγ) is indicative of severe pulmonary sarcoidosis: Possible involvement of interferon gamma (IFN-γ). Sarcoidosis Vasc. Diffus. Lung Dis 2006, 23, 93–100. [Google Scholar]
- Rosenbaum, J.T.; Pasadhika, S.; Crouser, E.D.; Choi, D.; Harrington, C.A.; Lewis, J.A.; Austin, C.R.; Diebel, T.N.; Vance, E.E.; Braziel, R.M.; et al. Hypothesis: Sarcoidosis is a STAT1-mediated disease. Clin. Immunol 2009, 132, 174–183. [Google Scholar]
- Moller, D.R.; Forman, J.D.; Liu, M.C.; Noble, P.W.; Greenlee, B.M.; Vyas, P.; Holden, D.A.; Forrester, J.M.; Lazarus, A.; Wysocka, M.; et al. Enhanced expression of IL-12 associated with Th1 cytokine profiles in active pulmonary sarcoidosis. J. Immunol 1996, 156, 4952–4960. [Google Scholar]
- Grohmann, U.; Belladonna, M.L.; Vacca, C.; Bianchi, R.; Fallarino, F.; Orabona, C.; Fioretti, M.C.; Puccetti, P. Positive regulatory role of IL-12 in macrophages and modulation by IFN-γ. J. Immunol 2001, 167, 221–227. [Google Scholar]
- Watford, W.T.; Hissong, B.D.; Bream, J.H.; Kanno, Y.; Muul, L.; O’Shea, J.J. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol. Rev 2004, 202, 139–156. [Google Scholar]
- Persky, M.E.; Murphy, K.M.; Farrar, J.D. IL-12, but not IFN-α, promotes STAT4 activation and Th1 development in murine CD4+ T cells expressing a chimeric murine/human Stat2 gene. J. Immunol 2005, 174, 294–301. [Google Scholar]
- Nishioka, Y.; Manabe, K.; Kishi, J.; Wang, W.; Inayama, M.; Azuma, M.; Sone, S. CXCL9 and 11 in patients with pulmonary sarcoidosis: A role of alveolar macrophages. Clin. Exp. Immunol 2007, 149, 317–326. [Google Scholar]
- Liu, J.; Guan, X.; Ma, X. Interferon regulatory factor 1 is an essential and direct transcriptional activator for interferon γ-induced RANTES/CCl5 expression in macrophages. J. Biol. Chem 2005, 280, 24347–24355. [Google Scholar]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol 2008, 8, 958–969. [Google Scholar]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig 2012, 122, 787–795. [Google Scholar]
- Sharif, M.N.; Sosic, D.; Rothlin, C.V.; Kelly, E.; Lemke, G.; Olson, E.N.; Ivashkiv, L.B. Twist mediates suppression of inflammation by type I IFNs and Axl. J. Exp. Med 2006, 203, 1891–1901. [Google Scholar]
- Loza, M.J.; Brodmerkel, C.; du Bois, R.M.; Judson, M.A.; Costabel, U.; Drent, M.; Kavuru, M.; Flavin, S.; Lo, K.H.; Barnathan, E.S.; et al. Inflammatory profile and response to anti-tumor necrosis factor therapy in patients with chronic pulmonary sarcoidosis. Clin. Vaccine Immunol 2011, 18, 931–939. [Google Scholar]
- Agostini, C.; Meneghin, A.; Semenzato, G. T-lymphocytes and cytokines in sarcoidosis. Curr. Opin. Pulm. Med 2002, 8, 435–440. [Google Scholar]
- Dai, H.; Guzman, J.; Chen, B.; Costabel, U. Production of soluble tumor necrosis factor receptors and tumor necrosis factor-α by alveolar macrophages in sarcoidosis and extrinsic allergic alveolitis. Chest 2005, 127, 251–256. [Google Scholar]
- Pozharskaya, V.; Torres-Gonzalez, E.; Rojas, M.; Gal, A.; Amin, M.; Dollard, S.; Roman, J.; Stecenko, A.A.; Mora, A.L. Twist: A regulator of epithelial-mesenchymal transition in lung fibrosis. PLoS One 2009, 4, e7559. [Google Scholar]
- Bridges, R.S.; Kass, D.; Loh, K.; Glackin, C.; Borczuk, A.C.; Greenberg, S. Gene expression profiling of pulmonary fibrosis identifies Twist1 as an antiapoptotic molecular “rectifier” of growth factor signaling. Am. J. Pathol 2009, 175, 2351–2361. [Google Scholar]
- Bouhlel, M.A.; Derudas, B.; Rigamonti, E.; DiFvart, R.; Brozek, J.; Haulon, S.; Zawadzki, C.; Jude, B.; Torpier, G.; Marx, N.; et al. PPARγ activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab 2007, 6, 137–143. [Google Scholar]
- American Thoracic Society. Statement on sarcoidosis. Am. J. Respir. Crit. Care Med 1999, 160, 736–755.
- Thomassen, M.J.; Buhrow, L.T.; Connors, M.J.; Kaneko, F.T.; Erzurum, S.C.; Kavuru, M.S. Nitric oxide inhibits inflammatory cytokine production by human alveolar macrophages. Am. J. Respir. Cell Mol. Biol 1997, 17, 279–283. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar]





| Gene Symbol | Gene Name | Fold Change |
|---|---|---|
| M1-associated Genes | ||
| IFNγ | Interferon-gamma | 7.13 |
| IL6 | Interleukin 6 | 2.83 |
| IL18R1 | Interleukin 18 receptor 1 | 4.59 |
| IL12Rb2 | Interleukin 12 receptor, beta 2 | 6.32 |
| STAT1 | Signal transducers and activators of transcription 1 | 2.05 |
| STAT4 | Signal transducers and activators of transcription 4 | 3.82 |
| CXCL11 | Interferon inducible T cell α chemoattractant (I-TAC) | 3.93 |
| CXCL10 | Interferon inducible protein 10, (IP-10) | 3.48 |
| CXCL9 | Monokine induced by interferonγ, (MIG) | 4.64 |
| CCL5 | Chemokine (C-C motif) (RANTES) | 5.77 |
| M2-associated Genes | ||
| IL10 | Interleukin 10 | NS * |
| IL1RA | Interleukin-1 receptor antagonist | NS * |
| CD36 | Member of the class B scavenger receptor | NS * |
| MMP2 | Matrix metalloproteinase 2 | NS * |
| MMP7 | Matrix metalloproteinase 7 | NS * |
| CCL24 | Chemokine (C-C motif) ligand 24 | −5.35 |
| CCL2 | Chemokine (C-C motif) ligand 2 | 2.71 |
| Characteristics | Sarcoidosis | Healthy Controls |
|---|---|---|
| (n = 23) | (n = 27) | |
| Age (year) | 44.8 ± 2.6 | 32.4 ± 1.4 |
| Gender | 15F/8M | 18F/9M |
| Self-reported race | 21AA/2C | 14AA/12C/1AI |
| Smokers | 0 (10 exsmokers) | 0 (1 exsmokers) |
| FVC % predicted | 80.6 ± 3.8 | – |
| CXR stage: 0–1 | 6* | – |
| CXR stage: 2–4 | 16 | – |
| BAL Macrophages (%) | 83.3 ± 2.8 | 95.5 ± 0.6 |
| BAL Lymphocytes (%) | 15.2 ± 2.8 | 4.0 ± 0.6 |
| BAL PMNs (%) | 1.6 ± 1.0 | 0.4 ± 0.2 |
| Main treatment indication(s) at time of bronchoscopy | Not treated (11) | – |
| pulmonary (9) | ||
| multiorgan-(dermal, ocular) (3) | ||
| Organ involvement | Lung (23) | – |
| Multiorgan (12) ** |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Barna, B.P.; Huizar, I.; Malur, A.; McPeek, M.; Marshall, I.; Jacob, M.; Dobbs, L.; Kavuru, M.S.; Thomassen, M.J. Carbon Nanotube-Induced Pulmonary Granulomatous Disease: Twist1 and Alveolar Macrophage M1 Activation. Int. J. Mol. Sci. 2013, 14, 23858-23871. https://doi.org/10.3390/ijms141223858
Barna BP, Huizar I, Malur A, McPeek M, Marshall I, Jacob M, Dobbs L, Kavuru MS, Thomassen MJ. Carbon Nanotube-Induced Pulmonary Granulomatous Disease: Twist1 and Alveolar Macrophage M1 Activation. International Journal of Molecular Sciences. 2013; 14(12):23858-23871. https://doi.org/10.3390/ijms141223858
Chicago/Turabian StyleBarna, Barbara P., Isham Huizar, Anagha Malur, Matthew McPeek, Irene Marshall, Mark Jacob, Larry Dobbs, Mani S. Kavuru, and Mary Jane Thomassen. 2013. "Carbon Nanotube-Induced Pulmonary Granulomatous Disease: Twist1 and Alveolar Macrophage M1 Activation" International Journal of Molecular Sciences 14, no. 12: 23858-23871. https://doi.org/10.3390/ijms141223858