Integration of C1 and C2 Metabolism in Trees
Abstract
:1. Introduction
2. Results
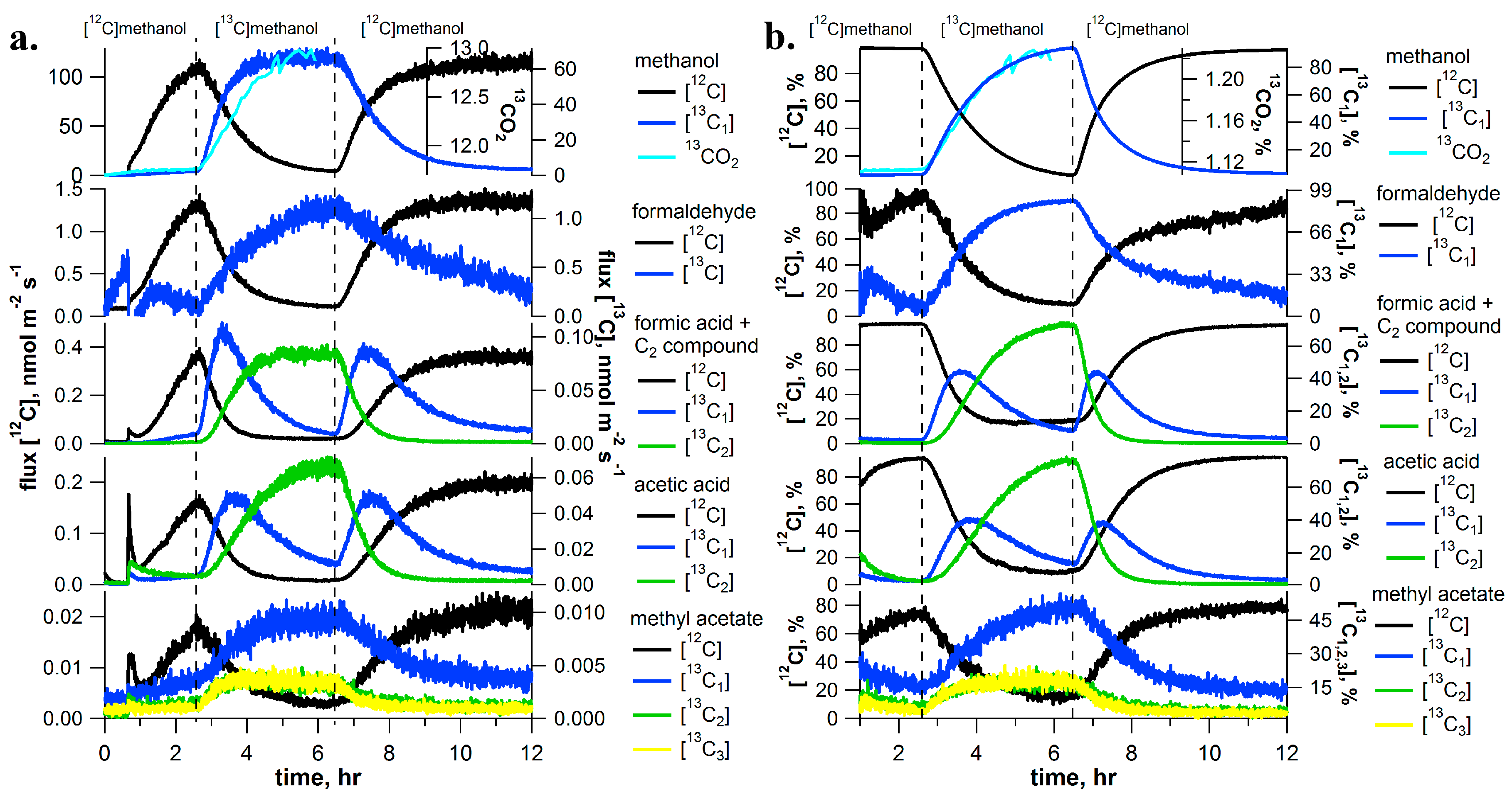
2.1. [13C]Methanol Pulse Chase Experiments
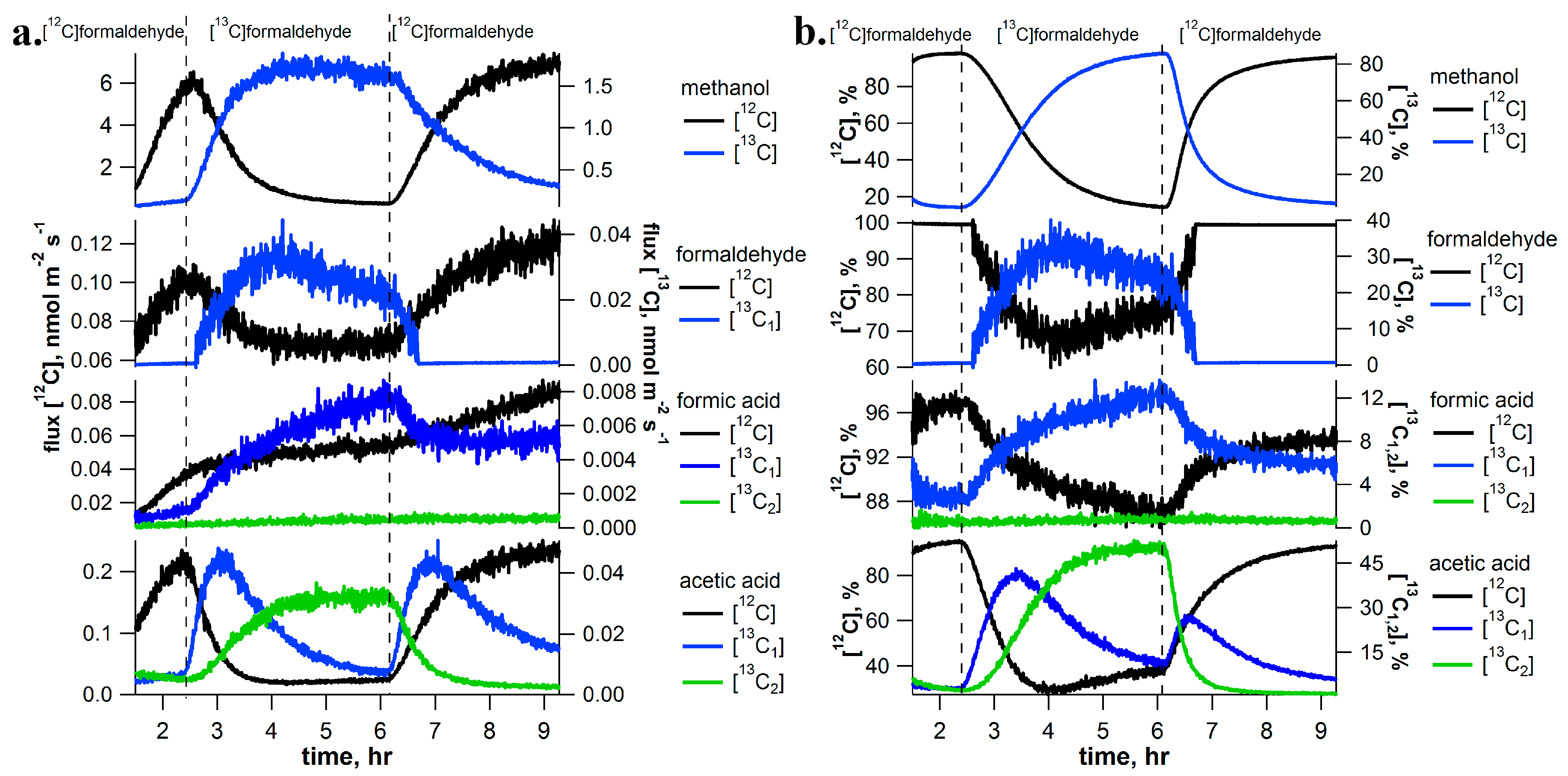
2.2. [13C]Formaldehyde Pulse-Chase Experiments
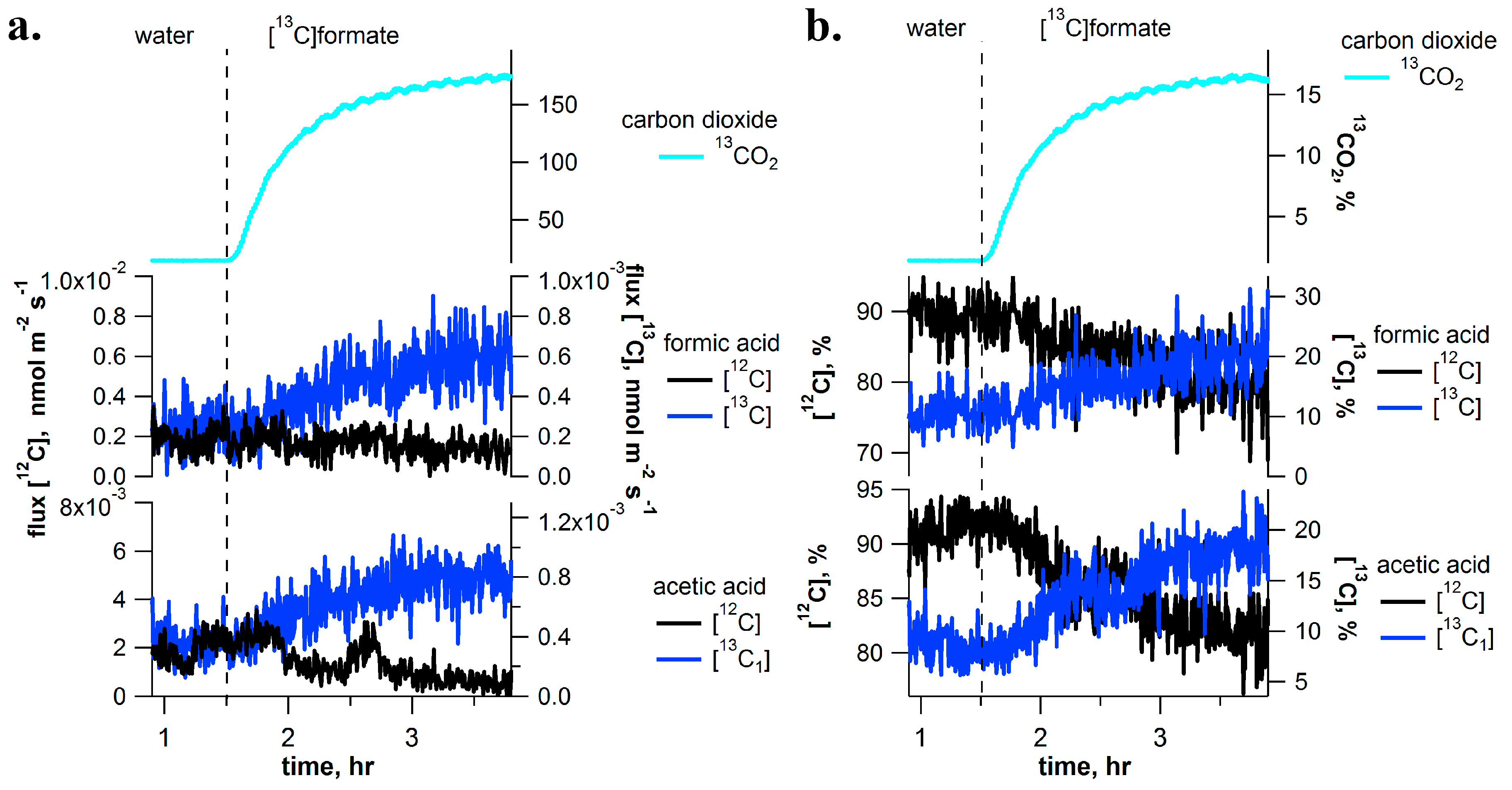
2.3. [13C]Formate Pulse-Chase Experiments
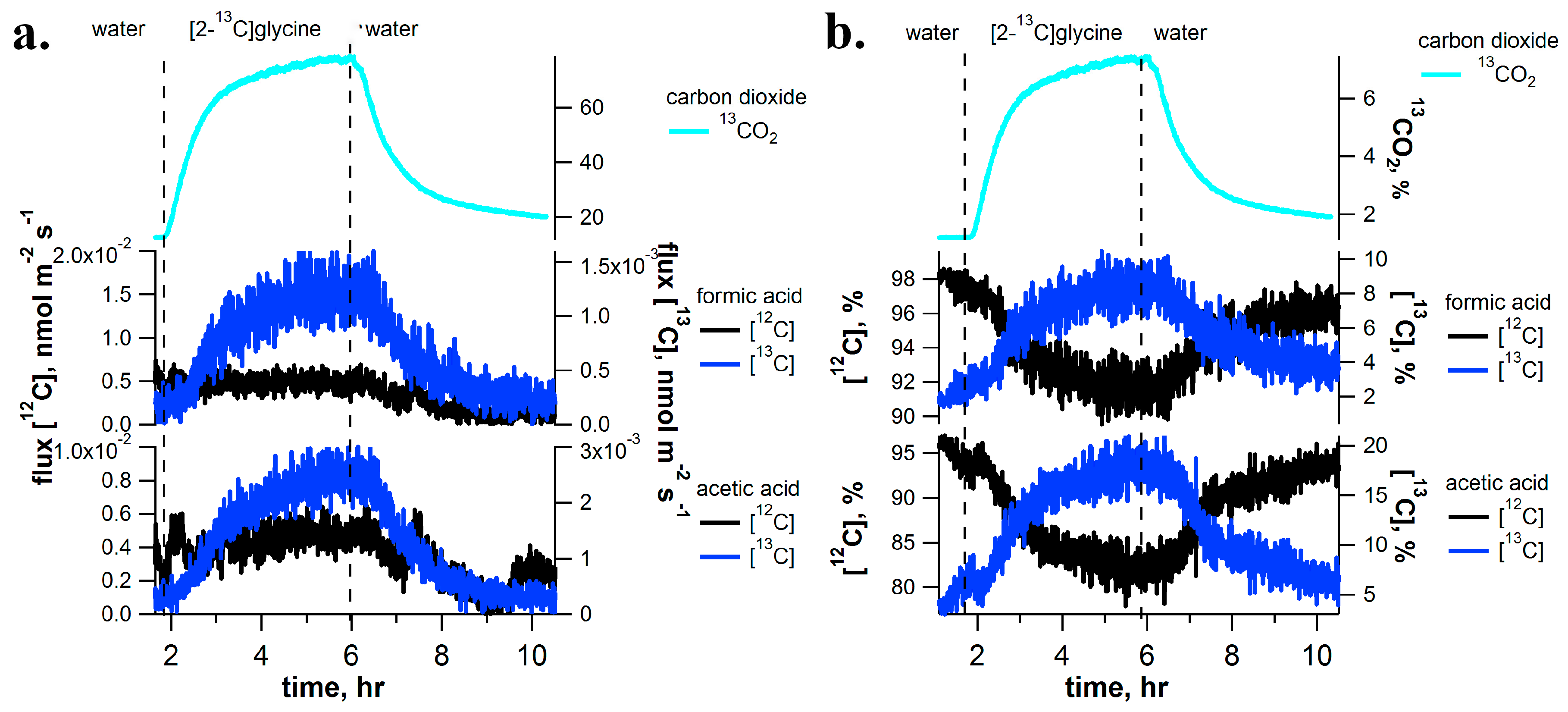
2.4. [2-13C]Glycine Pulse-Chase Experiments
2.5. [13C2]Acetate Pulse-Chase Experiments
2.6. 13C-Labeling Analysis of Isoprene (C5H8) Emissions and Its Dependence on 13CO2
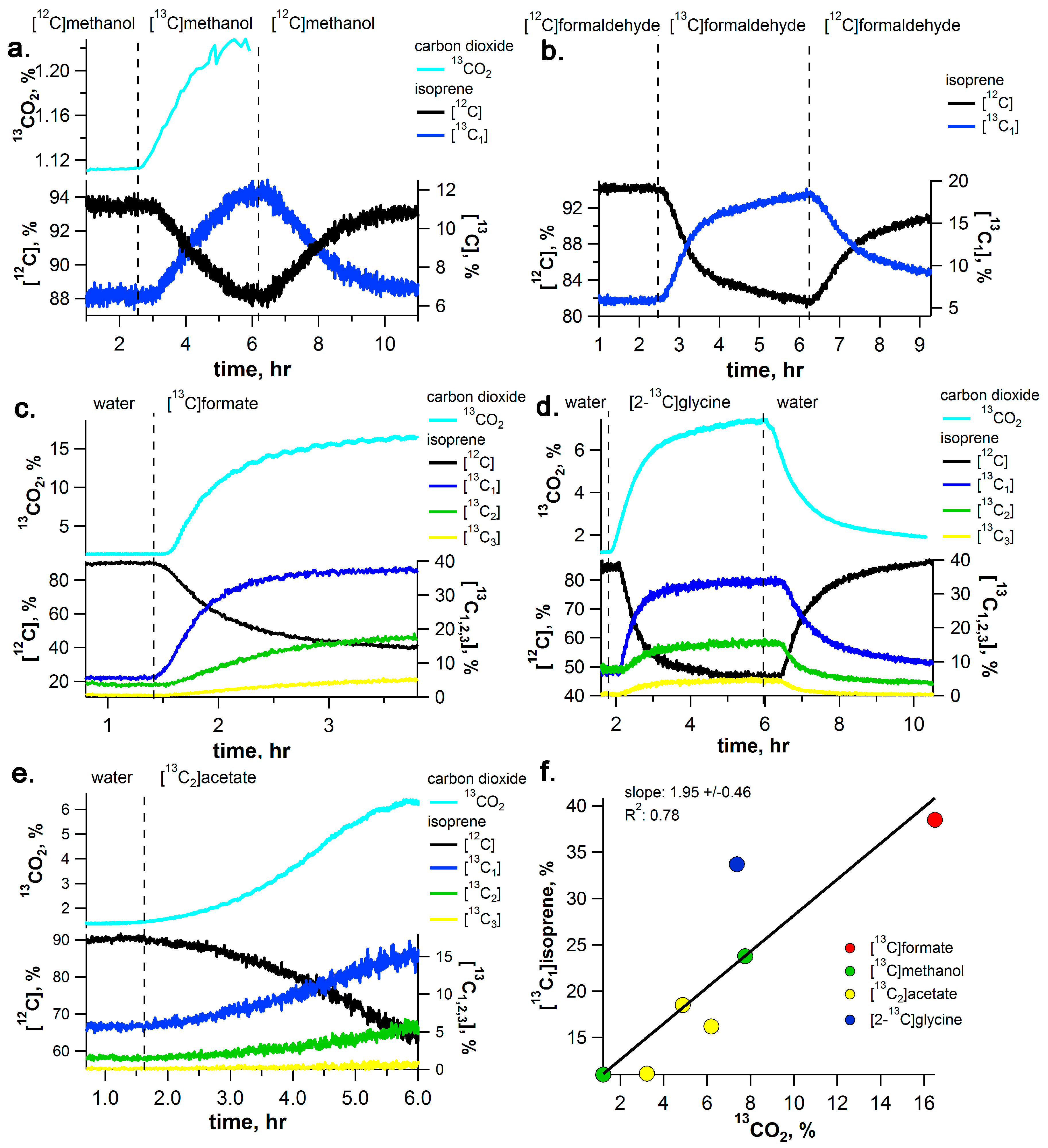
2.7. Quantitative Comparisons of Emission Rates of C1 and C2 Volatiles
3. Discussion
3.1. Dynamics of Labeling between [13C]Methanol and [13C]Formaldehyde Solutions
3.2. Reassimilation of CO2 from C1 Intermediates and Its Contribution as Carbon Source for Isoprene
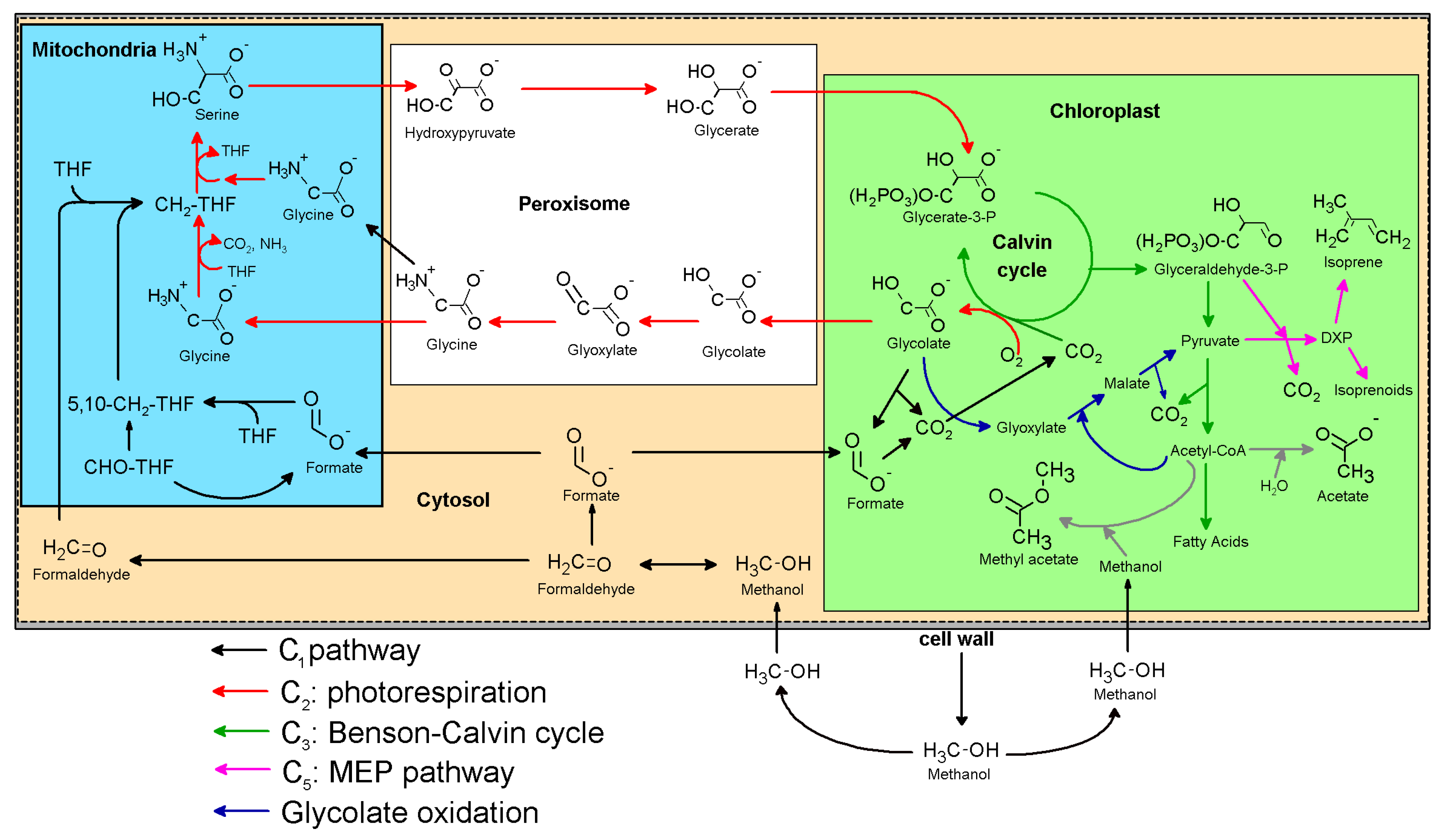
3.3. Integration of the C1 and C2 Metabolism
4. Materials and Methods
4.1. 13C Labeling Studies
4.2. Proton Transfer Reaction-Mass Spectrometry (PTR-MS)
4.3. Gas Chromatography-Mass Spectrometry (GC-MS)
4.4. CO2 Isotope Measurements
5. Conclusions
Supplementary Materials
Acknowledgments
Dedication
Author Contributions
Conflicts of Interest
References
- Jacob, D.J.; Field, B.D.; Li, Q.B.; Blake, D.R.; de Gouw, J.; Warneke, C.; Hansel, A.; Wisthaler, A.; Singh, H.B.; Guenther, A. Global budget of methanol: Constraints from atmospheric observations. J. Geophys. Res. Atmos. 2005, 110. [Google Scholar] [CrossRef] [Green Version]
- Stavrakou, T.; Guenther, A.; Razavi, A.; Clarisse, L.; Clerbaux, C.; Coheur, P.-F.; Hurtmans, D.; Karagulian, F.; Mazière, M.D.; Vigouroux, C. First space-based derivation of the global atmospheric methanol emission fluxes. Atmos. Chem. Phys. 2011, 11, 4873–4898. [Google Scholar] [CrossRef] [Green Version]
- Karl, T.; Guenther, A.; Lindinger, C.; Jordan, A.; Fall, R.; Lindinger, W. Eddy covariance measurements of oxygenated volatile organic compound fluxes from crop harvesting using a redesigned proton-transfer-reaction mass spectrometer. J. Geophys. Res. Atmos. 2001, 106, 24157–24167. [Google Scholar] [CrossRef]
- Schade, G.W.; Goldstein, A.H. Fluxes of oxygenated volatile organic compounds from a ponderosa pine plantation. J. Geophys. Res. Atmos. 2001, 106, 3111–3123. [Google Scholar] [CrossRef]
- Karl, T.; Guenther, A.; Spirig, C.; Hansel, A.; Fall, R. Seasonal variation of biogenic voc emissions above a mixed hardwood forest in northern michigan. Geophys. Res. Lett. 2003, 30. [Google Scholar] [CrossRef]
- Tie, X.; Guenther, A.; Holland, E. Biogenic methanol and its impacts on tropospheric oxidants. Geophys. Res. Lett. 2003, 30. [Google Scholar] [CrossRef]
- Palmer, P.I.; Jacob, D.J.; Fiore, A.M.; Martin, R.V.; Chance, K.; Kurosu, T.P. Mapping isoprene emissions over north america using formaldehyde column observations from space. J. Geophys. Res. Atmos. 2003, 108, 16. [Google Scholar] [CrossRef]
- Nemecek-Marshall, M.; MacDonald, R.C.; Franzen, J.J.; Wojciechowski, C.L.; Fall, R. Methanol emission from leaves (enzymatic detection of gas-phase methanol and relation of methanol fluxes to stomatal conductance and leaf development). Plant Physiol. 1995, 108, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Fall, R.; Benson, A.A. Leaf methanol—The simplest natural product from plants. Trends Plant Sci. 1996, 1, 296–301. [Google Scholar] [CrossRef]
- Frenkel, C.; Peters, J.S.; Tieman, D.M.; Tiznado, M.E.; Handa, A.K. Pectin methylesterase regulates methanol and ethanol accumulation in ripening tomato (lycopersicon esculentum) fruit. J. Biolog. Chem. 1998, 273, 4293–4295. [Google Scholar] [CrossRef]
- Hüve, K.; Christ, M.; Kleist, E.; Uerlings, R.; Niinemets, Ü.; Walter, A.; Wildt, J. Simultaneous growth and emission measurements demonstrate an interactive control of methanol release by leaf expansion and stomata. J. Exp. Bot. 2007, 58, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Jardine, K.J.; Jardine, A.B.; Souza, V.F.; Carneiro, V.; Ceron, J.V.; Gimenez, B.O.; Soares, C.P.; Durgante, F.M.; Higuchi, N.; Manzi, A.O.; et al. Methanol and isoprene emissions from the fast growing tropical pioneer species vismia guianensis (aubl.) pers. (hypericaceae) in the central amazon forest. Atmos. Chem. Phys. Discuss. 2016, 2016, 1–26. [Google Scholar] [CrossRef]
- Niinemets, U.; Reichstein, M. Controls on the emission of plant volatiles through stomata: Differential sensitivity of emission rates to stomatal closure explained. J. Geophys. Res. Atmos. 2003, 108, 4208. [Google Scholar] [CrossRef]
- Oikawa, P.Y.; Giebel, B.M.; da Silveira Lobo, O.; Li, L.; Timko, M.P.; Swart, P.K.; Riemer, D.D.; Mak, J.E.; Lerdau, M.T. Leaf and root pectin methylesterase activity and 13c/12c stable isotopic ratio measurements of methanol emissions give insight into methanol production in lycopersicon esculentum. New Phytol. 2011, 191, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Harley, P.; Greenberg, J.; Niinemets, U.; Guenther, A. Environmental controls over methanol emissions from leaves. Biogeosci. Discuss. 2007, 4, 2593–2640. [Google Scholar] [CrossRef]
- Bai, Y.-R.; Yang, P.; Su, Y.-Y.; He, Z.-L.; Ti, X.-N. Effect of exogenous methanol on glycolate oxidase and photorespiratory intermediates in cotton. J. Exp. Bot. 2014, 65, 5331–5338. [Google Scholar] [CrossRef] [PubMed]
- Cossins, E. The utilization of carbon-1 compounds by plants: I. The metabolism of methanol-c14 and its role in amino acid biosynthesis. Can. J. Biochem. 1964, 42, 1793–1802. [Google Scholar] [CrossRef]
- Gout, E.; Aubert, S.; Bligny, R.; Rébeillé, F.; Nonomura, A.R.; Benson, A.A.; Douce, R. Metabolism of methanol in plant cells. Carbon-13 nuclear magnetic resonance studies. Plant Physiol. 2000, 123, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Downie, A.; Miyazaki, S.; Bohnert, H.; John, P.; Coleman, J.; Parry, M.; Haslam, R. Expression profiling of the response of arabidopsis thaliana to methanol stimulation. Phytochemistry 2004, 65, 2305–2316. [Google Scholar] [CrossRef] [PubMed]
- Doman, N.G.; Romanova, A.K. Transformations of labeled formic acid, formaldehyde, methanol, & co2 absorbed by bean & barley leaves from air. Plant Physiol. 1962, 37, 833. [Google Scholar] [PubMed]
- Song, Z.B.; Xiao, S.Q.; You, L.; Wang, S.S.; Tan, H.; Li, K.Z.; Chen, L.M. C1 metabolism and the calvin cycle function simultaneously and independently during hcho metabolism and detoxification in arabidopsis thaliana treated with hcho solutions. Plant Cell Environ. 2013, 36, 1490–1506. [Google Scholar] [CrossRef] [PubMed]
- Igamberdiev, A.U.; Bykova, N.V.; Kleczkowski, L.A. Origins and metabolism of formate in higher plants. Plant Physiol. Biochem. 1999, 37, 503–513. [Google Scholar] [CrossRef]
- Hanson, A.D.; Gage, D.A.; Shachar-Hill, Y. Plant one-carbon metabolism and its engineering. Trends Plant Sci. 2000, 5, 206–213. [Google Scholar] [CrossRef]
- Whetten, R.; Sederoff, R. Lignin biosynthesis. Plant Cell 1995, 7, 1001. [Google Scholar] [CrossRef] [PubMed]
- Hanson, A.D.; Roje, S. One-carbon metabolism in higher plants. Ann. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Lindinger, W.; Hansel, A. Analysis of trace gases at ppb levels by proton transfer reaction mass spectrometry (PTR-MS). Plasma Sources Sci. Technol. 1997, 6, 111–117. [Google Scholar] [CrossRef]
- Šmejkalová, H.; Erb, T.J.; Fuchs, G. Methanol assimilation in methylobacterium extorquens am1: Demonstration of all enzymes and their regulation. PLoS ONE 2010, 5, e13001. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.D.; Carpenter, F.L. Interplanting inga edulis yields nitrogen benefits to terminalia amazonia. For. Ecol. Manag. 2006, 233, 344–351. [Google Scholar] [CrossRef]
- Shiraishi, T.; Fukusaki, E.-I.; Kobayashi, A. Formate dehydrogenase in rice plant: Growth stimulation effect of formate in rice plant. J. Biosci. Bioeng. 2000, 89, 241–246. [Google Scholar] [CrossRef]
- Olson, B.J.; Skavdahl, M.; Ramberg, H.; Osterman, J.C.; Markwell, J. Formate dehydrogenase in arabidopsis thaliana: Characterization and possible targeting to the chloroplast. Plant Sci. 2000, 159, 205–212. [Google Scholar] [CrossRef]
- Fukusaki, E.-I.; Ikeda, T.; Shiraishi, T.; Nishikawa, T.; Kobayashi, A. Formate dehydrogenase gene of arabidopsis thaliana is induced by formaldehyde and not by formic acid. J. Biosci. Bioeng. 2000, 90, 691–693. [Google Scholar] [CrossRef]
- Li, R.; Ziola, B.; King, J. Purification and characterization of formate dehydrogenase from arabidopsis thaliana. J. Plant Physiol. 2000, 157, 161–167. [Google Scholar] [CrossRef]
- Busch, F.A.; Sage, T.L.; Cousins, A.B.; Sage, R.F. C3 plants enhance rates of photosynthesis by reassimilating photorespired and respired co2. Plant Cell Environ. 2013, 36, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Jardine, K.J.; Chambers, J.; Alves, E.G.; Teixeira, A.; Garcia, S.; Holm, J.; Higuchi, N.; Manzi, A.; Abrell, L.; Fuentes, J.D.; et al. Dynamic balancing of isoprene carbon sources reflects photosynthetic and photorespiratory responses to temperature stress. Plant Physiol. 2014, 166, 2051–2064. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, H.; Hilgers, U.; Weidner, M. Assimilation and metabolism of formaldehyde by leaves appear unlikely to be of value for indoor air purification. New Phytol. 2000, 147, 307–315. [Google Scholar] [CrossRef]
- Li, R.; Moore, M.; King, J. Investigating the regulation of one-carbon metabolism in arabidopsis thaliana. Plant Cell Physiol. 2003, 44, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Bloemen, J.; Anne McGuire, M.; Aubrey, D.P.; Teskey, R.O.; Steppe, K. Internal recycling of respired co2 may be important for plant functioning under changing climate regimes. Plant Signal. Behav. 2013, 8, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Randall, D.D. Pyruvate dehydrogenase complex from chloroplasts of pisum sativum l. Plant Physiol. 1979, 64, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Peterhansel, C.; Maurino, V.G. Photorespiration redesigned. Plant Physiol. 2011, 155, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Wingler, A.; Lea, P.J.; Leegood, R.C. Photorespiratory metabolism of glyoxylate and formate in glycine-accumulating mutants of barley and amaranthus edulis. Planta 1999, 207, 518–526. [Google Scholar] [CrossRef]
- Eastmond, P.J.; Graham, I.A. Re-examining the role of the glyoxylate cycle in oilseeds. Trends Plant Sci. 2001, 6, 72–78. [Google Scholar] [CrossRef]
- Wei, P.; Yu, B. Roles of methanol on plants and its physiological mechanisms. Acta Bot. Boreal Occident Sin. 2014, 34, 416–422. [Google Scholar]
- Nonomura, A.; Benson, A. The path of carbon in photosynthesis: Improved crop yields with methanol. Proc. Natl. Acad. Sci. USA 1992, 89, 9794–9798. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, I.; Dorta, F.; Espinoza, V.; Jiménez, E.; Mercado, A.; Peña-Cortés, H. Effects of foliar and root applications of methanol on the growth of arabidopsis, tobacco, and tomato plants. J. Plant Growth Regul. 2006, 25, 30–44. [Google Scholar] [CrossRef]
- Jardine, K.J.; Henderson, W.M.; Huxman, T.E.; Abrell, L. Dynamic solution injection: A new method for preparing pptv-ppbv standard atmospheres of volatile organic compounds. Atmos. Meas. Tech. 2010, 3, 1569–1576. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jardine, K.J.; Fernandes de Souza, V.; Oikawa, P.; Higuchi, N.; Bill, M.; Porras, R.; Niinemets, Ü.; Chambers, J.Q. Integration of C1 and C2 Metabolism in Trees. Int. J. Mol. Sci. 2017, 18, 2045. https://doi.org/10.3390/ijms18102045
Jardine KJ, Fernandes de Souza V, Oikawa P, Higuchi N, Bill M, Porras R, Niinemets Ü, Chambers JQ. Integration of C1 and C2 Metabolism in Trees. International Journal of Molecular Sciences. 2017; 18(10):2045. https://doi.org/10.3390/ijms18102045
Chicago/Turabian StyleJardine, Kolby J., Vinicius Fernandes de Souza, Patty Oikawa, Niro Higuchi, Markus Bill, Rachel Porras, Ülo Niinemets, and Jeffrey Q. Chambers. 2017. "Integration of C1 and C2 Metabolism in Trees" International Journal of Molecular Sciences 18, no. 10: 2045. https://doi.org/10.3390/ijms18102045







