Microwave Deposition of Palladium Catalysts on Graphite Spheres and Reduced Graphene Oxide Sheets for Electrochemical Glucose Sensing
Abstract
:1. Introduction
2. Experimental Section
2.1. Pulse Microwave Synthesis of Pd Catalysts
2.2. Characterization and GOR Activity of Pd Catalysts
3. Results and Discussion
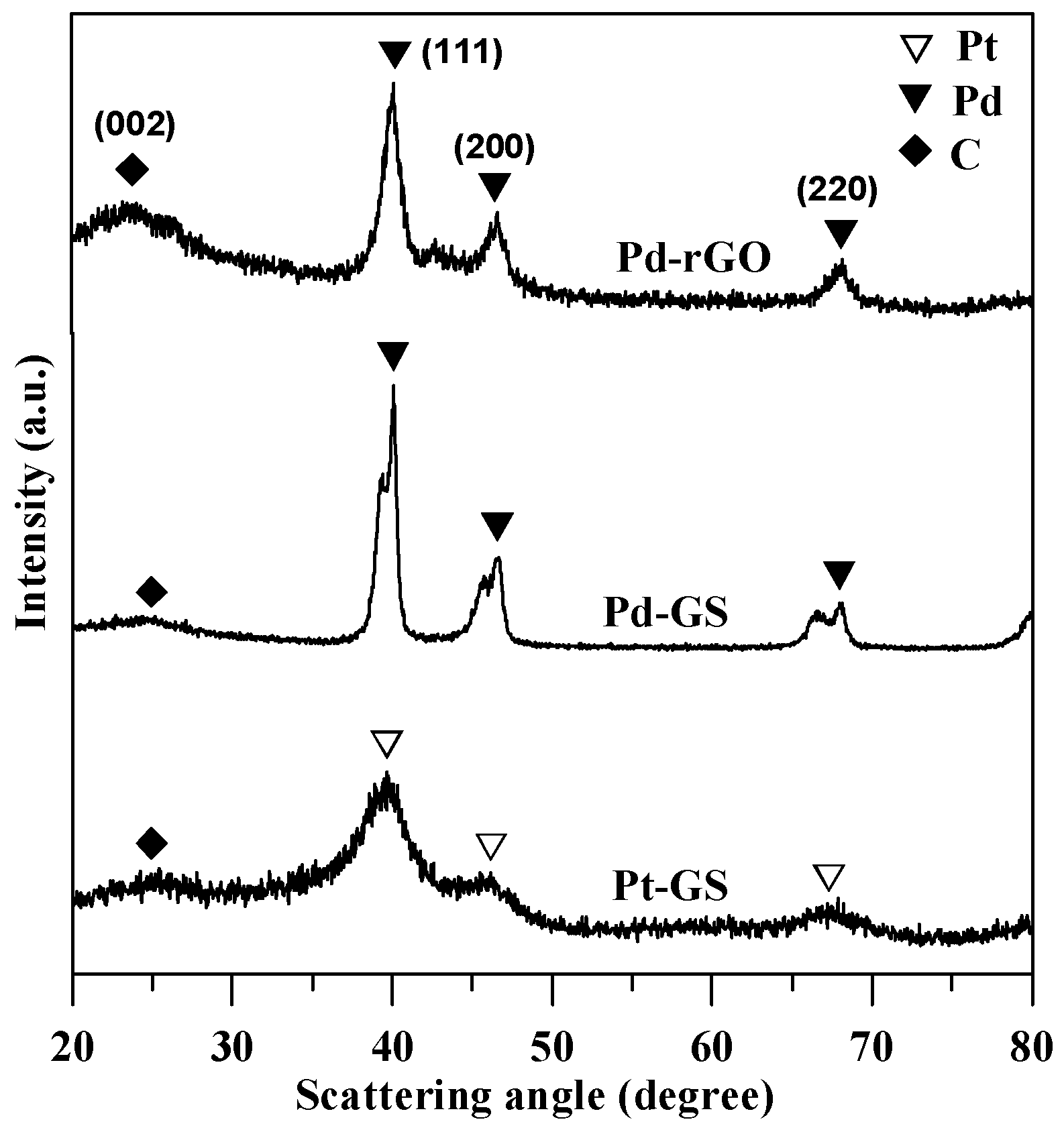
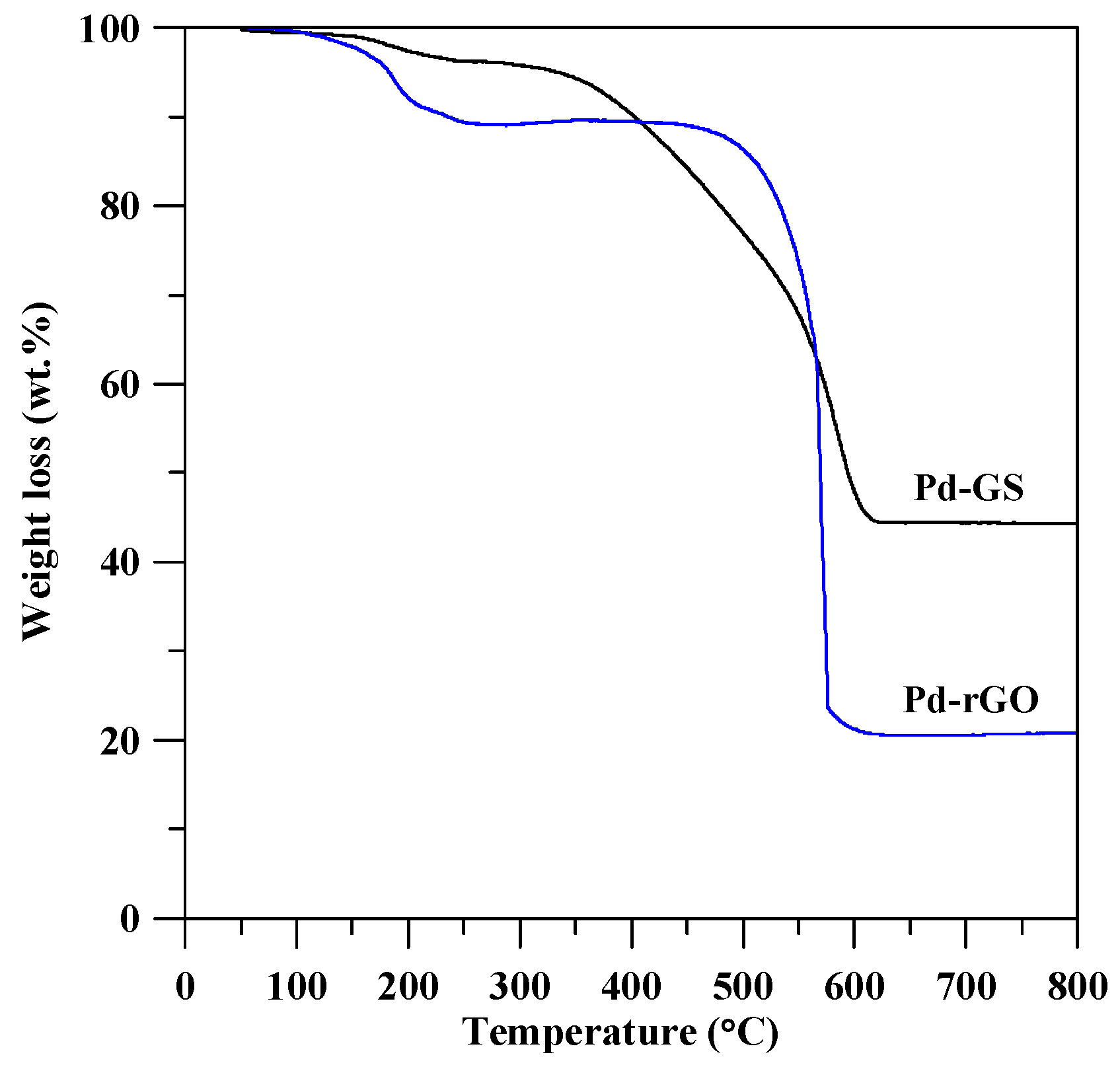
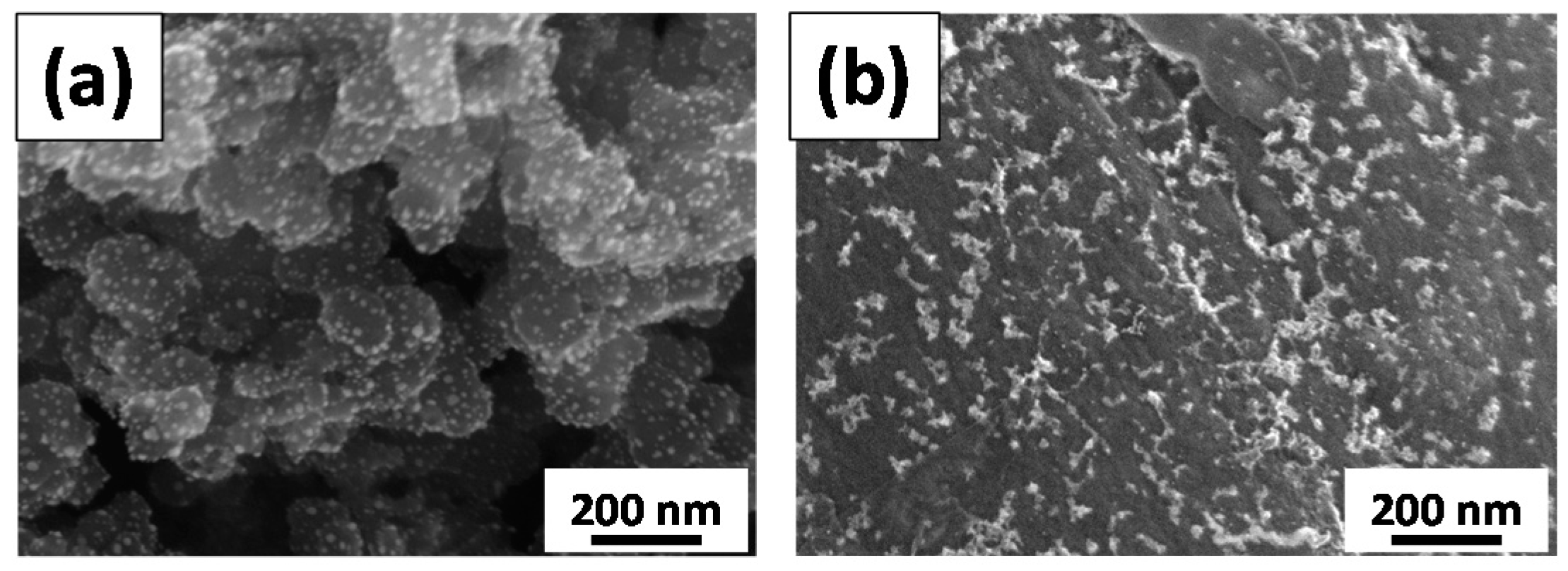
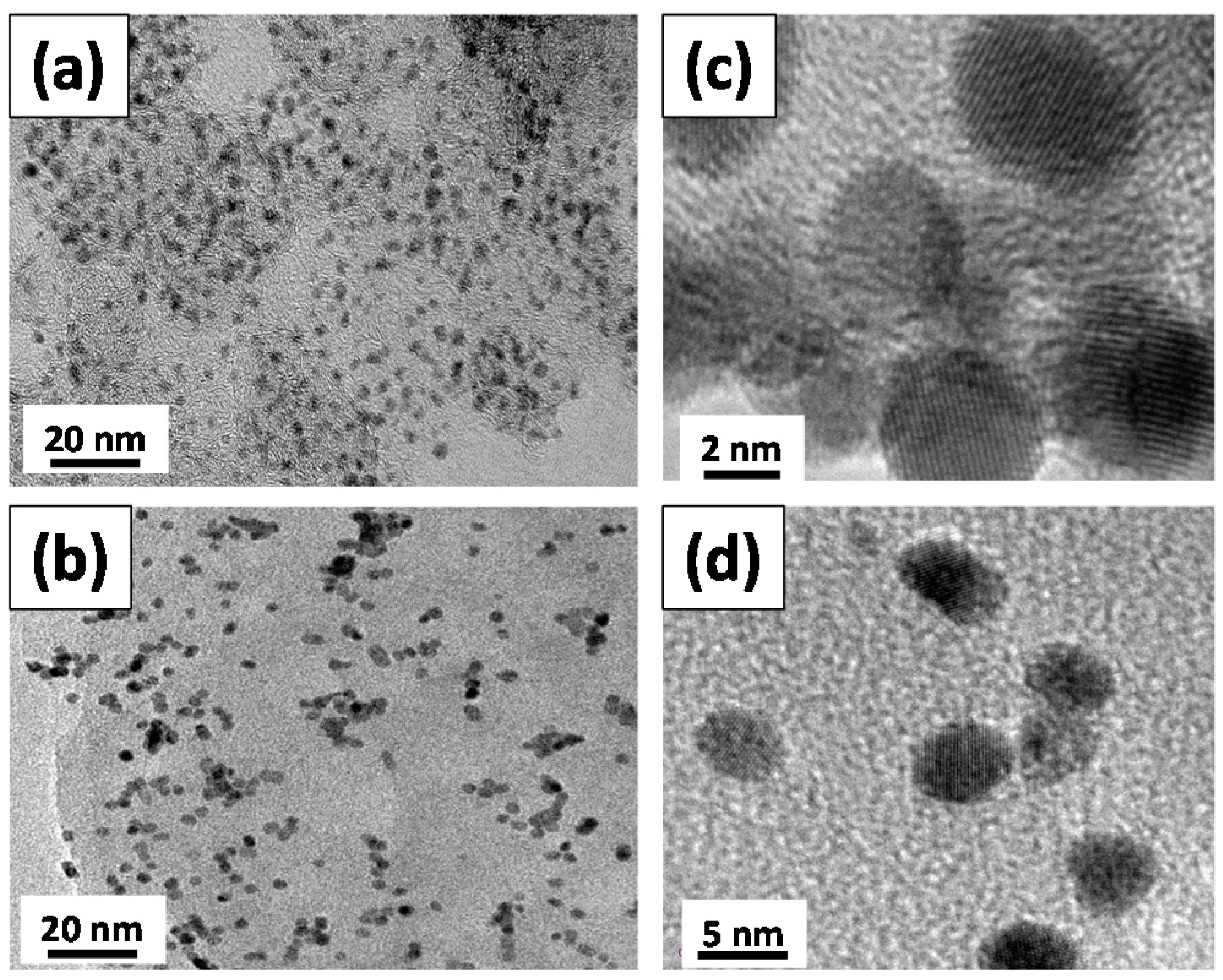
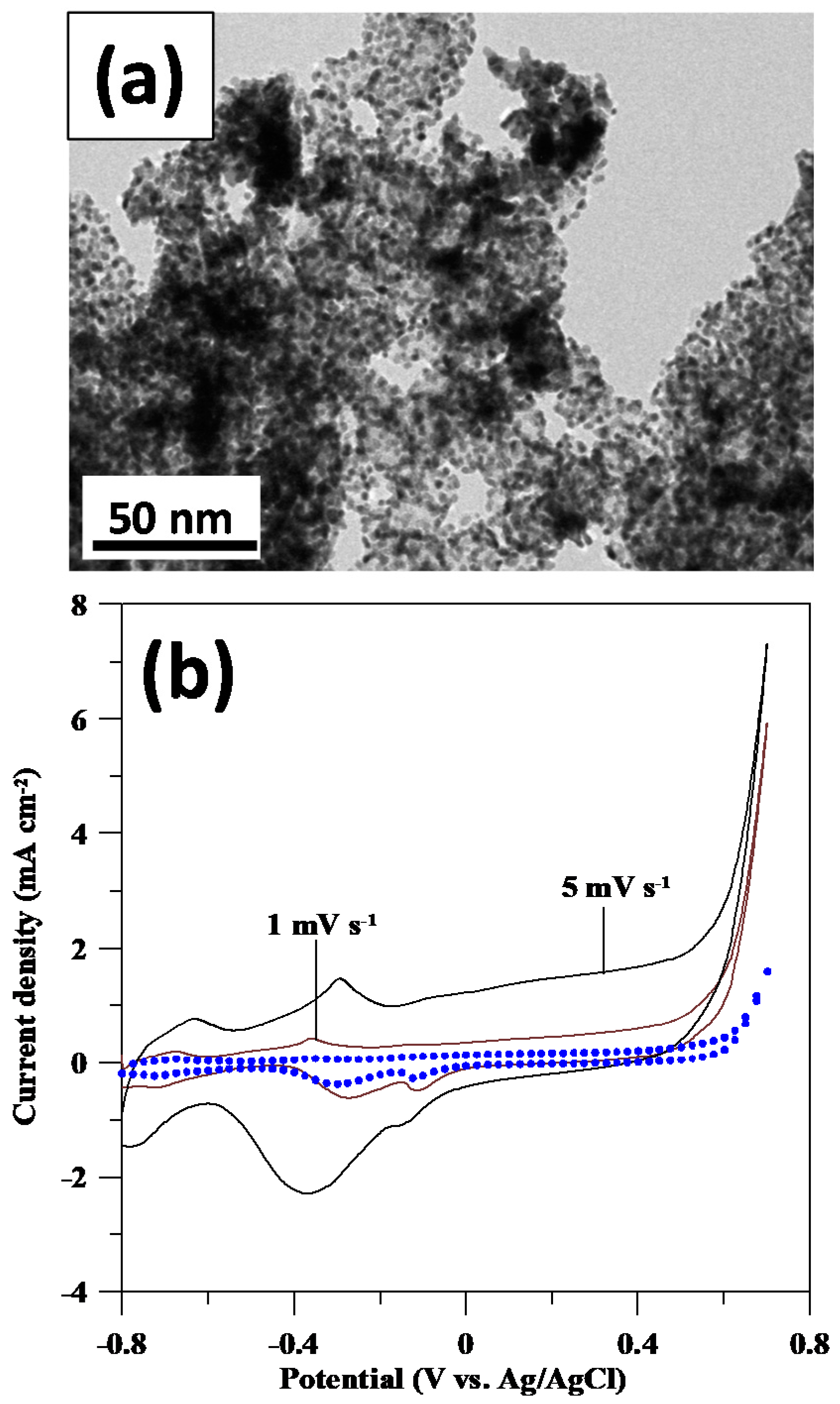
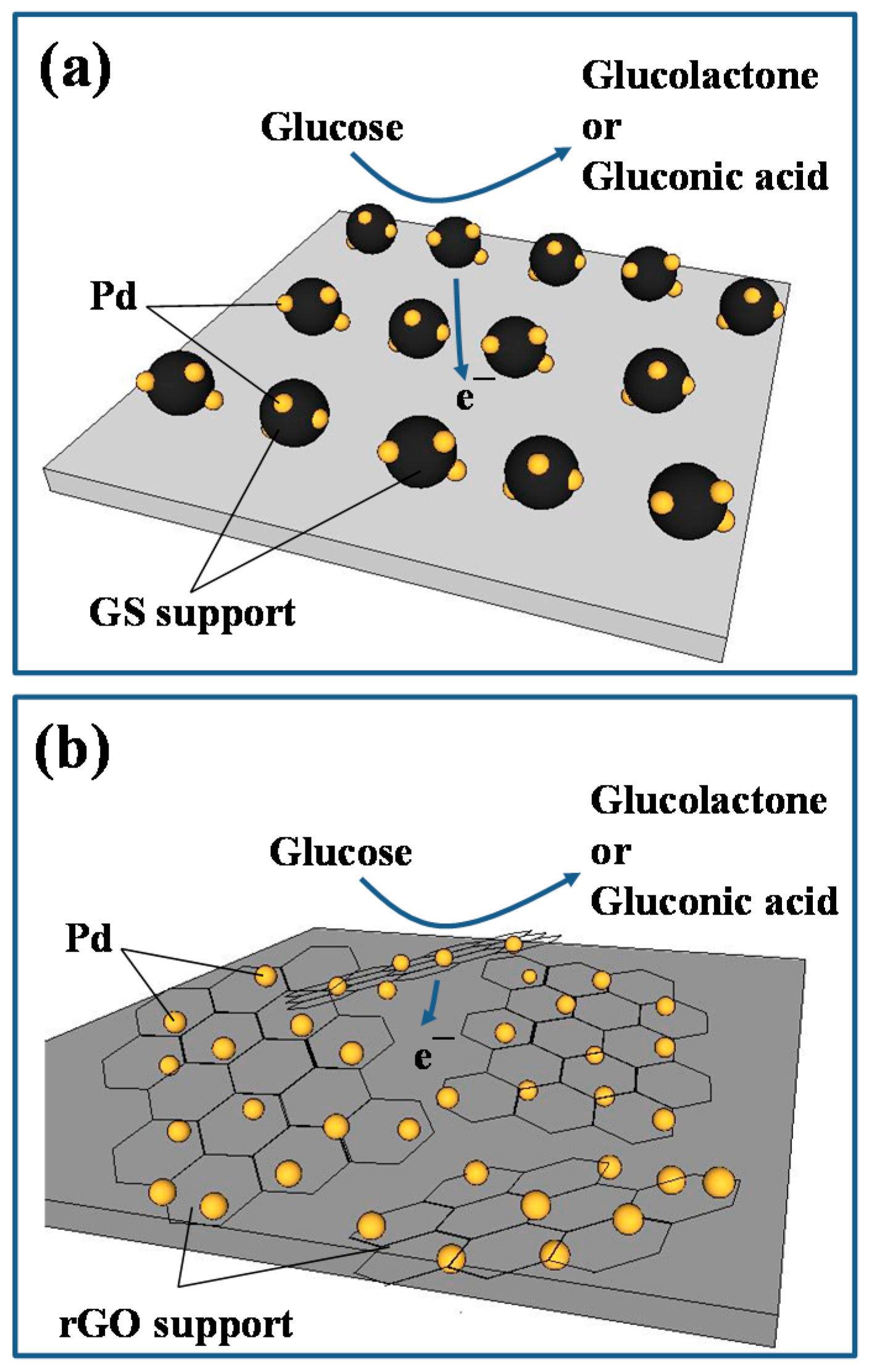
3.1. Physiochemical Properties of Pd Catalysts
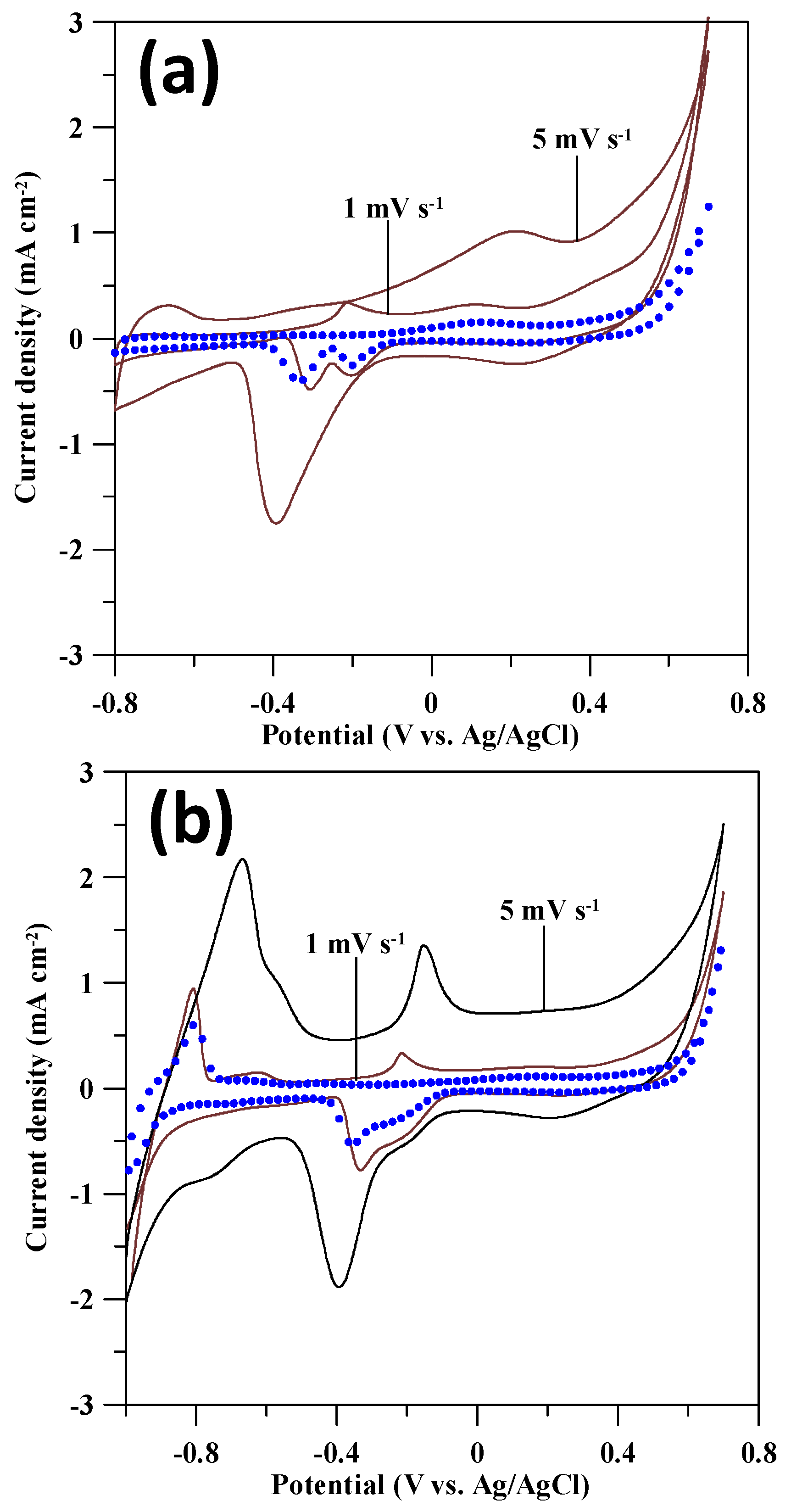
3.2. GOR Activity on Pd Catalysts
3.3. Sensitivity of Pd Catalysts
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, A.; Holt-Hindle, P. Platinum-based nanostructured materials: Synthesis, properties, and applications. Chem. Rev. 2010, 110, 3767–3804. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Hu, G.; Nitze, F.; Barzegar, H.R.; Sharifi, T.; Tai, C.-W.; Wågberg, T. Synthesis of palladium/helical carbon nanofiber hybrid nanostructures and their application for hydrogen peroxide and glucose detection. ACS Appl. Mater. Interfaces 2013, 5, 12017–12022. [Google Scholar] [CrossRef] [PubMed]
- Meher, S.K.; Rao, G.R. Archetypal sandwich-structured CuO for high performance non-enzymatic sensing of glucose. Nanoscale 2013, 5, 2089–2099. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Guo, S.; Ma, H.; Shan, D.; Yang, S.; Gong, J. Nickel oxide microfibers immobilized onto electrode by electrospinning and calcination for nonenzymatic glucose sensor and effect of calcination temperature on the performance. Biosens. Bioelectron. 2011, 26, 2756–2760. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wang, Y.; Zhao, Y.; Xu, C. Non-enzymatic glucose sensor based on three dimensional nickel oxide for enhanced sensitivity. Anal. Methods 2013, 5, 1644–1647. [Google Scholar] [CrossRef]
- Ye, D.; Liang, G.; Li, H.; Luo, J.; Zhang, S.; Chen, H.; Kong, J. A novel nonenzymatic sensor based on CuO nanoneedle/graphene/carbon nanofiber modified electrode for probing glucose in Saliva. Talanta 2013, 116, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xu, L.; Zhou, C.; Xing, R.; Dai, Q.; Liu, D.; Song, H. Synthesis of graphene oxide based CuO nanoparticles composite electrode for highly enhanced nonenzymatic glucose detection. ACS Appl. Mater. Interfaces 2013, 5, 12928–12934. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Lin, C.L.; Chen, L.C. Functionalized carbon nanomaterial supported palladium nano-catalysts for electrocatalytic glucose oxidation reaction. Electrochim. Acta 2015, 152, 408–416. [Google Scholar] [CrossRef]
- Su, Y.; Luo, B.; Zhang, J.Z. Controllable cobalt oxide/Au hierarchically nanostructured electrode for nonenzymatic glucose sensing. Anal. Chem. 2016, 88, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wang, N. A novel non-enzymatic glucose sensor modified with Fe2O3 nanowire arrays. Analyst 2011, 136, 4241–4246. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-T.; Chen, Y.-F.; Yu, P.-Y. Deposition of binary Pd–Rh catalysts on nanostructured carbon supports for non-enzymatic glucose oxidation. Int. J. Hydrogen Energy 2015, 40, 14857–14865. [Google Scholar] [CrossRef]
- Xi, J.; Xie, C.; Zhang, Y.; Wang, L.; Xizo, J.; Duan, X.; Ren, J.; Xiao, F.; Wang, S. Pd nanoparticles decorated N-Doped graphene quantum Dots@N-Doped carbon hollow nanospheres with high electrochemical sensing performance in cancer detection. ACS Appl. Mater. Interfaces 2016, 8, 22563–22568. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ju, H. Reagentless glucose biosensor based on direct electron transfer of glucose oxidase immobilized on colloidal gold modified carbon paste electrode. Biosens. Bioelectron. 2003, 19, 177–183. [Google Scholar] [CrossRef]
- Lien, C.H.; Chen, J.C.; Hu, C.C.; Wong, D.S.H. Cathodic deposition of binary nickel-cobalt hydroxide for non-enzymatic glucose sensing. J. Taiwan Inst. Chem. Eng. 2014, 45, 846–851. [Google Scholar] [CrossRef]
- Dong, J.; Ren, L.; Zhang, Y.; Cui, X.; Hu, P.; Xu, J. Direct electrodeposition of cable-like CuO@Cu nanowires array for non-enzymatic sensing. Talanta 2015, 132, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Basu, D.; Basu, S. A study on direct glucose and fructose alkaline fuel cell. Electrochim. Acta 2010, 55, 5775–5779. [Google Scholar] [CrossRef]
- Akter, R.; Rhee, C.K.; Rahman, M.A. A stable and sensitive voltammetric immunosensor based on a new non-enzymatic label. Biosens. Bioelectron. 2013, 50, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, G.; Yao, A.; Xiao, Y.; Du, J.; Guo, Y.; Xiao, D.; Hu, Q.; Choi, M.M.F. A sensitive AgNPs/CuO nanofibers non-enzymatic glucose sensor based on electrospinning technology. Sens. Actuators B Chem. 2014, 195, 431–438. [Google Scholar] [CrossRef]
- Ye, J.S.; Chen, C.W.; Lee, C.L. Pd Nanocube as non-enzymatic glucose sensor. Sens. Actuators B Chem. 2015, 208, 569–574. [Google Scholar] [CrossRef]
- Cai, Z.X.; Liu, C.C.; Wu, G.H.; Chen, X.M.; Chen, X. Palladium nanoparticles deposit on multi-walled carbon nanotubes and their catalytic applications for electrooxidation of ethanol and glucose. Electrochim. Acta 2013, 112, 756–762. [Google Scholar] [CrossRef]
- Kadirgan, F.; Beyhan, S.; Atilan, T. Preparation and characterization of nano-sized Pt–Pd/C catalysts and comparison of their electro-activity toward methanol and ethanol oxidation. Int. J. Hydrogen Energy 2009, 34, 4312–4320. [Google Scholar] [CrossRef]
- Steigerwalt, E.S.; Deluga, G.A.; Lukehart, C.M. Pt–Ru/Carbon fiber nanocomposites: Synthesis, characterization, and performance as anode catalysts of direct methanol fuel cells. A search for exceptional performance. J. Phys. Chem. B 2002, 106, 760–766. [Google Scholar] [CrossRef]
- He, Q.; Shyam, B.; Nishijima, M.; Yang, X.; Koel, B.; Ernst, F.; Ramaker, D.; Mukerjee, S. Highly stable Pt-Au@Ru/C catalyst nanoparticles for methanol electro-oxidation. J. Phys. Chem. C 2013, 117, 1457–1467. [Google Scholar] [CrossRef]
- Meng, L.; Jin, J.; Yang, G.X.; Lu, T.H.; Zhang, H.; Cai, C.X. Nonenzymatic electrochemical detection of glucose based on palladium-single-walled carbon nanotube hybrid nanostructures. Anal. Chem. 2009, 81, 7271–7280. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Cheng, J.-S.; Liu, X.-F.; Bai, H.-T.; Jiang, J.-H. Palladium nanoparticle/chitosan-grafted graphene nanocomposites for construction of a glucose biosensor. Biosens. Bioelectron. 2011, 26, 3456–3463. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.K.; Prokhorov, E.; Bahena, D.; Esparza, R.; Meyyappan, M. Chitosan-covered Pd@Pt core-shell nanocubes for direct electron transfer in electrochemical enzymatic glucose biosensor. ACS Omega 2017, 2, 1896–1904. [Google Scholar] [CrossRef]
- Ismail, N.S.; Le, Q.H.; Yoshikawa, H.; Saito, M.; Tamiya, E. Development of non-enzymatic electrochemical glucose sensor based on graphene oxide nanoribbon–gold nanoparticle hybrid. Electrochim. Acta 2014, 146, 98–105. [Google Scholar] [CrossRef]
- Fu, S.; Fan, G.; Yang, L.; Li, F. Non-enzymatic glucose sensor based on au nanoparticles decorated ternary Ni-Al layered double hydroxide/single-walled carbon nanotubes/graphene nanocomposite. Electrochim. Acta 2015, 152, 146–154. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.-D.; Gu, S.; Zhang, H. Microwave Deposition of Palladium Catalysts on Graphite Spheres and Reduced Graphene Oxide Sheets for Electrochemical Glucose Sensing. Sensors 2017, 17, 2163. https://doi.org/10.3390/s17102163
Xie J-D, Gu S, Zhang H. Microwave Deposition of Palladium Catalysts on Graphite Spheres and Reduced Graphene Oxide Sheets for Electrochemical Glucose Sensing. Sensors. 2017; 17(10):2163. https://doi.org/10.3390/s17102163
Chicago/Turabian StyleXie, Jian-De, Siyong Gu, and Houan Zhang. 2017. "Microwave Deposition of Palladium Catalysts on Graphite Spheres and Reduced Graphene Oxide Sheets for Electrochemical Glucose Sensing" Sensors 17, no. 10: 2163. https://doi.org/10.3390/s17102163




