Effect of the Biofilm Age and Starvation on Acid Tolerance of Biofilm Formed by Streptococcus mutans Isolated from Caries-Active and Caries-Free Adults
Abstract
:1. Introduction
2. Results
2.1. Effect of ATR on Acid Tolerance of S. mutans Biofilm
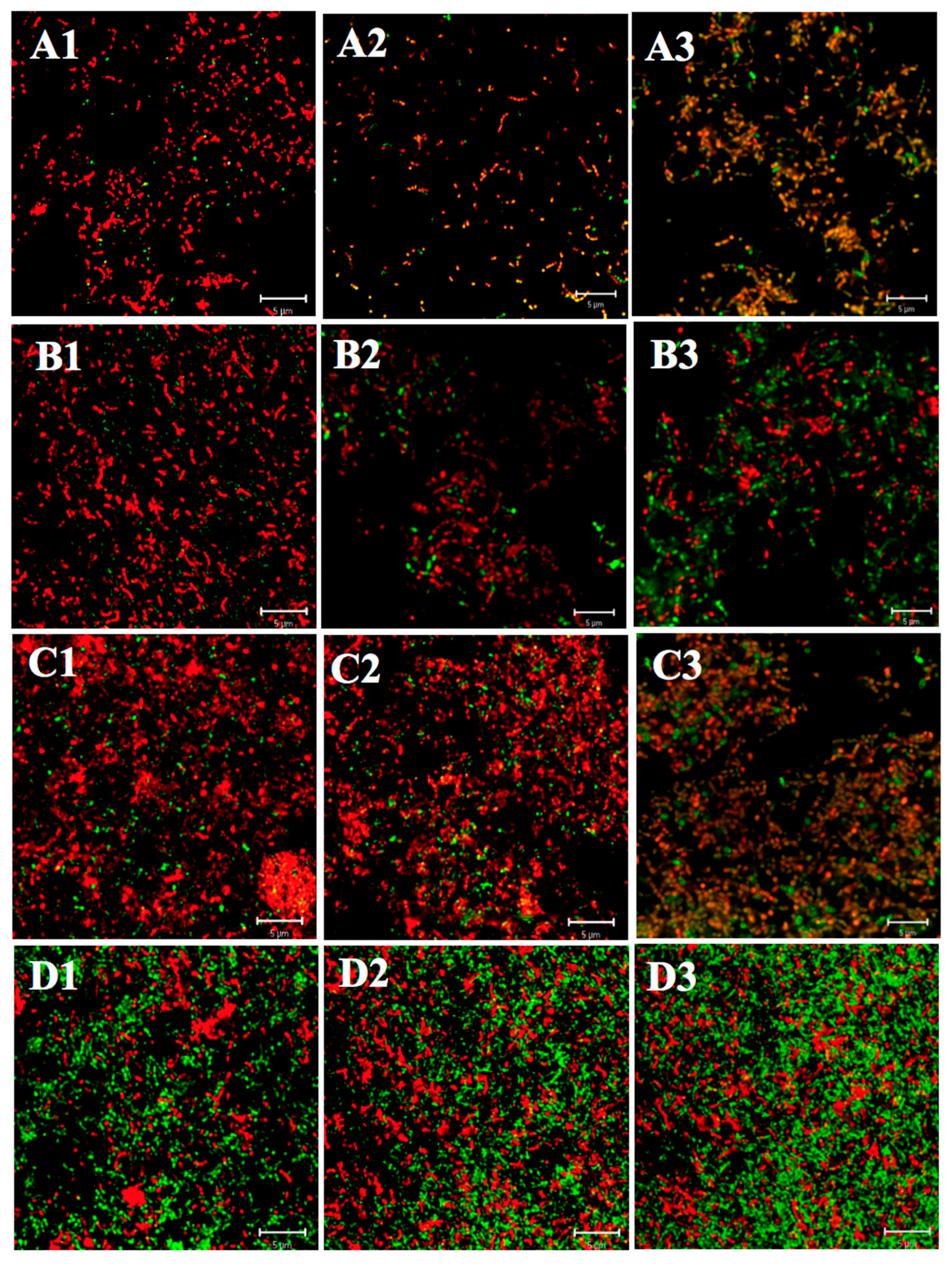
2.1.1. Differences in Acid Tolerance between the Acid-Adapted and Non-Adapted Biofilms
2.1.2. Differences in Acid Tolerance between Young and Mature Biofilms
2.1.3. Differences in Acid Tolerance on ATR among Three Strains
2.2. Effect of Starvation on Acid Tolerance of S. mutans Biofilm
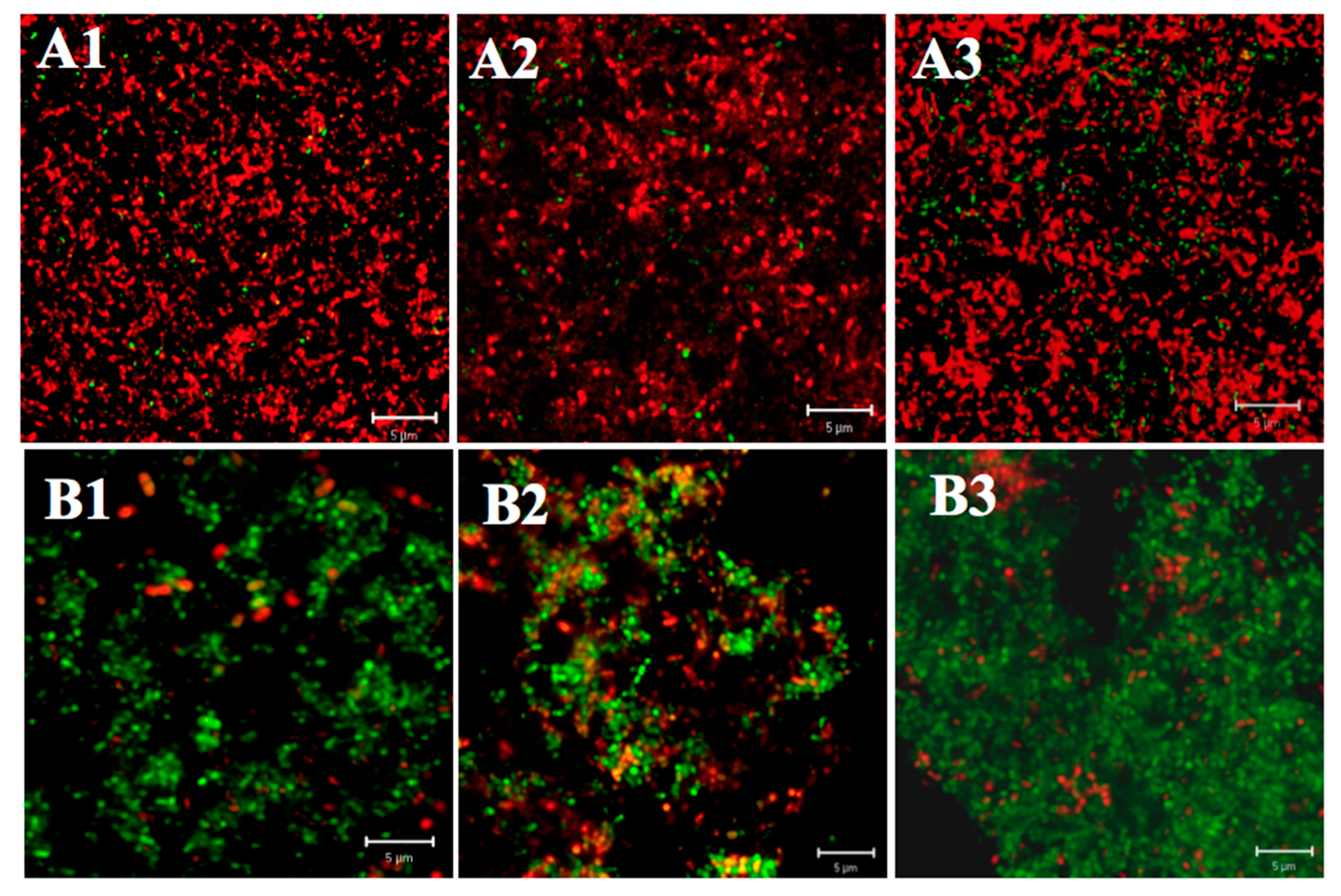
2.2.1. Differences in Acid Tolerance between the Starved and Non-Starved Biofilms
2.2.2. Differences in Acid Tolerance on Starvation among Three Strains
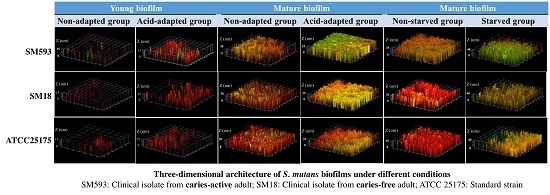
2.3. Three-Dimensional Reconstructions
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. Formation of Young and Mature Biofilms
4.3. Acid-Adaptive Response of Young and Mature Biofilms
4.4. Acid Shock of Starved/Non-Starved Biofilms
4.5. Fluorescence Staining for Biofilms (Viability Staining)
4.6. CLSM Observation of Biofilms and Image Analysis
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ATCC | American Type Culture Collection |
| ANOVA | One way analysis of variance |
| ATR | Acid tolerance response |
| CLSM | Confocal laser scanning microscopy |
| DMFT | decayed, missing, and filled teeth |
| DFT | decayed and filled teeth |
| MS | Mutans Streptococcus |
| OD | Optical density |
| PBS | Phosphate-buffered saline |
| PCR | Polymerase chain reaction |
| PI | Propidium iodide |
| TPY | Tryptone-polypeptone-yeast extract |
| SD | Standard deviation |
| SPSS | Statistical Package for Social Science |
References
- Featherstone, J.D. The continuum of dental caries—Evidence for a dynamic disease process. J. Dent. Res. 2004, 83, C39–C42. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J.D. Dental caries: A dynamic disease process. Aust. Dent. J. 2008, 53, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, M.; Tam, A.; Steinberg, D. Expression of biofilm-associated genes of Streptococcus mutans in response to glucose and sucrose. J. Med. Microbiol. 2007, 56, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, J. Bacterial metabolism in dental biofilms. Adv. Dent. Res. 1997, 11, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tao, Y.; Yu, L.; Zhuang, P.; Zhi, Q.; Zhou, Y.; Lin, H. Analysis of Small RNAs in Streptococcus mutans under Acid Stress-A New Insight for Caries Research. Int. J. Mol. Sci. 2016, 17, 1529. [Google Scholar] [CrossRef] [PubMed]
- Svensater, G.; Larsson, U.B.; Greif, E.C.; Cvitkovitch, D.G.; Hamilton, I.R. Acid tolerance response and survival by oral bacteria. Oral Microbiol. Immunol. 1997, 12, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; McLean, J.S.; Lux, R.; He, X.; Shi, W. The well-coordinated linkage between acidogenicity and aciduricity via insoluble glucans on the surface of Streptococcus mutans. Sci. Rep. 2015, 5, 18015. [Google Scholar] [CrossRef] [PubMed]
- Landini, P.; Antoniani, D.; Burgess, J.G.; Nijland, R. Molecular mechanisms of compounds affecting bacterial biofilm formation and dispersal. Appl. Microbiol. Biotechnol. 2010, 86, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, M. The physiological activity of bacteria attached to solid surfaces. Adv. Micro Physiol. 1991, 32, 53–85. [Google Scholar]
- Burne, R.A. Oral streptococci... products of their environment. J. Dent. Res. 1998, 77, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Welin, J.; Wilkins, J.C.; Beighton, D.; Wrzesinski, K.; Fey, S.J.; Mose-Larsen, P.; Hamilton, I.R.; Svensater, G. Effect of acid shock on protein expression by biofilm cells of Streptococcus mutans. FEMS Microbiol. Lett. 2003, 227, 287–293. [Google Scholar] [CrossRef]
- Arnold, K.W.; Kaspar, C.W. Starvation—And stationary-phase-induced acid tolerance in Escherichia coli O157:H7. Appl. Environ. Microbiol. 1995, 61, 2037–2039. [Google Scholar] [PubMed]
- Zhu, M.; Takenaka, S.; Sato, M.; Hoshino, E. Influence of starvation and biofilm formation on acid resistance of Streptococcus mutans. Oral Microbiol. Immunol. 2001, 16, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Washio, J.; Takahashi, N. Metabolomic Studies of Oral Biofilm, Oral Cancer, and Beyond. Int. J. Mol. Sci. 2016, 17, 870. [Google Scholar] [CrossRef] [PubMed]
- Lembo, F.L.; Longo, P.L.; Ota-Tsuzuki, C.; Rodrigues, C.R.; Mayer, M.P. Genotypic and phenotypic analysis of Streptococcus mutans from different oral cavity sites of caries-free and caries-active children. Oral Microbiol. Immunol. 2007, 22, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.H.; Shi, J.N.; Zhang, Y.; Liu, X.D.; Duan, J.; Wei, S. Identification of genetic differences between two clinical isolates of Streptococcus mutans by suppression subtractive hybridization. Oral Microbiol. Immunol. 2006, 21, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.H.; Wang, H.L.; Liu, X.D.; Duan, J. Identification of protein differences between two clinical isolates of Streptococcus mutans by proteomic analysis. Oral Microbiol. Immunol. 2008, 23, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Loesche, W.J. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 1986, 50, 353–380. [Google Scholar] [PubMed]
- Van Houte, J.; Jordan, H.V.; Laraway, R.; Kent, R.; Soparkar, P.M.; DePaola, P.F. Association of the microbial flora of dental plaque and saliva with human root-surface caries. J. Dent. Res. 1990, 69, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Giacaman, R.A.; Araneda, E.; Padilla, C. Association between biofilm-forming isolates of mutans streptococci and caries experience in adults. Arch. Oral Biol. 2010, 55, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, T.; Chen, Z.; Zhan, L.; Yang, J. Evaluation of cariogenic potential of Streptococcus mutans isolated from caries-free and -active persons: Adherence properties to saliva-coated hydroxyapatite. Hua Xi Kou Qiang Yi Xue Za Zhi 2000, 18, 416–418. [Google Scholar] [PubMed]
- Huang, X.; Liu, T.; Yang, J.; Chen, Z.; Liu, J. Evaluation of cariogenic potential of Streptococcus mutans isolated from caries-free and -active persons: Abilities to synthesize water-soluble and—Insoluble glucans. Hua Xi Kou Qiang Yi Xue Za Zhi 2000, 18, 419–421. [Google Scholar] [PubMed]
- Huang, X.; Liu, T.; Chen, G. [Typing of Streptococcus mutans (serotype C) by arbitrarily primed polymerase chain reaction]. Zhonghua Kou Qiang Yi Xue Za Zhi 2001, 36, 281–284. [Google Scholar] [PubMed]
- Huang, X.J.; Liu, T.J.; Cai, Z.Y.; Chen, Z.; Yang, J.B.; Liu, J.G. [Evaluation of the in vitro cariogenic potential of Streptococcus mutans (serotype C) strains isolated from caries-free and -active people: The ability of acidogenicity]. Sichuan Da Xue Xue Bao Yi Xue Ban 2004, 35, 520–521. [Google Scholar] [PubMed]
- Belli, W.A.; Marquis, R.E. Adaptation of Streptococcus mutans and Enterococcus hirae to acid stress in continuous culture. Appl. Environ. Microbiol. 1991, 57, 1134–1138. [Google Scholar] [PubMed]
- Hamilton, I.R.; Buckley, N.D. Adaptation by Streptococcus mutans to acid tolerance. Oral Microbiol. Immunol. 1991, 6, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Motegi, M.; Takagi, Y.; Yonezawa, H.; Hanada, N.; Terajima, J.; Watanabe, H.; Senpuku, H. Assessment of genes associated with Streptococcus mutans biofilm morphology. Appl. Environ. Microbiol. 2006, 72, 6277–6287. [Google Scholar] [CrossRef] [PubMed]
- Svensater, G.; Sjogreen, B.; Hamilton, I.R. Multiple stress responses in Streptococcus mutans and the induction of general and stress-specific proteins. Microbiology 2000, 146, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, J.C.; Homer, K.A.; Beighton, D. Analysis of Streptococcus mutans proteins modulated by culture under acidic conditions. Appl. Environ. Microbiol. 2002, 68, 2382–2390. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Stojicic, S.; Haapasalo, M. Antimicrobial efficacy of chlorhexidine against bacteria in biofilms at different stages of development. J. Endod. 2011, 37, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhao, J.; de la Fuente-Nunez, C.; Wang, Z.; Hancock, R.E.; Roberts, C.R.; Ma, J.; Li, J.; Haapasalo, M.; Wang, Q. Experimental and Theoretical Investigation of Multispecies Oral Biofilm Resistance to Chlorhexidine Treatment. Sci. Rep. 2016, 6, 27537. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Cheng, K.J.; Geesey, G.G.; Ladd, T.I.; Nickel, J.C.; Dasgupta, M.; Marrie, T.J. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 1987, 41, 435–464. [Google Scholar] [CrossRef] [PubMed]
- Sonia Goodacre, C.C.; Slattery, C. Cambridge VCE Health and Human Development Units 1 and 2 App, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Welin-Neilands, J.; Svensater, G. Acid tolerance of biofilm cells of Streptococcus mutans. Appl. Environ. Microbiol. 2007, 73, 5633–5638. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Huang, X.; Zhang, C.; Cai, Z.; Zou, T. Morphological and proteomic analyses of the biofilms generated by Streptococcus mutans isolated from caries-active and caries-free adults. J. Dent. Sci. 2015, 10, 206–215. [Google Scholar] [CrossRef]
- Parsek, M.R.; Greenberg, E.P. Acyl-homoserine lactone quorum sensing in gram-negative bacteria: A signaling mechanism involved in associations with higher organisms. Proc. Natl. Acad. Sci. USA 2000, 97, 8789–8793. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Lau, P.C.; Tang, N.; Svensater, G.; Ellen, R.P.; Cvitkovitch, D.G. Novel two-component regulatory system involved in biofilm formation and acid resistance in Streptococcus mutans. J. Bacteriol. 2002, 184, 6333–6342. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.A.; Petersen, F.C.; Scheie, A.A. Biofilm formation and autoinducer-2 signaling in Streptococcus intermedius: Role of thermal and pH factors. Oral Microbiol. Immunol. 2008, 23, 492–497. [Google Scholar] [CrossRef] [PubMed]
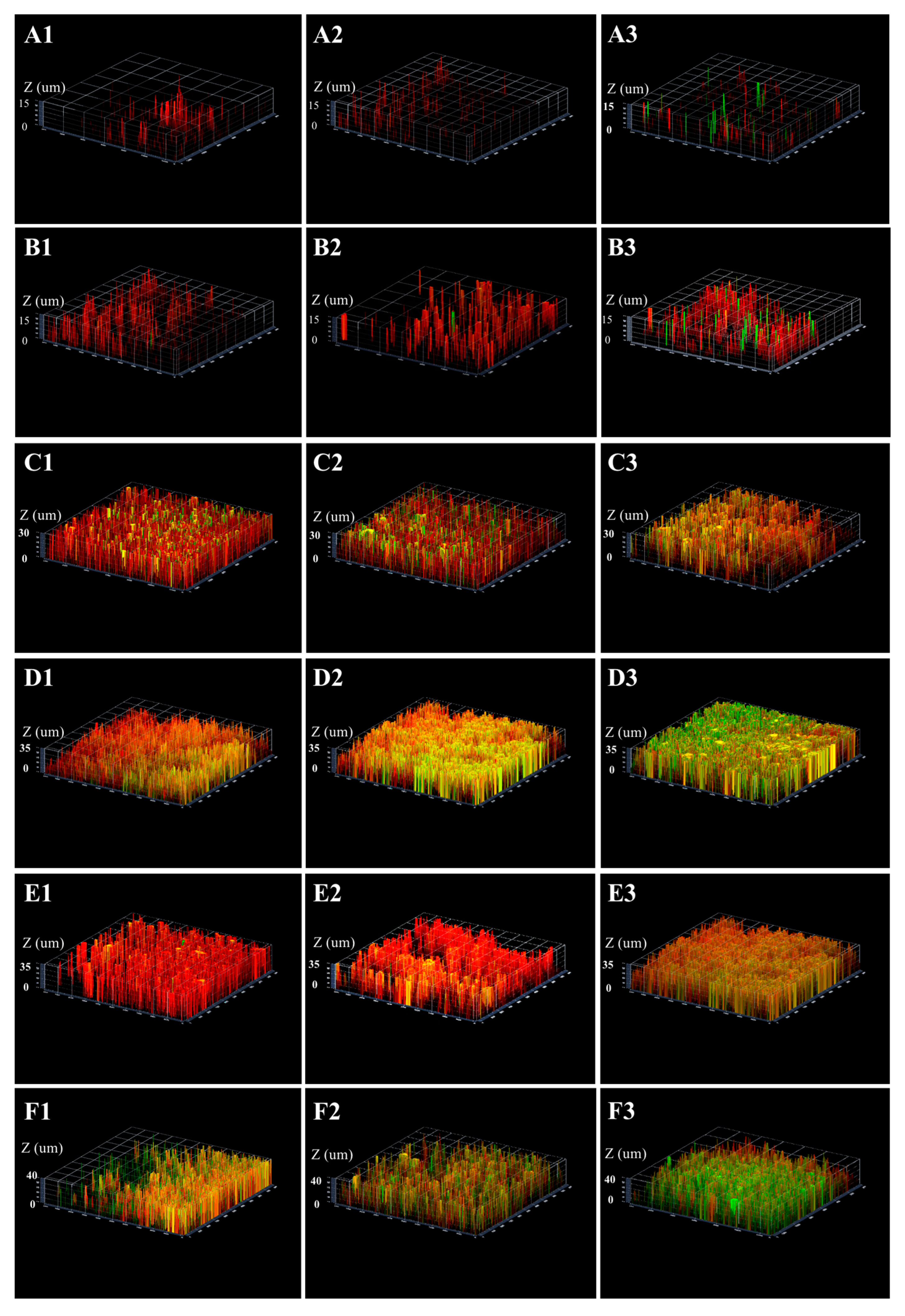
| Strains | Outer | Middle | Inner | |||
|---|---|---|---|---|---|---|
| Non-Adapted | Acid-Adapted | Non-Adapted | Acid-Adapted | Non-Adapted | Acid-Adapted | |
| ATCC25175 | 3.15 ± 0.67 | 11.12 ± 0.85 | 13.27 ± 1.17 | 24.17 ± 1.01 | 4.34 ± 0.91 | 11.85 ± 0.75 |
| SM18 | 2.56 ± 0.79 | 10.91 ± 0.92 | 12.45 ± 0.95 | 21.42 ± 0.95 | 3.23 ± 0.96 | 10.99 ± 0.81 |
| SM593 | 7.72 ± 0.57 | 15.41 ± 0.96 | 22.56 ± 0.87 | 42.37 ± 0.44 | 8.51 ± 0.91 | 15.65 ± 0.65 |
| Strains | Outer | Middle | Inner | |||
|---|---|---|---|---|---|---|
| Non-Adapted | Acid-Adapted | Non-Adapted | Acid-Adapted | Non-Adapted | Acid-Adapted | |
| ATCC25175 | 12.26 ± 0.22 | 18.43 ± 1.23 | 29.72 ± 0.97 | 49.88 ± 0.87 | 12.49 ± 0.74 | 19.76 ± 0.76 |
| SM18 | 11.48 ± 0.75 | 17.52 ± 1.11 | 28.71 ± 1.04 | 47.79 ± 0.83 | 13.42 ± 0.85 | 18.52 ± 1.16 |
| SM593 | 16.51 ± 0.96 | 31.19 ± 1.06 | 38.73 ± 0.74 | 68.75 ± 0.95 | 17.31 ± 0.76 | 34.45 ± 1.36 |
| Strains | Outer | Middle | Inner | |||
|---|---|---|---|---|---|---|
| Non-Starved | Starved | Non-Starved | Starved | Non-Starved | Starved | |
| ATCC25175 | 13.36 ± 1.05 | 21. 67 ± 0.66 | 27.21 ± 1.05 | 47.88 ± 0.87 | 14.77 ± 0.76 | 23.58 ± 0.88 |
| SM18 | 11.39 ± 1.10 | 22.47 ± 0.91 | 26.16 ± 1.15 | 47.24 ± 1.25 | 12.14 ± 0.21 | 23.28 ± 1.07 |
| SM593 | 21.47 ± 1.30 | 41.59 ± 0.99 | 39.28 ± 0.89 | 61.31 ± 0.76 | 23.11 ± 0.31 | 43.35 ± 0.92 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Chen, S.; Zhang, C.; Zhao, X.; Huang, X.; Cai, Z. Effect of the Biofilm Age and Starvation on Acid Tolerance of Biofilm Formed by Streptococcus mutans Isolated from Caries-Active and Caries-Free Adults. Int. J. Mol. Sci. 2017, 18, 713. https://doi.org/10.3390/ijms18040713
Jiang S, Chen S, Zhang C, Zhao X, Huang X, Cai Z. Effect of the Biofilm Age and Starvation on Acid Tolerance of Biofilm Formed by Streptococcus mutans Isolated from Caries-Active and Caries-Free Adults. International Journal of Molecular Sciences. 2017; 18(4):713. https://doi.org/10.3390/ijms18040713
Chicago/Turabian StyleJiang, Shan, Shuai Chen, Chengfei Zhang, Xingfu Zhao, Xiaojing Huang, and Zhiyu Cai. 2017. "Effect of the Biofilm Age and Starvation on Acid Tolerance of Biofilm Formed by Streptococcus mutans Isolated from Caries-Active and Caries-Free Adults" International Journal of Molecular Sciences 18, no. 4: 713. https://doi.org/10.3390/ijms18040713