Block of the Mevalonate Pathway Triggers Oxidative and Inflammatory Molecular Mechanisms Modulated by Exogenous Isoprenoid Compounds
Abstract
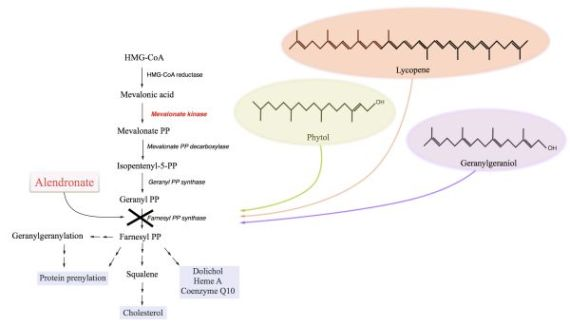
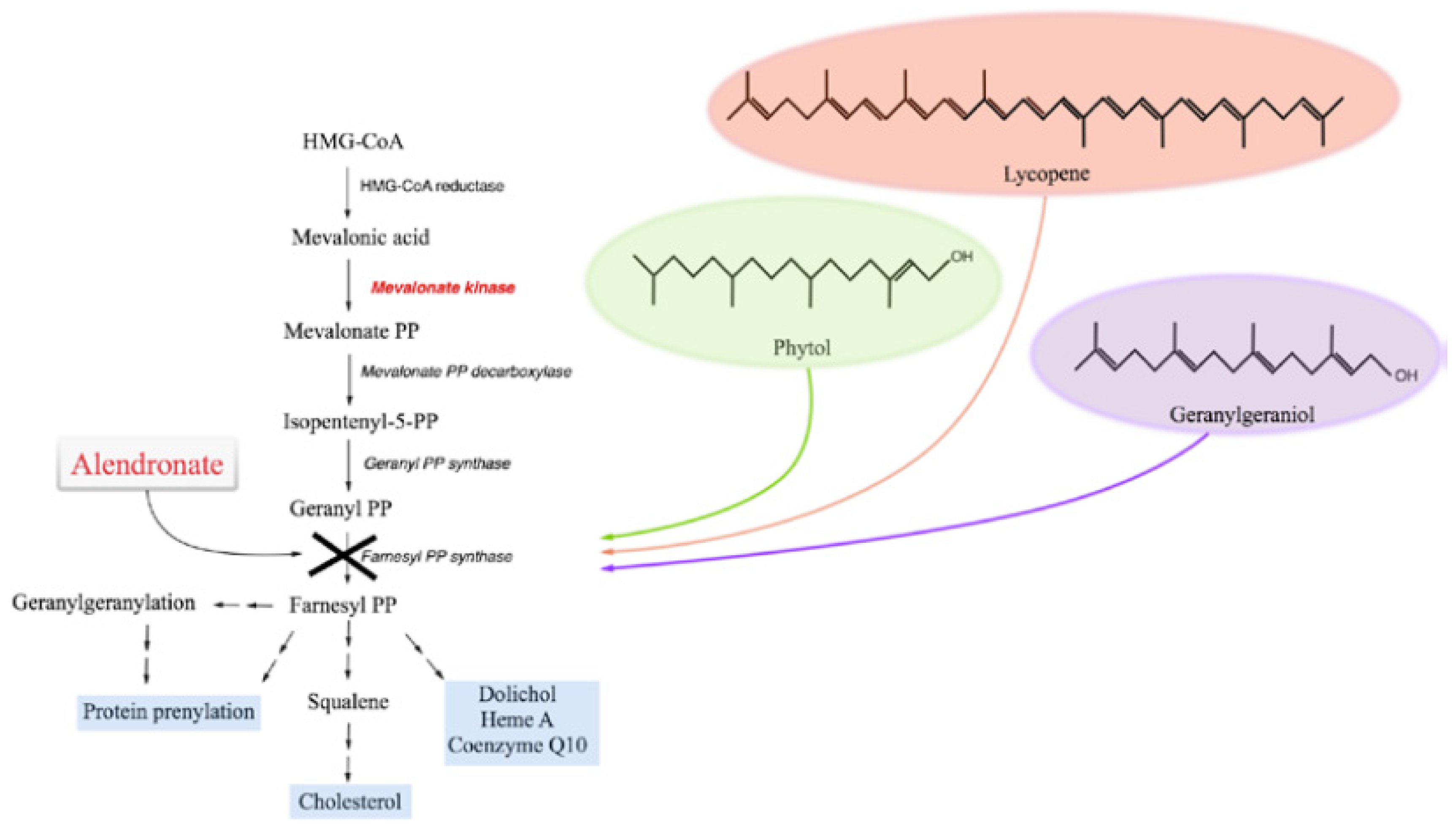
:1. Introduction
2. Results and Discussion
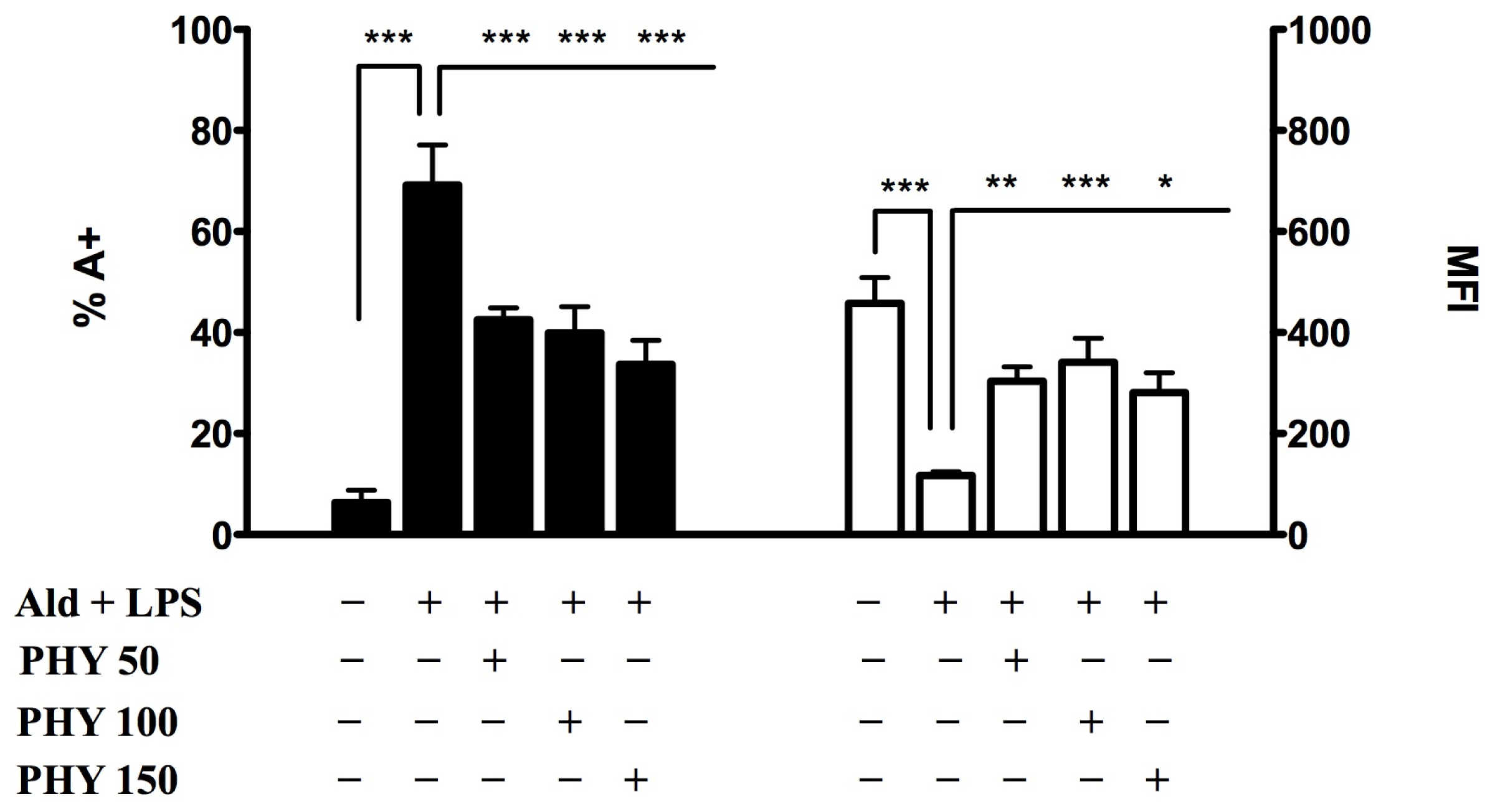
2.1. The Block of the Mevalonate Pathway Induces Programmed Cell Death (PCD) and Mitochondrial Dysfunction
2.1.1. Phytol
2.1.2. Geranylgeraniol
2.1.3. Lycopene
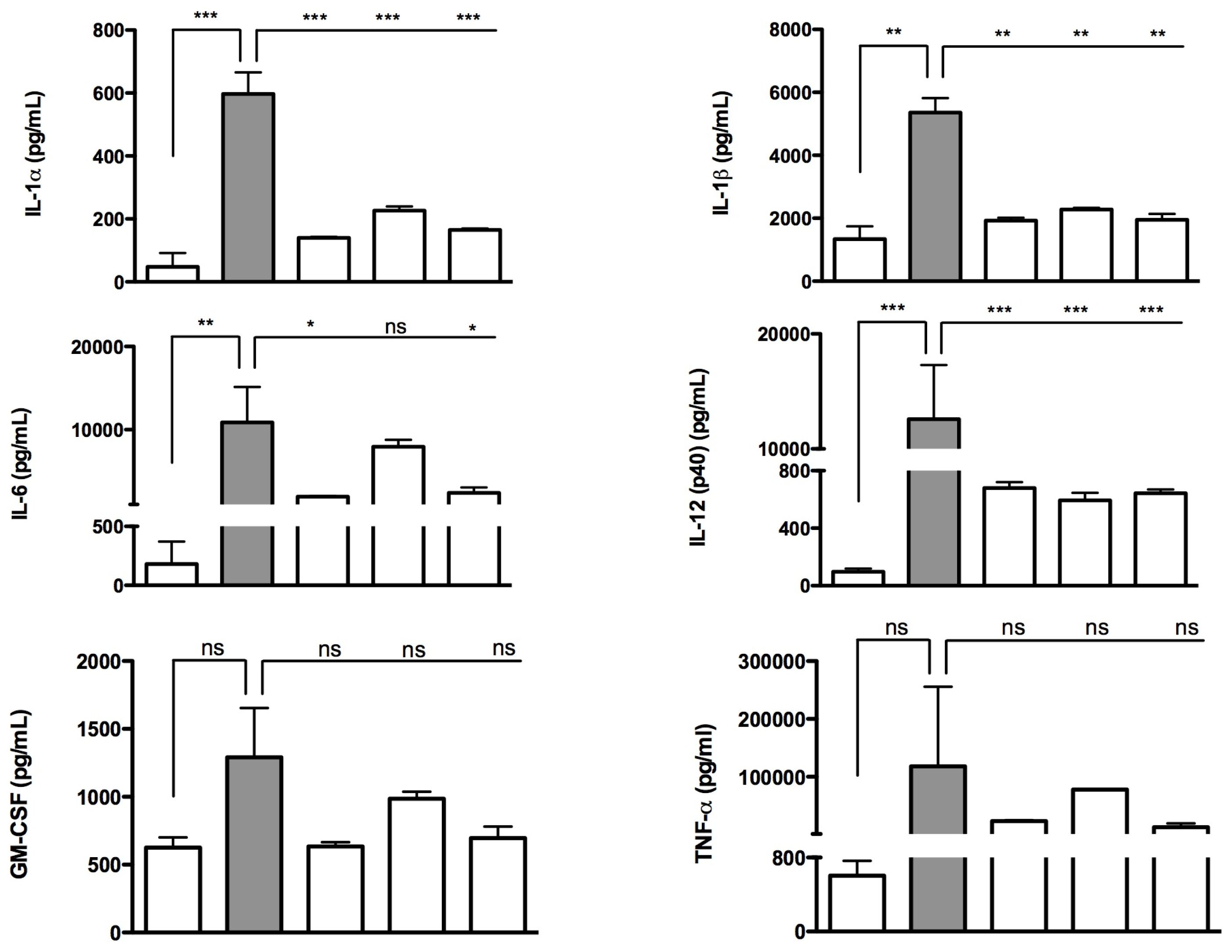
2.2. Effects of Isoprenoids on Cytokine Profile
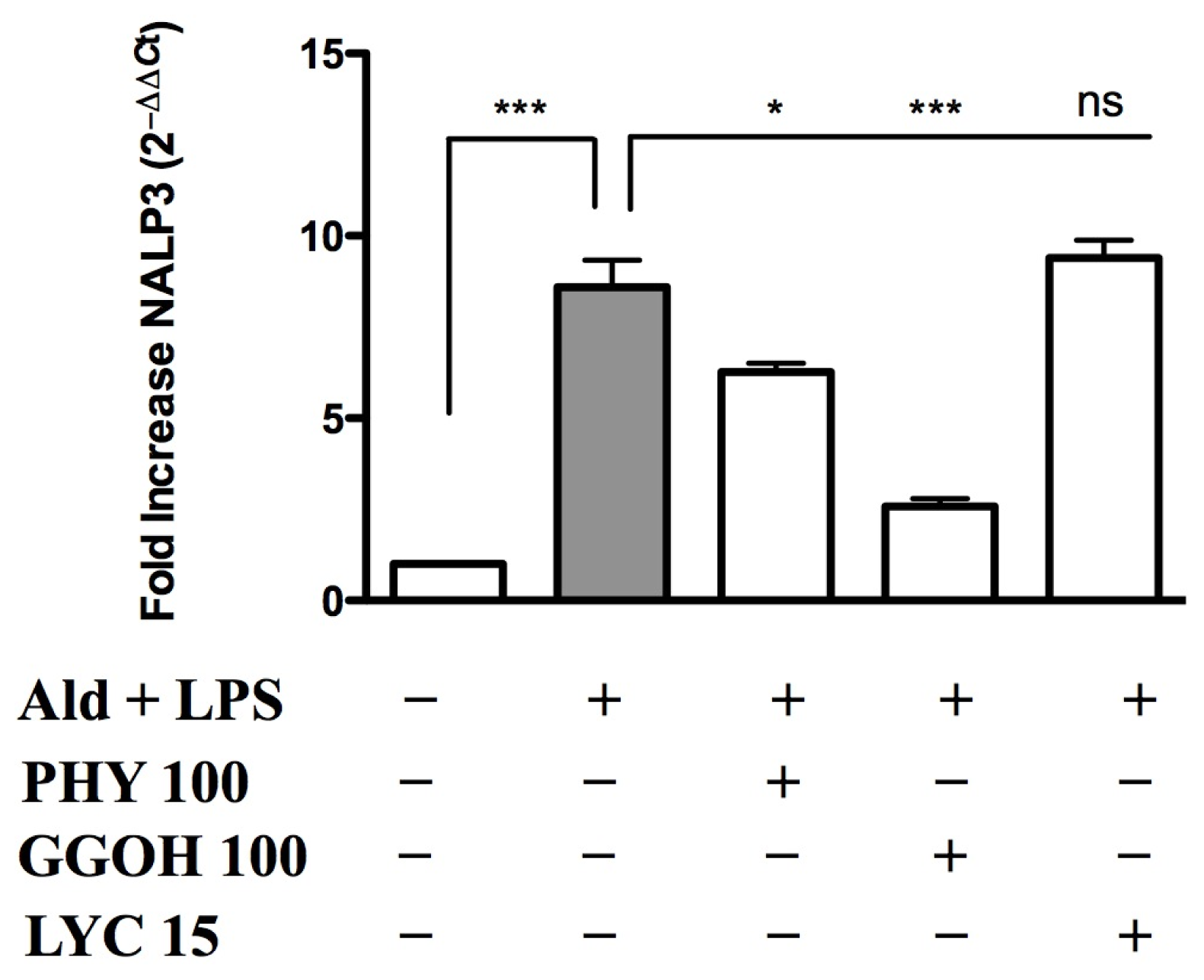
2.3. Inflammasome (NALP3) Expression in the Presence of Anti-Inflammatory/Anti-Oxidant Compounds
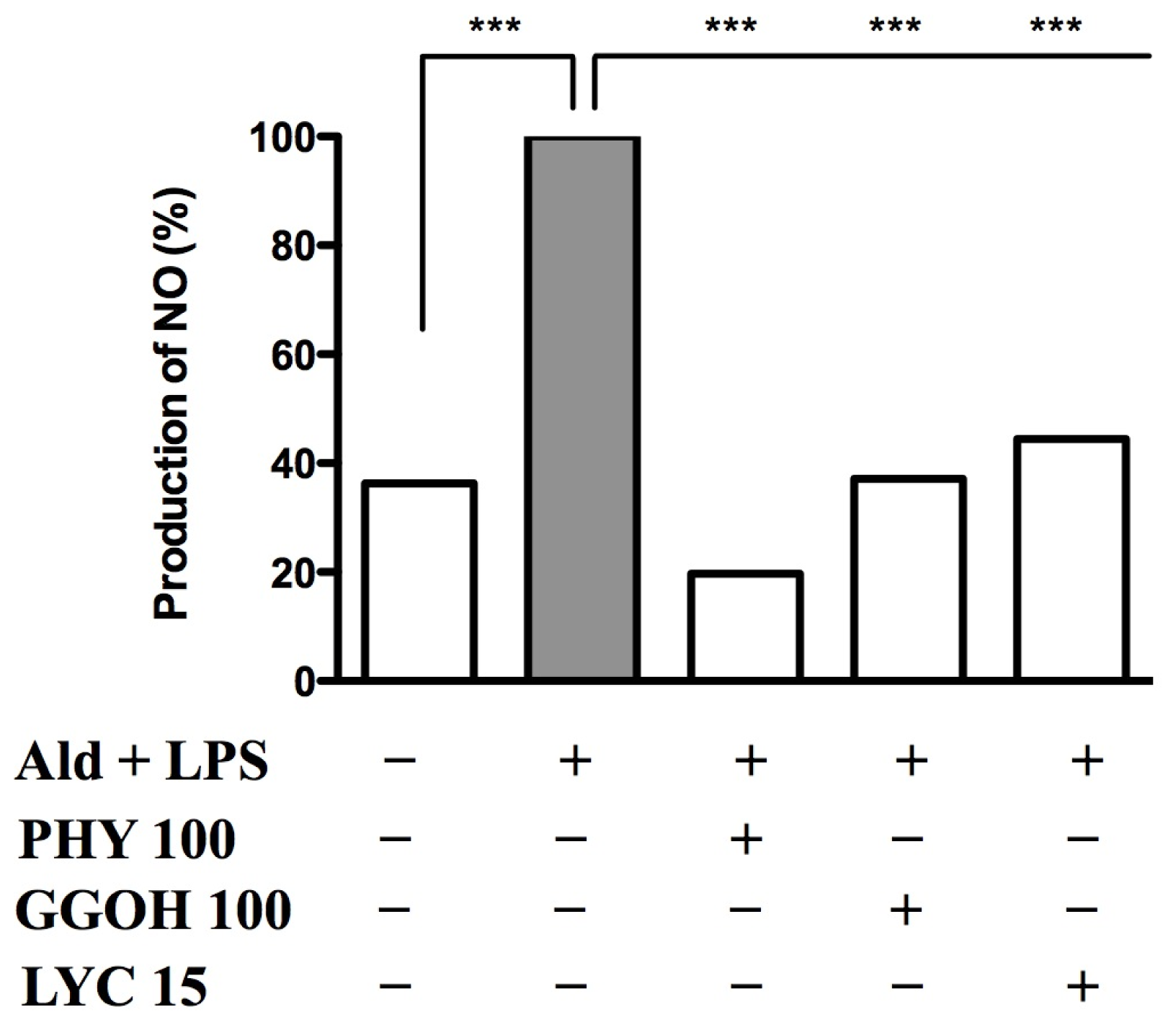
2.4. Nitric Oxide Release after Blocking the Pathway and Rescuing it with Exogenous Isoprenoids
3. Experimental Section
3.1. Chemicals
3.2. Raw 264.7 Cell Culture
3.3. Evaluation of Programmed Cell Death
3.4. Mean Fluorescence Intensity (MFI)
3.5. Measurement of Cytokines in Cell Culture Supernatants
3.6. Nucleotide-Binding Oligomerization-Domain Protein-Like Receptors 3(NALP3) RNA Isolation and Real Time-PCR (Polymerase Chain Reaction)
3.7. Nitric Oxide Production on Raw 264.7 Cell Cultures
3.8. Data Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
- Author ContributionsP.M.T., G.K., S.C. and A.M. designed the experimental plan, performed the analysis of the results and wrote the manuscript; M.G. and G.C. carried out the experiments on mRNA expression; P.M.T. and G.K. carried out the cytokines’ release assays; E.V. performed the flow cytometric analysis and helped with the acquisition and interpretation of data; A.K. carried out the statistical analysis and revised the language grammar and style. All authors read and approved the final manuscript.
References
- Clayton, P.T. Disorders of cholesterol biosynthesis. Arch. Dis. Child 1998, 78, 185–189. [Google Scholar]
- Moebius, F.F.; Fitzky, B.U.; Glossmann, H. Genetic defects in postsqualene cholesterol biosynthesis. Trends Endocrinol. Metab 2000, 11, 106–114. [Google Scholar]
- Goldstein, J.L.; Brown, M.S. Regulation of the mevalonate pathway. Nature 1990, 343, 425–430. [Google Scholar]
- Drenth, J.P.; Haagsma, C.J.; van der Meer, J.W. Hyperimmunoglobulinemia D and periodic fever syndrome. The clinical spectrum in a series of 50 patients. International Hyper-IgD Study Group. Medicine 1994, 73, 133–144. [Google Scholar]
- Houten, S.M.; Frenkel, J.; Rijkers, G.T.; Wanders, R.J.; Kuis, W.; Waterham, H.R. Temperature dependence of mutant mevalonate kinase activity as a pathogenic factor in hyper-IgD and periodic fever syndrome. Hum. Mol. Genet 2002, 11, 3115–3124. [Google Scholar]
- Marcuzzi, A.; Zanin, V.; Kleiner, G.; Monasta, L.; Crovella, S. Mouse model of mevalonate kinase deficiency: Comparison of cytokine and chemokine profile with that of human patients. Pediatr. Res 2013, 74, 266–271. [Google Scholar]
- Kuijk, L.M.; Beekman, J.M.; Koster, J.; Waterham, H.R.; Frenkel, J.; Coffer, P.J. HMG-CoA reductase inhibition induces IL-1β release through Rac1/PI3K/PKB-dependent caspase-1 activation. Blood 2008, 112, 3563–3573. [Google Scholar]
- Kuijk, L.M.; Mandey, S.H.; Schellens, I.; Waterham, H.R.; Rijkers, G.T.; Coffer, P.J.; Frenkel, J. Statin synergizes with LPS to induce IL-1β release by THP-1 cells through activation of caspase-1. Mol. Immunol 2008, 45, 2158–2165. [Google Scholar]
- Frenkel, J.; Rijkers, G.T.; Mandey, S.H.; Buurman, S.W.; Houten, S.M.; Wanders, R.J.; Waterham, H.R.; Kuis, W. Lack of isoprenoid products raises ex vivo interleukin-1β secretion in hyperimmunoglobulinemia D and periodic fever syndrome. Arthritis Rheum 2002, 46, 2794–2803. [Google Scholar]
- Marcuzzi, A.; Crovella, S.; Monasta, L.; Vecchi Brumatti, L.; Gattorno, M.; Frenkel, J. Mevalonate kinase deficiency: Disclosing the role of mevalonate pathway modulation in inflammation. Curr. Pharm. Des 2012, 18, 5746–5752. [Google Scholar]
- Tricarico, P.M.; Marcuzzi, A.; Piscianz, E.; Monasta, L.; Crovella, S.; Kleiner, G. Mevalonate kinase deficiency and neuroinflammation: Balance between apoptosis and pyroptosis. Int. J. Mol. Sci 2013, 14, 23274–23288. [Google Scholar]
- Celec, P.; Behuliak, M. The lack of non-steroid isoprenoids causes oxidative stress in patients with mevalonic aciduria. Med. Hypotheses 2008, 70, 938–940. [Google Scholar]
- Van der Burgh, R.; Nijhuis, L.; Pervolaraki, K.; Compeer, E.B.; Jongeneel, L.H.; van Gijn, M.; Coffer, P.J.; Murphy, M.P.; Mastroberardino, P.G.; Frenkel, J.; Boes, M. Defects in mitochondrial clearance predispose human monocytes to Interleukin-1β hypersecretion. 2014. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar]
- Tiwari, M.; Kakkar, P. Plant derived antioxidants—Geraniol and camphene protect rat alveolar macrophages against t-BHP induced oxidative stress. Toxicol. in Vitro 2009, 23, 295–301. [Google Scholar]
- Yonar, M.E. Protective effect of lycopene on oxidative stress and antioxidant status in Cyprinus carpio during cypermethrin exposure. Environ. Toxicol 2013, 28, 609–616. [Google Scholar]
- Marcuzzi, A.; Tommasini, A.; Crovella, S.; Pontillo, A. Natural isoprenoids inhibit LPS-induced-production of cytokines and nitric oxide in aminobisphosphonate-treated monocytes. Int. Immunopharmacol 2010, 10, 639–642. [Google Scholar]
- Furniss, B.S.; Hannaford, A.J.; Smith, P.W.G.; Tatchell, A.R. Vogel’s Textbook of Practical Organic Chemistry, 5th ed.; Longman Scientific & Technical: Essex, UK, 1989; pp. 1019–1020. [Google Scholar]
- Marcuzzi, A.; Zanin, V.; Piscianz, E.; Tricarico, P.M.; Vuch, J.; Girardelli, M.; Monasta, L.; Bianco, A.M.; Crovella, S. Lovastatin-induced apoptosis is modulated by geranylgeraniol in a neuroblastoma cell line. Int. J. Dev. Neurosci 2012, 30, 451–456. [Google Scholar]
- Hung, C.F.; Huang, T.F.; Chen, B.H.; Shieh, J.M.; Wu, P.H.; Wu, W.B. Lycopene inhibits TNF-α-induced endothelial ICAM-1 expression and monocyte-endothelial adhesion. Eur. J. Pharmacol 2008, 586, 275–282. [Google Scholar]
- Marcuzzi, A.; Tricarico, P.M.; Piscianz, E.; Kleiner, G.; Brumatti, L.V.; Crovella, S. Lovastatin induces apoptosis through the mitochondrial pathway in an undifferentiated SH-SY5Y neuroblastoma cell line. Cell Death Dis 2013, 4, e585. [Google Scholar]
- Drenth, J.P.; van Deuren, M.; van der Ven-Jongekrijg, J.; Schalkwijk, C.G.; van der Meer, J.W. Cytokine activation during attacks of the hyperimmunoglobulinemia D and periodic fever syndrome. Blood 1995, 85, 3586–3593. [Google Scholar]
- Normand, S.; Massonnet, B.; Delwail, A.; Favot, L.; Cuisset, L.; Grateau, G.; Morel, F.; Silvain, C.; Lecron, J.C. Specific increase in caspase-1 activity and secretion of IL-1 family cytokines: A putative link between mevalonate kinase deficiency and inflammation. Eur. Cytokine Netw 2009, 20, 101–107. [Google Scholar]
- Moudgil, K.D.; Choubey, D. Cytokines in autoimmunity: Role in induction, regulation, and treatment. J. Interf. Cytokine Res 2011, 3, 695–703. [Google Scholar]
- Schuijs, M.J.; Willart, M.A.; Hammad, H.; Lambrecht, B.N. Cytokine targets in airway inflammation. Curr. Opin. Pharmacol 2013, 13, 351–361. [Google Scholar]
- Pontillo, A.; Paoluzzi, E.; Crovella, S. The inhibition of mevalonate pathway induces upregulation of NALP3 expression: New insight in the pathogenesis of mevalonate kinase deficiency. Eur. J. Hum. Genet 2010, 18, 844–847. [Google Scholar]
- Kleiner, G.; Celsi, F.; Tricarico, P.M.; Zacchigna, S.; Crovella, S.; Marcuzzi, A. Systemic and neuronal inflammatory markers in a mouse model of mevalonate kinase deficiency: A strain-comparative study. In Vivo 2013, 27, 715–722. [Google Scholar]
- Duncan, A.J.; Heales, S.J. Nitric oxide and neurological disorders. Mol. Aspects Med 2005, 26, 67–96. [Google Scholar]
- Ungvari, Z.; Gupte, S.A.; Recchia, F.A.; Batkai, S.; Pacher, P. Role of oxidative-nitrosative stress and downstream pathways in various forms of cardiomyopathy and heart failure. Curr. Vasc. Pharmacol 2005, 3, 221–229. [Google Scholar]



© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tricarico, P.M.; Kleiner, G.; Valencic, E.; Campisciano, G.; Girardelli, M.; Crovella, S.; Knowles, A.; Marcuzzi, A. Block of the Mevalonate Pathway Triggers Oxidative and Inflammatory Molecular Mechanisms Modulated by Exogenous Isoprenoid Compounds. Int. J. Mol. Sci. 2014, 15, 6843-6856. https://doi.org/10.3390/ijms15046843
Tricarico PM, Kleiner G, Valencic E, Campisciano G, Girardelli M, Crovella S, Knowles A, Marcuzzi A. Block of the Mevalonate Pathway Triggers Oxidative and Inflammatory Molecular Mechanisms Modulated by Exogenous Isoprenoid Compounds. International Journal of Molecular Sciences. 2014; 15(4):6843-6856. https://doi.org/10.3390/ijms15046843
Chicago/Turabian StyleTricarico, Paola Maura, Giulio Kleiner, Erica Valencic, Giuseppina Campisciano, Martina Girardelli, Sergio Crovella, Alessandra Knowles, and Annalisa Marcuzzi. 2014. "Block of the Mevalonate Pathway Triggers Oxidative and Inflammatory Molecular Mechanisms Modulated by Exogenous Isoprenoid Compounds" International Journal of Molecular Sciences 15, no. 4: 6843-6856. https://doi.org/10.3390/ijms15046843