A Waist-Worn Inertial Measurement Unit for Long-Term Monitoring of Parkinson’s Disease Patients
Abstract
:1. Introduction
2. Currently Existing Inertial Systems
2.1. Dataloggers
2.2. Open Source Dataloggers
2.3. Parkinson’s Disease Monitoring
3. Requirements of the System
3.1. Clinical Protocol
- Stage 0: In this stage, a baseline exploration is performed by clinicians. They will ask patients to come into doctor’s office to receive an information sheet and the consent form. Then a series of tests are performed and questionnaires are filled in with the aim of mapping the severity of the disease symptoms by means of the UPDRS [43] and Hoehn & Yahr scale [44]. A total of 25 patients will be recruited in two parts (12 patients the first part, 13 patients the second part) for the data collection after providing informed consent according to the Declaration of Helsinki.
- Stage 1: This stage’s main goal is devoted to acquiring inertial data during patients’ activities of daily living in order to adapt two personalized machine learning classifiers to detect bradykinetic gait and freezing of gait. Thus, in this stage patients receive the inertial system and wear it during 3 days at waking hours, at least 10 h, in which the system acquires inertial data at 50 Hz. The fourth day patients go to doctor’s office, where he/she executes, with the sensor in the waist, a series of tests also video-recorded in order to have a gold-standard. The aim of this experiment is to perform a laboratory test of the required real-time algorithms from the inertial system, so that bradykinetic gait and FoG real-time detections are compared to the video recordings. Experiments and results of this test are shown in Section 6 and Section 7, respectively.
- Stage 2: The main objective in this stage is to personalize the RAS system according to patients’ preference and test the system under laboratory conditions. One of the main advantages of the MASPARK system is the capability of providing an actuation once a FoG episode or a bradykinetic gait state is detected. This actuation relies on RAS which enhances gait whenever these symptoms appear [45,46]. Hence, the user is invited to test the headset in laboratory conditions. The headsets will produce different RAS and clinicians will evaluate which one is more effective.
- Stage 3: This stage is dedicated to test the effectivity of the RAS cues in the ADL of patients administered through the MASPARK system (Inertial sensor, smartphone, and headset). Patients will use the 9 × 3 device in two periods of 4 days each, a 4-day period with the actuating system enabled and the other period being the system disabled. Different measures, such as the number of FoG episodes, will be compared among both periods to determine the effectivity of the approach.
- Stage 4: In this stage, patients will use the system during 30 days continuously. At night, patients will charge the 9 × 3 with a standard mobile phone charger (micro-B USB connector). The system will send RAS to the headsets when it detects a FoG or bradykinesia episode, as in Stage 3. The main goal is to test the system in long periods, check usability and analyse patient’s quality of life before and after the use of the system over a longer period.
3.2. Hardware Requirements
- Accelerometer 1 (axes X, Y and Z): 12 bytes.
- Accelerometer 2 (axes X, Y and Z): 12 bytes.
- Accelerometer 3 (axes X, Y and Z): 12 bytes.
- Gyroscope (axes X, Y and Z): 12 bytes.
- Magnetometer (axes X, Y and Z): 12 bytes.
- Barometer: 4 bytes
- Device temperature (accelerometers 1, 2, 3, and barometer): 16 bytes
- Battery status: 4 bytes
- Real-time registers (year, month, day, hour, minute, and second): 6 bytes
- Sample counters: 4 bytes
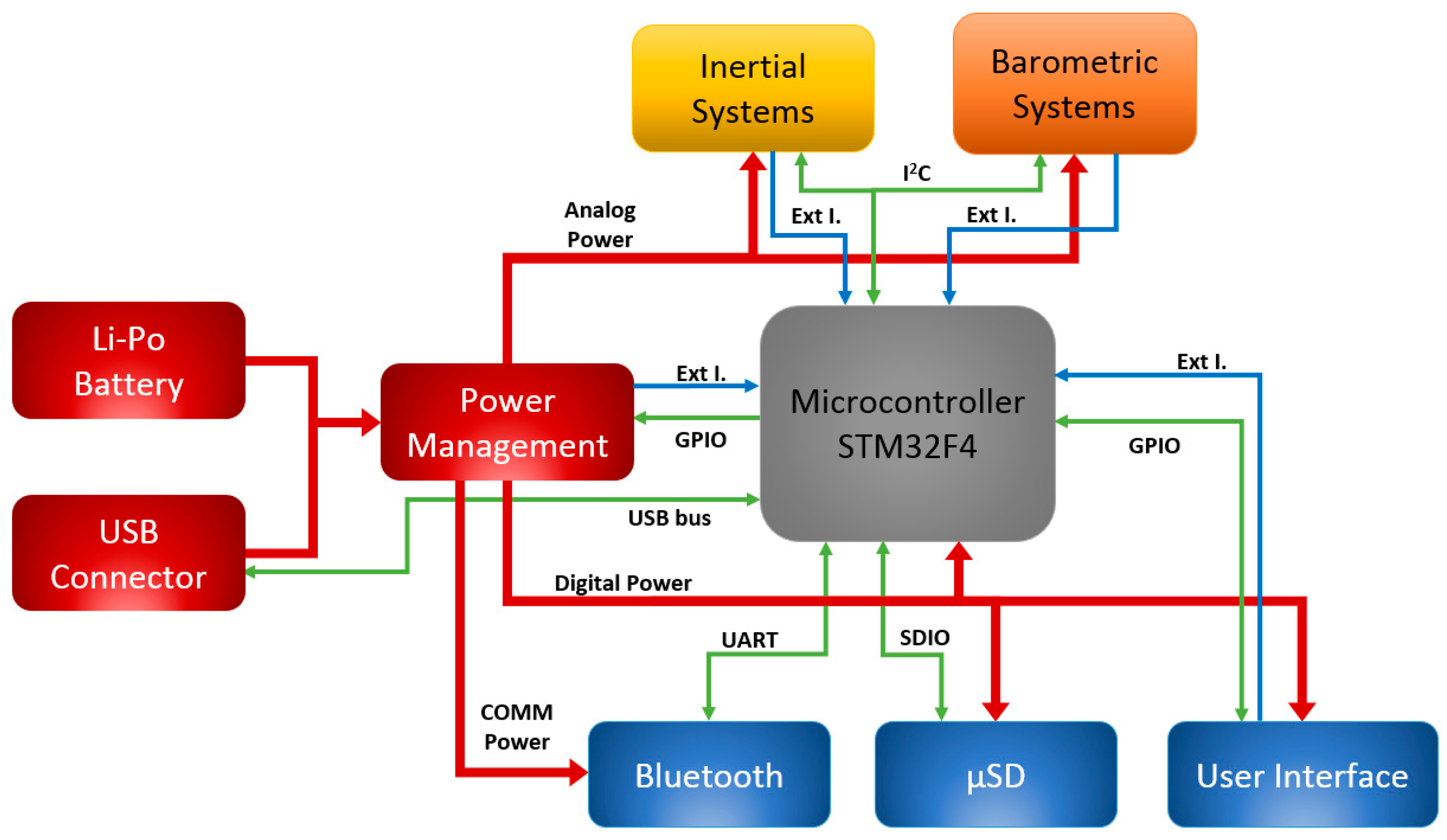
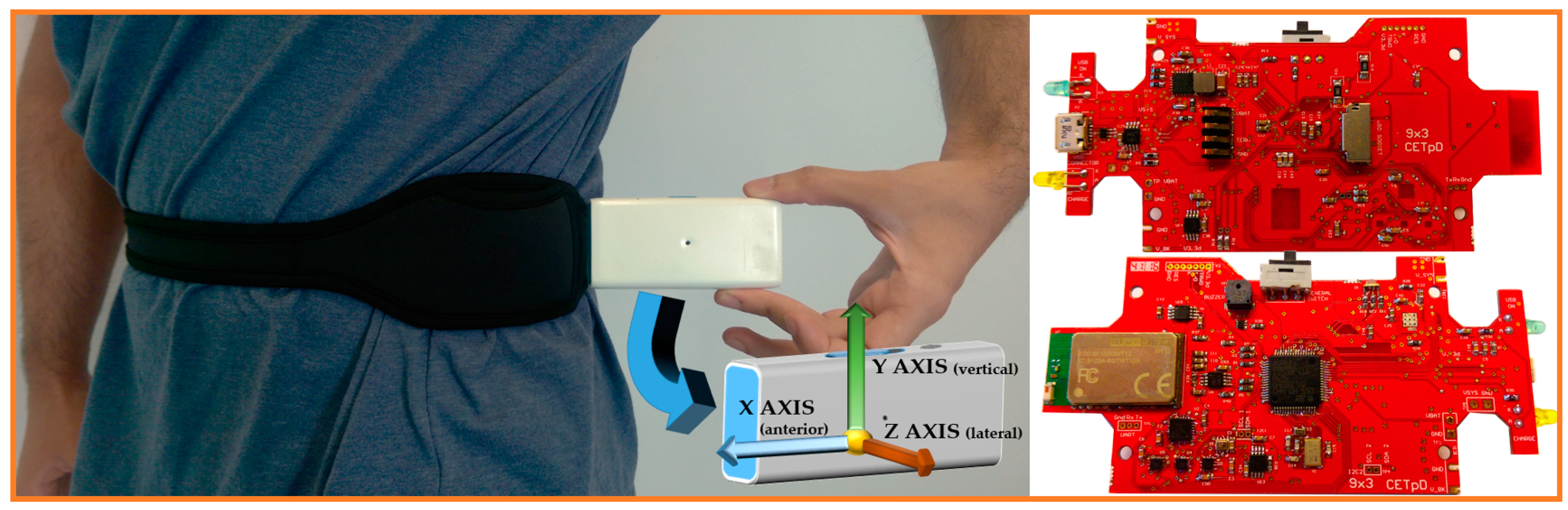
4. 9 × 3 Hardware Architecture
4.1. Microcontroller
4.2. Sensors
4.2.1. LSM9DS0 Description
4.2.2. LIS2DH Description
4.2.3. Barometric System Description
4.3. Communication and Storage Units
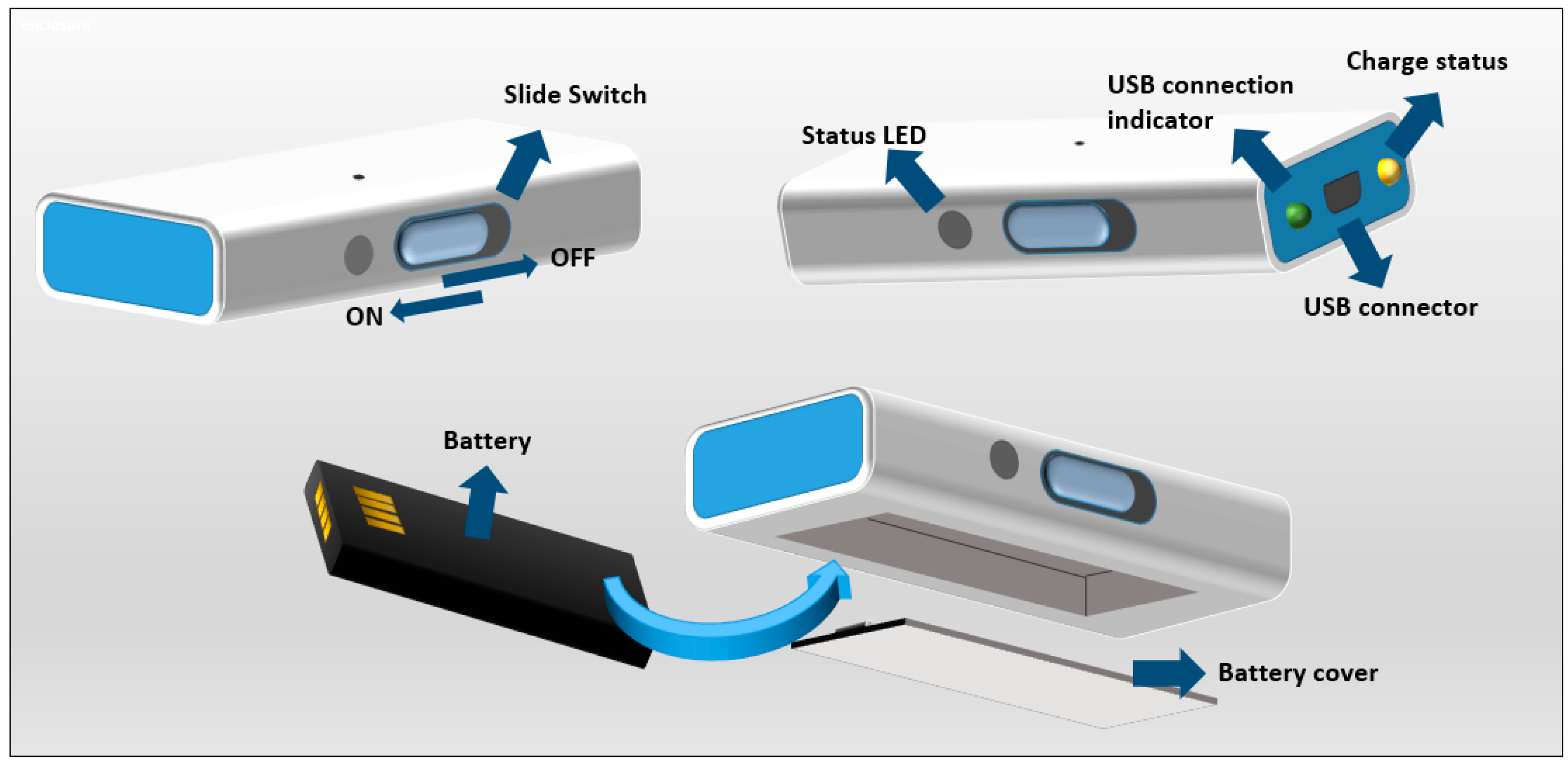
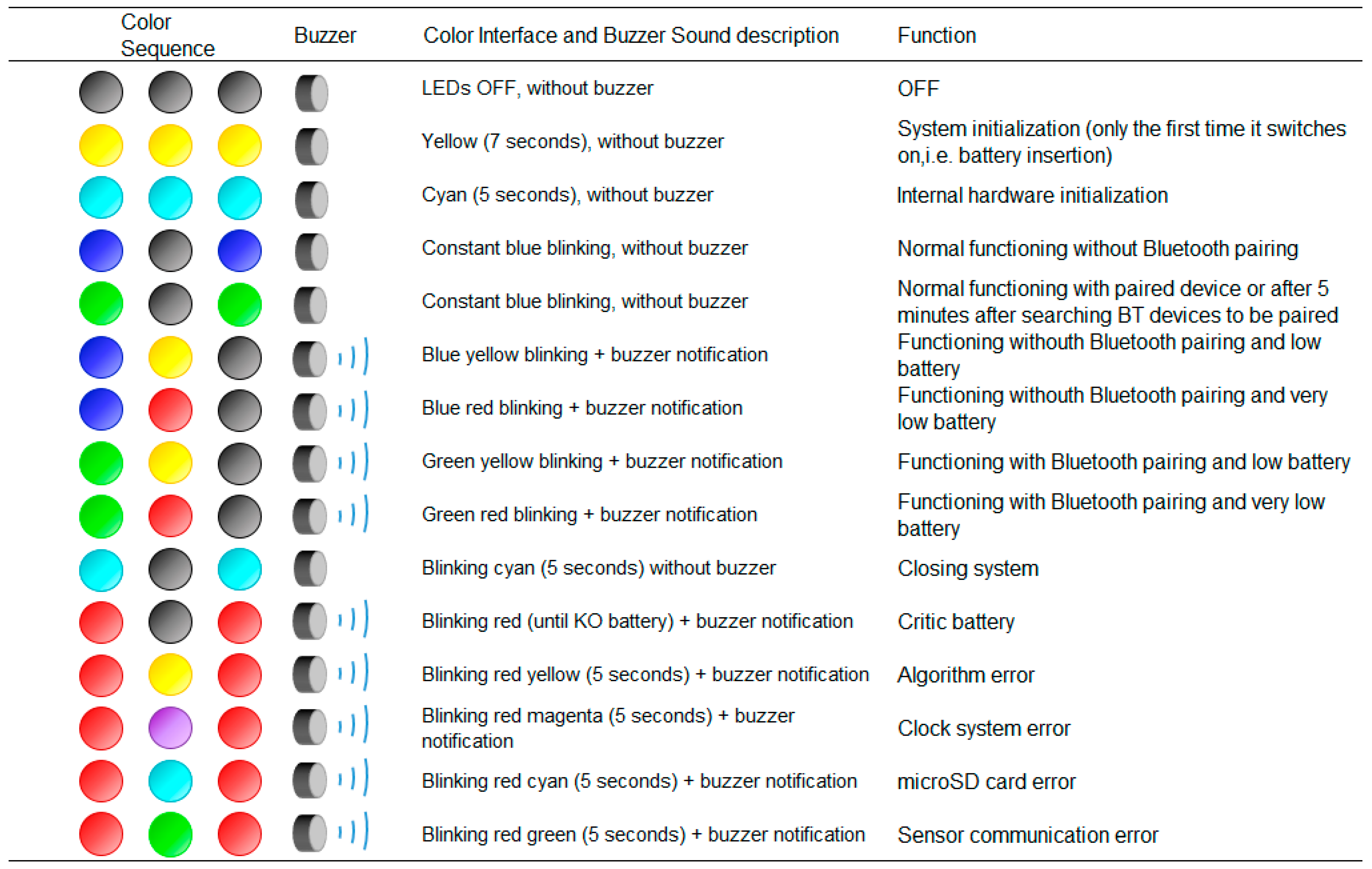
4.4. User Interface
5. Firmware
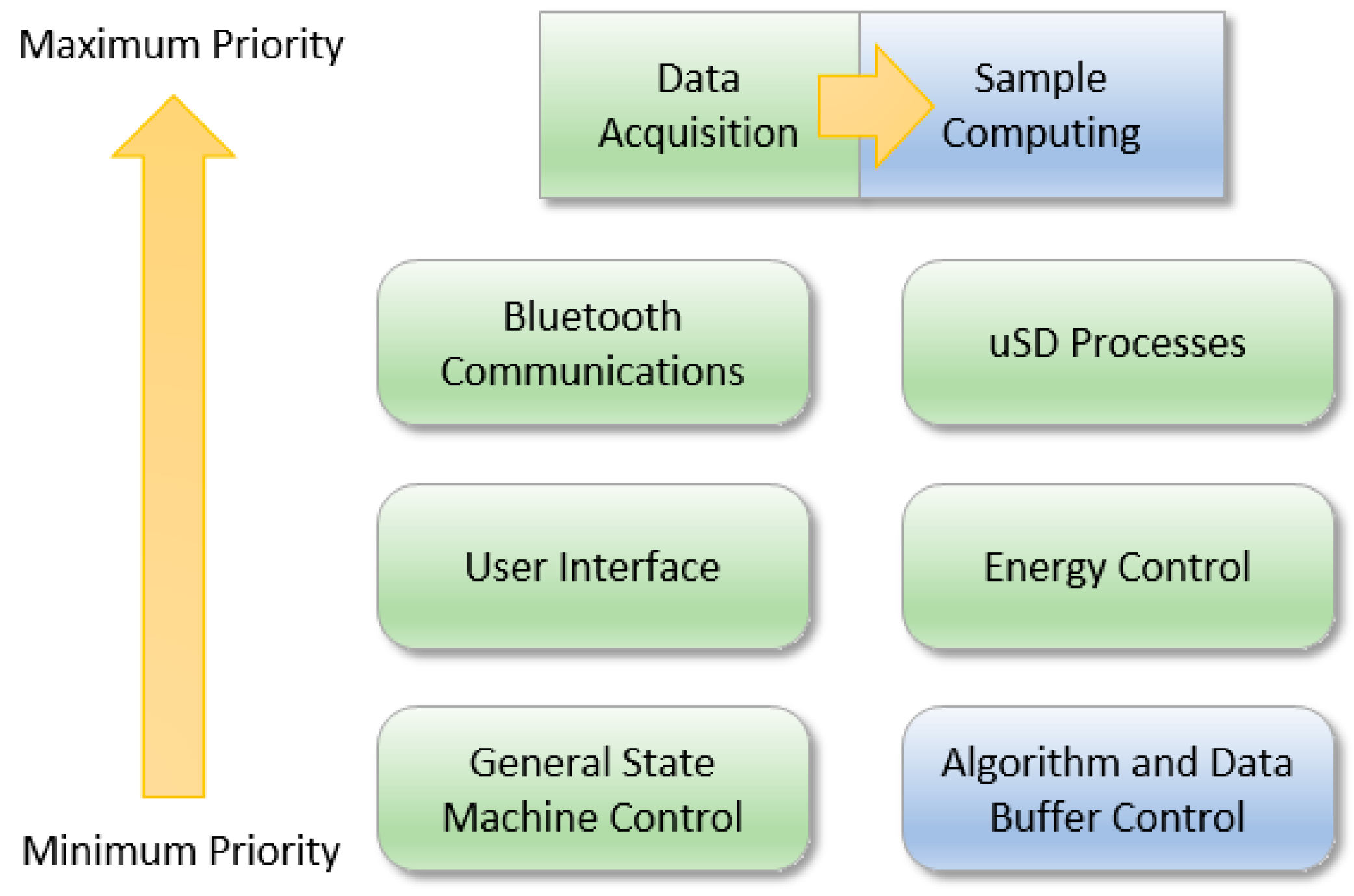
5.1. Peripheral Microcontroller Management
5.2. Embedded Algorithms
6. Experiments
6.1. Hardware Experiments
- Test 1: Consumption evaluation
- Test 2: Autonomy evaluation (Algorithms)
- Test 3: Autonomy evaluation (Data capture)
- Test 4: Timing evaluation
- Sending a message through Bluetooth every 15 min to ensure Bluetooth communication between the smartphone and the 9 × 3 device.
- Storing algorithmic information within the microSD every minute.
- Storing date, time and battery level every minute.
- Computing algorithms at each window of data (FoG and bradykinetic gait). It requires windowing, filtering, feature extraction and SVM classification in real-time.
- Sampling data frequency set to 40 Hz.
- Receive date and time at the beginning from another device via Bluetooth.
- Store inertial data and other parameters (3 accelerometers, gyroscope, magnetometer, barometer, temperature, sample counter, battery level, date and time) within the microSD at 50 Hz.
6.2. Experiments in the Clinical Environment
7. Results
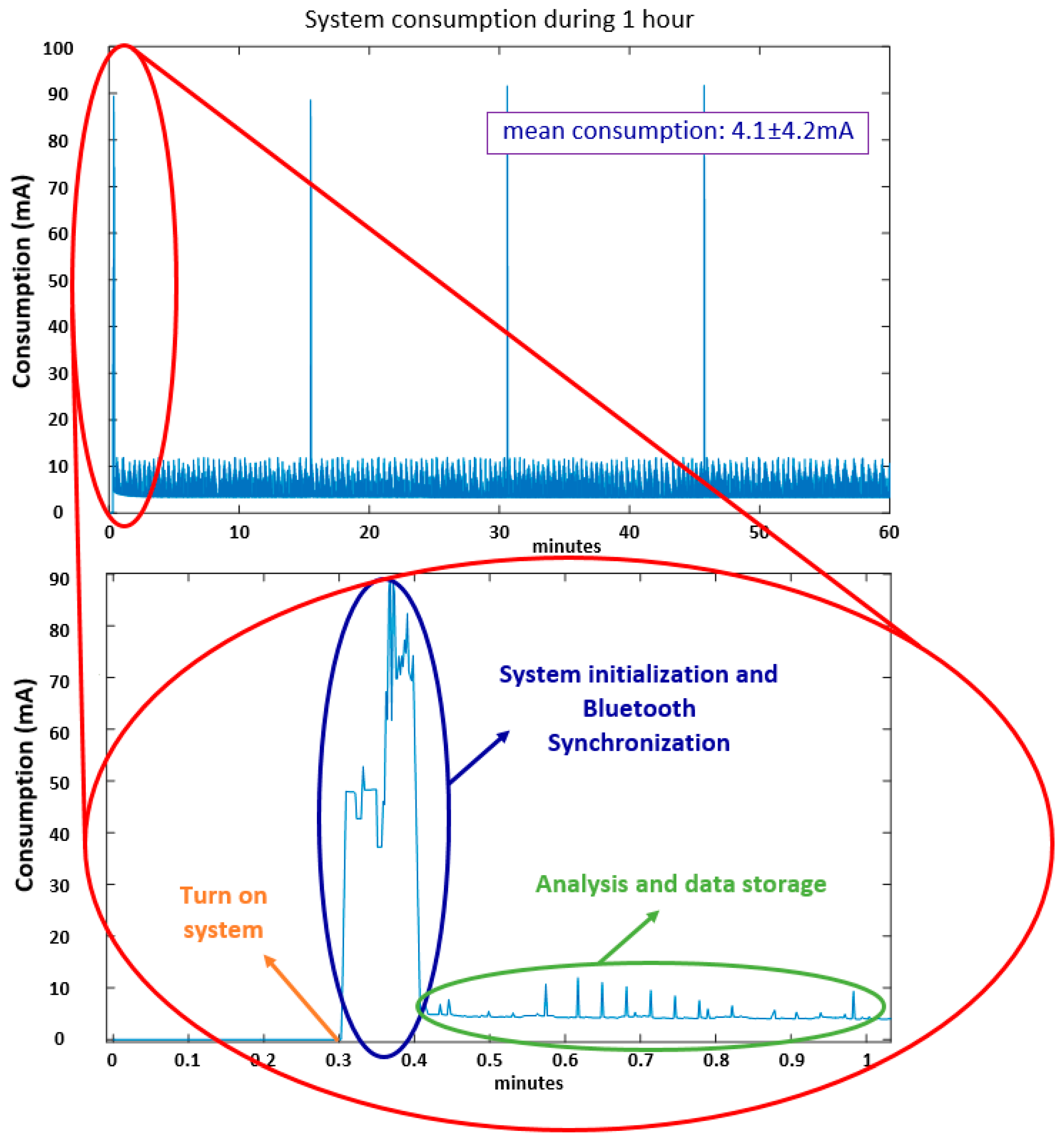
7.1. Power Consumption and Autonomy Tests
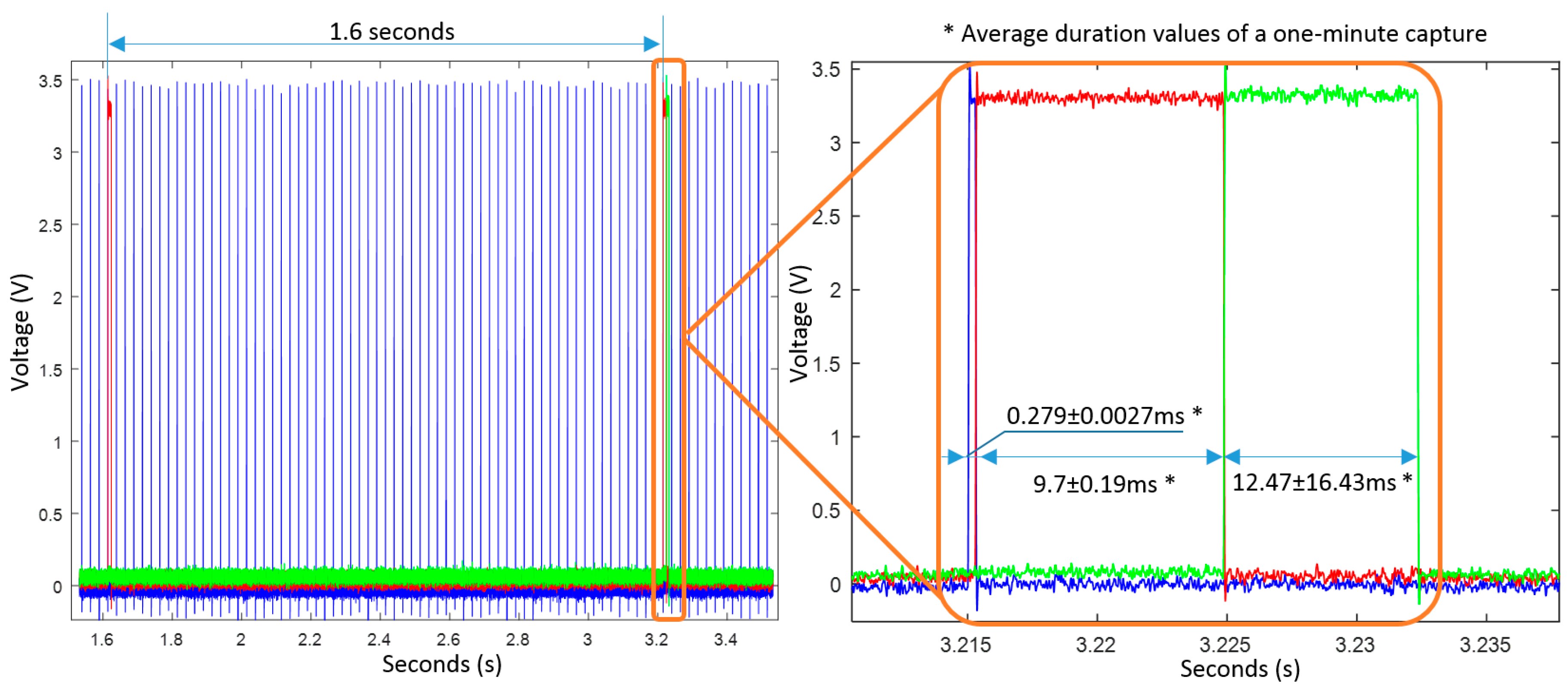
7.2. Timing Analysis of the Algorithms
7.3. Algorithm Evaluation
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ozdemir, A.T.; Barshan, B. Detecting falls with wearable sensors using machine learning techniques. Sensors 2014, 14, 10691–10708. [Google Scholar] [CrossRef] [PubMed]
- Bourke, A.K.; O’Brien, J.V.; Lyons, G.M. Evaluation of a threshold-based tri-axial accelerometer fall detection algorithm. Gait Posture 2007, 26, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Molinero, A.; Samà, A.; Pérez-Martínez, D.A.; Pérez López, C.; Romagosa, J.; Bayés, À.; Sanz, P.; Calopa, M.; Gálvez-Barrón, C.; de Mingo, E.; et al. Validation of a Portable Device for Mapping Motor and Gait Disturbances in Parkinson’s Disease. JMIR mHealth uHealth 2015, 3, e9. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-H.; Chung, P.-C.; Hsu, Y.-L.; Pai, M.-C.; Lin, C.-W. Inertial-Sensor-Based Balance Analysis System for Patients with Alzheimer’s Disease. In Proceedings of the Conference on Technologies and Applications of Artificial Intelligence, Taipei, Taiwan, 6–8 December 2013; pp. 128–133. [Google Scholar]
- Chung, P.-C.; Hsu, Y.-L.; Wang, C.-Y.; Lin, C.-W.; Wang, J.-S.; Pai, M.-C. Gait analysis for patients with Alzheimer’s disease using a triaxial accelerometer. In Proceedings of the IEEE International Symposium on Circuits and Systems, Seoul, Korea, 20–23 May 2012; pp. 1323–1326. [Google Scholar]
- Mizuike, C.; Ohgi, S.; Morita, S. Analysis of stroke patient walking dynamics using a tri-axial accelerometer. Gait Posture 2009, 30, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Fulk, G.D.; Sazonov, E. Using Sensors to Measure Activity in People with Stroke. Top. Stroke Rehabil. 2012, 18, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Londral, A.; Pinto, S.; de Carvalho, M. Markers for upper limb dysfunction in Amyotrophic Lateral Sclerosis using analysis of typing activity. Clin. Neurophysiol. 2016, 127, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, N.; Ayazi, F.; Najafi, K.; Member, S. Micromachined Inertial Sensors. Proc. IEEE 1998, 86, 1640–1659. [Google Scholar] [CrossRef]
- Yang, C.C.; Hsu, Y.L. A review of accelerometry-based wearable motion detectors for physical activity monitoring. Sensors 2010, 10, 7772–7788. [Google Scholar] [CrossRef] [PubMed]
- Najafi, B.; Aminian, K.; Loew, F.; Blanc, Y.; Robert, P.A. Measurement of stand-sit and sit-stand transitions using a miniature gyroscope and its application in fall risk evaluation in the elderly. IEEE Trans. Biomed. Eng. 2002, 49, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Mathie, M.J.; Coster, A.C.F.; Lovell, N.H.; Celler, B.G. Accelerometry: Providing an integrated, practical method for long-term, ambulatory monitoring of human movement. Physiol. Meas. 2004, 25, 1–20. [Google Scholar] [CrossRef]
- Gouwanda, D.; Senanayake, S.M. Periodical gait asymmetry assessment using real-time wireless gyroscopes gait monitoring system. J. Med. Eng. Technol. 2011, 35, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, S.; Héliot, R.A. A Magnetometer-Based Approach for Studying Human Movements. IEEE Trans. Biomed. Eng. 2007, 54, 1353–1355. [Google Scholar] [CrossRef] [PubMed]
- Moncada-Torres, A.; Leuenberger, K.; Gonzenbach, R.; Luft, A.; Gassert, R. Activity classification based on inertial and barometric pressure sensors at different anatomical locations. Physiol. Meas. 2014, 35, 1245–1263. [Google Scholar] [CrossRef] [PubMed]
- Tolkiehn, M.; Atallah, L.; Lo, B.; Yang, G.-Z. Direction sensitive fall detection using a triaxial accelerometer and a barometric pressure sensor. In Proceedings of the 33rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 369–372. [Google Scholar]
- Rodríguez-Martín, D.; Pérez-López, C.; Samà, A.; Cabestany, J.; Català, A. A Wearable Inertial Measurement Unit for Long-Term Monitoring in the Dependency Care Area. Sensors 2013, 13, 14079–14104. [Google Scholar] [CrossRef] [PubMed]
- Freezing in Parkinson’s Disease: Improving Quality of Life with an Automatic Control System- La Marató de TV3 (MASPARK). Available online: http://www.fundacionquironsalud.org/en/research/projects/freezing-in-parkinson-s-disease-improving-quality-of-life-w (accessed on 10 April 2017).
- Shimmer Sensing. Available online: www.shimmersensing.com (accessed on 15 July 2016).
- Gaitup. Available online: http://www.gaitup.com/ (accessed on 4 August 2016).
- Yost Labs, 3-SpaceTM Data Logger. Available online: https://yostlabs.com/product/3-space-data-logger/ (accessed on 17 October 2016).
- MTw Awinda. Wireless Motion Tracker. Xsens. Available online: https://www.xsens.com/download/pdf/documentation/mtw2-awinda/MTw2-Awinda.pdf (accessed on 17 October 2016).
- MTi 100-Series. Available online: https://www.xsens.com/products/mti-100-series/ (accessed on 5 October 2016).
- KineO Electro Goniometer; Datasheet; Technoconcept Inc.: Mane, France, 2011.
- Wimu. RealTrack Systems. Available online: http://www.realtracksystems.com/index.php?option=com_content&view=article&id=5&Itemid=131&lang=en (accessed on 17 October 2016).
- Lord MicroStrain Inc. Inertial Sensors 3DM-GX1. Technical Product Overview. Available online: http://www.microstrain.com/inertial/3DM-GX1 (accessed on 17 October 2016).
- Dynaport MM. Available online: McRoberts http://www.mcroberts.nl/products/movemonitor/ (accessed on 17 October 2016).
- BioRadio TM. Available online: https://glneurotech.com/bioradio/bioradio-specifications/ (accessed on 21 September 2016).
- RT6 Research Activity Monitor. Available online: https://www.stayhealthy.com/en_us/main/research_activity_monitor (accessed on 17 October 2016).
- PALtechnologies. ActivPAL. Available online: http://www.paltech.plus.com/activPALBrochure.1001.40417.1.6.pdf (accessed on 17 October 2016).
- x-IMU. IO Technologies. Available online: http://x-io.co.uk/x-imu/ (accessed on 26 September 2016).
- STT-IWS. High-End Wi-Fi IMU for Developers. Available online: http://www.stt-systems.com/products/inertial-motion-capture/stt-iws/ (accessed on 17 October 2016).
- Massé, F.; Bourke, A.K.; Chardonnens, J.; Paraschiv-Ionescu, A.; Aminian, K. Suitability of commercial barometric pressure sensors to distinguish sitting and standing activities for wearable monitoring. Med. Eng. Phys. 2014, 36, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Varesano Projects. FreeIMU. Available online: http://www.varesano.net/projects/hardware/FreeIMU (accessed on 26 September 2016).
- Parkinson’s KinetiGraphTM. Available online: http://www.globalkineticscorporation.com/product.php (accessed on 1 September 2016).
- Kinesia Objective Assessment. Available online: http://glneurotech.com/kinesia/products/kinesia-360/ (accessed on 1 September 2016).
- Griffiths, R.I.; Kotschet, K.; Arfon, S.; Xu, Z.M.; Johnson, W.; Drago, J.; Evans, A.; Kempster, P.; Raghav, S.; Horne, M.K. Automated assessment of bradykinesia and dyskinesia in Parkinson’s disease. J. Parkinsons Dis. 2012, 2, 47–55. [Google Scholar] [PubMed]
- Pulliam, C.L.; Eichenseer, S.R.; Goetz, C.G.; Waln, O.; Hunter, C.B.; Jankovic, J.; Vaillancourt, D.E.; Giuffrida, J.P.; Heldman, D.A. Continuous in-home monitoring of essential tremor. Parkinsonism Relat. Disord. 2014, 20, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Mera, T.O.; Burack, M.A.; Giuffrida, J.P. Objective Motion Sensor Assessment Highly Correlated with Scores of Global Levodopa-Induced Dyskinesia in Parkinson’s Disease. J. Parkinsons Dis. 2013, 3, 399–407. [Google Scholar] [PubMed]
- Kinesia ONETM Product Overview. Available online: http://glneurotech.com/kinesia/products/kinesia-one/ (accessed on 1 September 2016).
- Pérez-López, C.; Samà, A.; Rodríguez-Martín, D.; Català, A.; Cabestany, J.; de Mingo, E.; Rodríguez-Molinero, A. Monitoring Motor Fluctuations in Parkinson’s Disease Using a Waist-Worn Inertial Sensor. In Advances in Computational Intelligence: International Work Conference on Artificial Neural Networks; Lecture Notes on Computer Science; Springer International Publishing AG: Palma de Mallorca, Spain, 2015; pp. 461–474. [Google Scholar]
- Bächlin, M.; Roggen, D.; Plotnik, M.; Hausdorff, J.M.; Giladi, N.; Tröster, G. Online Detection of Freezing of Gait in Parkinson’s Disease Patients: A Performance Characterization. In Proceedings of the 4th International Conference on Body Area Networks, Los Angeles, CA, USA, 1–3 April 2009. [Google Scholar]
- Movement Disorders Society Task Force on Rating Scales for Parkinson’s Disease. The Unified Parkinson’s Disease Rating Scale (UPDRS): Status and Recommendations. Mov. Disord. 2003, 18, 738–750. [Google Scholar]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression, and mortality. Neurology 1967, 17, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Arias, P.; Cudeiro, J. Effect of rhythmic auditory stimulation on gait in Parkinsonian patients with and without freezing of gait. PLoS ONE 2010, 5, e9675. [Google Scholar] [CrossRef] [PubMed]
- Nieuwboer, A.; Baker, K.; Willems, A.-M.; Jones, D.; Spildooren, J.; Lim, I.; Kwakkel, G.; Van Wegen, E.; Rochester, L. The short-term effects of different cueing modalities on turn speed in people with Parkinson’s disease. Neurorehabil. Neural Repair 2009, 23, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Gjoreski, H.; Lustrek, M.; Gams, M. Accelerometer Placement for Posture Recognition and Fall Detection. In Proceedings of the 2011 Seventh International Conference on Intelligent Environments, Nothingham, UK, 25–26 July 2011; pp. 47–54. [Google Scholar]
- Mathie, M.J.; Basilakis, J.; Celler, B.G. A system for monitoring posture and physical activity using accelerometers. In Proceedings of the 23rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Istanbul, Turkey, 25–28 October 2001; Volume 4, pp. 3654–3657. [Google Scholar]
- Zhou, S.; Shan, Q.; Fei, F.; Li, W.J.; Wu, P.C.K.; Meng, B.; Chan, C.K.H.; Liou, J.Y.J. Gesture recognition for interactive controllers using MEMS motion sensors. In Proceedings of the 2009 4th IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Shenzhen, China, 5–8 January 2009; pp. 935–940. [Google Scholar]
- Pérez-López, C. Monitorización Ambulatoria de Fluctuaciones y Síntomas Motores Mediante Acelerómetros: Contribución a la Diagnosis y Seguimiento de la Enfermedad de Parkinson. Ph.D. Thesis, Universitat Politécnica de Catalunya, Barcelona, Spain, 2016. [Google Scholar]
- ST Microelectronics, Inc. STM32F415xx,STM32417xx Data Sheet; ST Microelectronics, Inc.: Ginebra, Switzerland, 2016. [Google Scholar]
- STMicroelectronics. LSM9DS0. iNEMO Inertial Module: 3D Accelerometer, 3D Gyroscope, 3D Magnetometer; DocID024763 Rev 2; STMicroelectronics: Ginebra, Switzerland, 2013. [Google Scholar]
- STMicroelectronics LIS2DH. MEMS Digital Output Motion Sensor: Ultra Low-Power High Performance 3-Axes Femto Accelerometer. Available online: http://www.st.com/en/mems-and-sensors/lis2dh.html (accessed on 6 March 2017).
- Robert Bosch GmbH. Datasheet BMP280 Digital Pressure Sensor; Robert Bosch GmbH: Stuttgart, Germany, 2015. [Google Scholar]
- STMicroelectronics. LPS25H.MEMS Pressure Sensor: 260-1260 hPa Absolute Digital Output Barometer; DocID023722 Rev 5; STMicroelectronics: Ginebra, Switzerland, 2016. [Google Scholar]
- TE Connectivity Inc. MS5637-02BA03. Low Voltage Barometric Pressure Sensor; TE Connectivity Inc., Sensor Solutions: Schaffhausen, Switzerland, 2015. [Google Scholar]
- ST Microelectronics, Inc. LIS2DH Data Sheet; ST Microelectronics, Inc.: Geneva, Switzerland, 2012. [Google Scholar]
- West, J.B.; Lahiri, S.; Maret, K.H.; Peters, R.M.; Pizzo, C.J. Barometric pressures at extreme altitudes on Mt. Everest: Physiological significance. J. Appl. Physiol. 1983, 54, 1188–1194. [Google Scholar] [PubMed]
- Najafi, B.; Aminian, K.; Paraschiv-Ionescu, A.; Loew, F.; Bula, C.J.; Robert, P. Ambulatory system for human motion analysis using a kinematic sensor: Monitoring of daily physical activity in the elderly. IEEE Trans. Biomed. Eng. 2003, 50, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martín, D.; Samà, A.; Pérez-López, C.; Català, A.; Mestre, B.; Alcaine, S.; Bayès, À. Comparison of features, window sizes and classifiers in detecting Freezing of Gait in patients with Parkinson’s Disease through a waist-worn accelerometer. In Proceedings of the 19th International Conference of the Catalan Association for Artificial Intelligence, Barcelona, Spain, 19–21 October 2016. [Google Scholar]
- Pérez-López, C.; Samà, A.; Rodríguez-Martín, D.; Moreno-Aróstegui, J.M.; Cabestany, J.; Bayes, A.; Mestre, B.; Alcaine, S.; Quispe, P.; Laighin, G.Ó.; et al. Dopaminergic-induced dyskinesia assessment based on a single belt-worn accelerometer. Artif. Intell. Med. 2016, 67, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Sayeed, T.; Samà, A.; Català, A.; Rodríguez-Molinero, A.; Cabestany, J. Adapted step length estimators for patients with Parkinson’s disease using a lateral belt worn accelerometer. Technol. Health Care 2015, 23, 179–194. [Google Scholar] [PubMed]
- Rodríguez-Martín, D.; Samà, A.; Pérez-López, C.; Català, A.; Moreno Arostegui, J.M.; Cabestany, J.; Bayés, À.; Alcaine, S.; Mestre, B.; Prats, A.; et al. Home detection of freezing of gait using support vector machines through a single waist-worn triaxial accelerometer. PLoS ONE 2017, 12, e0171764. [Google Scholar] [CrossRef] [PubMed]
- Samà, A.; Pérez-López, C.; Rodríguez-Martín, D.; Català, A.; Moreno-Aróstegui, J.M.; Cabestany, J.; de Mingo, E.; Rodríguez-Molinero, A. Estimating bradykinesia severity in Parkinson’s disease by analysing gait through a waist-worn sensor. Comput. Biol. Med. 2017, 84, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martín, D.; Samà, A.; Pérez-López, C.; Català, A.; Cabestany, J.; Rodríguez-Molinero, A. Posture Detection with waist-worn Accelerometer: An application to improve Freezing of Gait detection in Parkinson’s disease patients. In Recent Advances in Ambient Assisted Living—Bridging Assistive Technologies, e-Health and Personalized Health Care; Chen, W., Augusto, J.C., Seoane, F., Lehocki, F., Wolf, K.-H., Arends, J., Constantin Ungureanu, R.W., Eds.; IOS Press: Eindhoven, The Netherlands, 2015; Volume 20, pp. 3–17. [Google Scholar]
- Pérez-López, C.; Samà, A.; Rodríguez-Martín, D.; Català, A.; Cabestany, J.; Moreno-Arostegui, J.; de Mingo, E.; Rodríguez-Molinero, A. Assessing Motor Fluctuations in Parkinson’s Disease Patients Based on a Single Inertial Sensor. Sensors 2016, 16, 2132. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, J.D.; Balash, Y.; Gurevich, T.; Bartels, A.L.; Hausdorff, J.M.; Giladi, N. Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson’s disease. Eur. J. Neurol. 2003, 10, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Okuma, Y. Freezing of gait in Parkinson’s disease. J. Neurol. 2006, 253 (Suppl. 7), VII/27–VII/32. [Google Scholar] [CrossRef] [PubMed]
- Keysight Technologies 34405A Digital Multimeter, 5½ Digit; Datasheet; Keysight Technologies Inc.: Santa Rosa, CA, USA.
- VARTA Storage GmbH. VARTA Product Information; VARTA Storage GmbH—Rev No. 7; VARTA Storage GmbH: Nürnberger, Germany, 2017. [Google Scholar]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Geebelen, D.; Suykens, J.A.K.; Vandewalle, J. Reducing the number of support vectors of SVM classifiers using the smoothed separable case approximation. IEEE Trans. Neural Netw. Learn. Syst. 2012, 23, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Nieuwboer, A.; De Weerdt, W.; Dom, R.; Lesaffre, E. A frequency and correlation analysis of motor deficits in Parkinson patients. Disabil. Rehabil. 1998, 20, 142–150. [Google Scholar] [CrossRef] [PubMed]


| Name | Manufacturer | Sample Freq.* (Hz) | Autonomy Info.* | Size (mm3) | Weight (g) | Storage Unit | Wireless | Acc * | Gyr * | Mag * | Barometric Pressure | GPS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shimmer 3 [19] | Shimmer | 50 | 450 mAh | 51 × 34 × 14 | 23.6 | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Physilog 4 Gold [20] | Gaitup (EPFL) | 500 | 21 h | 50 × 37× 9.2 | 19 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Physilog 4 Silver [20] | Gaitup (EPFL) | 500 | 21 h | 50 × 37 × 9.2 | 19 | Yes | Yes | Yes | Yes | Yes | Yes | No |
| 3-space™ Sensor Datalogger [21] | Yost Labs | 475 | 5 h | 35 × 60 × 15 | 28 | Yes | Yes | Yes | Yes | Yes | No | No |
| MTw Awinda [22] | Xsens | 1000 | 6 h | 47 × 30 × 13 | 16 | No | Yes | Yes | Yes | Yes | No | No |
| MTi-G-710 GNSS [23] | Xsens | 375 | 675–950 mW | 57 × 42 × 23.5 | 55 | No | No | Yes | Yes | Yes | Yes | No |
| KineO [24] | Technoconcept | 100 | 4 h | 49 × 38 × 19 | 25 | Yes | No | Yes | Yes | Yes | No | No |
| Wimu [25] | Realtrack Systems | 1000 | 360 mAh | 85 × 48 × 15 | 60 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 3DM-GX4 [26] | MicroStrain | 1000 | 100 mA | 36 × 24.4 × 11.1 | 16.5 | No | No | Yes | Yes | No | Yes | No |
| Dynaport MM [27] | McRoberts | 200 | 14 days | 106.6 × 58 × 11.5 | 55 | No | Yes | Yes | Yes | Yes | Yes | No |
| BioRadio [28] | GLNeuroTech | 250 | 8 h | 100 × 60 × 20 | 113.4 | Yes | No | Yes | Yes | No | No | No |
| Research Tracker 6 [29] | Stayhealthy | 20 | 25 h | 51 × 51 × 13 | 51 | Yes | No | Yes | Yes | No | No | No |
| activPAL3 [30] | paltechnologies | 10 | 10 days | 53 × 35 × 7 | 15 | No | No | Yes | No | No | No | No |
| x-IMU [31] | x-IO Technologies | 512 | 150 mA | 57 × 38 × 21 | 49 | Yes | Yes | Yes | Yes | Yes | No | No |
| STT-IWS [32] | STT-Systems | 400 | 3.5 h | 56 × 38.5 × 18 | 46 | Yes | Yes | Yes | Yes | Yes | Yes | No |
| 9 × 2 (2013) [17] | UPC-CETpD | 200 | 36.8 h | 99 × 53 × 19 | 78 | Yes | Yes | Yes | Yes | Yes | No | No |
| Stage 0 | Stage 1 | Stage 2 | Stage 3 | Stage 4 | |||
|---|---|---|---|---|---|---|---|
| Baseline exploration | Data capture at patients home | Laboratory validation | RAS personalization | Use system at home without RAS | Washout period | Use system at home with RAS | Use system at home with RAS |
| Patient’s visit | 3 days | Patient’s visit | Patient’s visit | 4 days | 30 days | 4 days | 30 days |
| Microcontroller Features | dsPIC33FJ128MC804 (9 × 2) | STM32F415RG (9 × 3) |
|---|---|---|
| Maximum Speed (Hz) | 80 | 168 |
| Flash Memory (kB) | 128 | 1024 |
| RAM memory (kB) | 16 | 192 + 4 (DMA) |
| DMA streams | 8 | 16 |
| Consumption at full work (mA) * | 65 | 43 |
| Consumption in Idle mode (mA) * | 34 | 9 |
| Consumption in Sleep mode (mA) * | 0.01 | 0.004 |
| SDIO | No | Yes |
| I2C Bus | 2 | 3 |
| Computing method | Fixed point | Floating point |
| Computing performance | 40 MIPS | 210DMIPS (Dhrystone 2.1) |
| Parameters | BMP280 | LPS25H | MS5637 |
|---|---|---|---|
| Range (mbar) | 300–1100 | 260–1260 | 10–2000 |
| Relative accuracy (mbar) | 0.12 | 0.1 | 0.1 |
| Absolute accuracy (mbar) | 1 | 1 | 4 |
| Resolution RMS (mbar) | 0.0016 | 0.000244 | 0.016 |
| Pressure Noise (mbar) | 0.0013 | 0.01 | 0.5 |
| Compensation | External | Internal | External |
| Size (mm3) | 2 × 2.5 × 0.95 | 2.5 × 2.5 × 1 | 3 × 3 × 0.9 |
| Consumption @1 Hz (μA) | 2.7 | 25 | 20.1 |
| Maximum Data Rate (Hz) | 26.7 | 25 | 60 |
| Oversampling | 16 | 512 | 8192 |
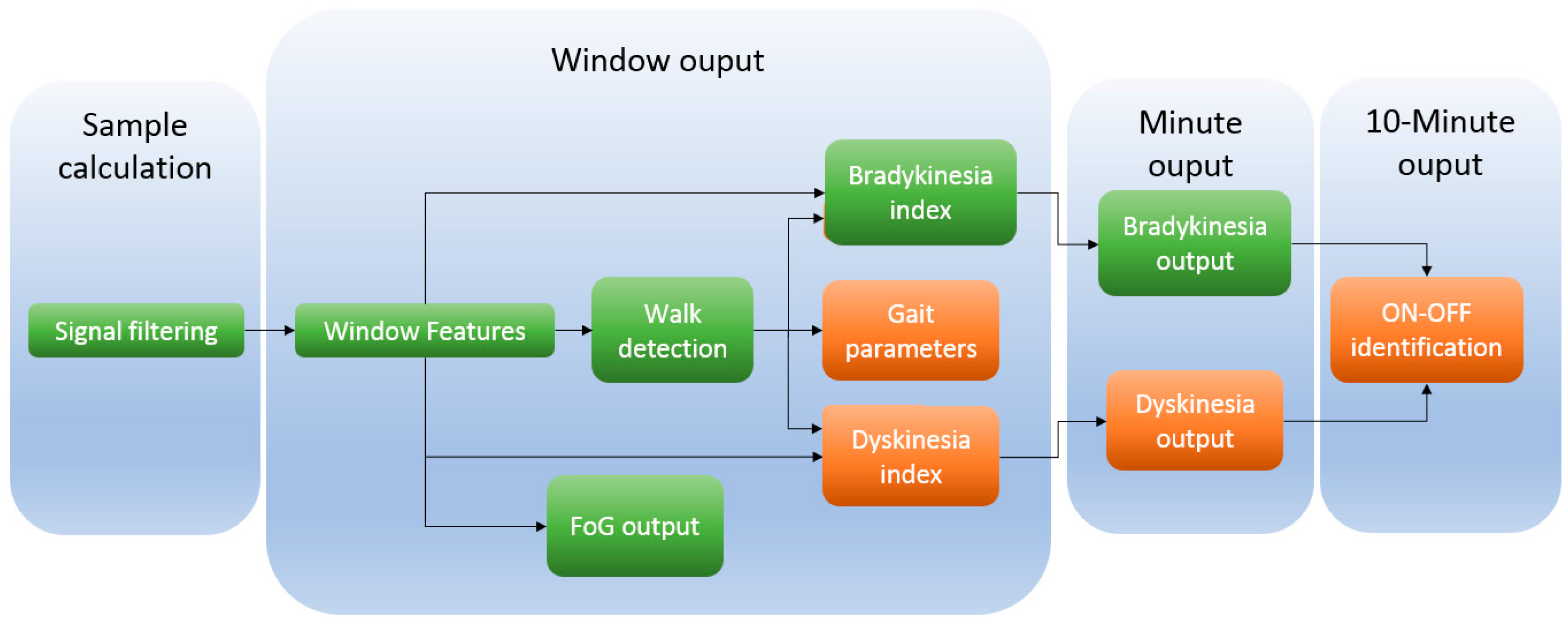
| Algorithm Block | Algorithm | Temporal Level * |
|---|---|---|
| 2nd order filters [3,41,60,61,62,63,64] | All | Sample calculation |
| Mean 3 accelerometer axes [3,41,60,63,64] | Brady, FoG, Gait | Window output |
| Standard deviation [3,41,60,63,64] | Brady, FoG, Gait | Window output |
| STFT—Band 1 [3,41,61,62,63,64] | Gait, Dysk, Brady | Window output |
| STFT—Band 2 [3,41,61,62,63,64] | Gait, Dysk, Brady | Window output |
| STFT—dyskinetic band [61] | Dyskinesia | Window output |
| STFT—non-continuous movement band [61] | Dyskinesia | Window output |
| STFT—Postural Transition band [60,61,63] | Dyskinesia, FoG | Window output |
| SVM Walk [3,41,61,62,64] | Brady, Dysk, Gait | Window output |
| Dyskinesia tree-based classifier [61] | Dyskinesia | Window output |
| Step detector [3,41,62,64] | Brady, Gait | Window output |
| Stride detector [3,41,62,64] | Brady, Gait | Window output |
| Cadence Estimation [62] | Gait | Window output |
| Step length [62] | Gait | Window output |
| Step velocity [62] | Gait | Window output |
| Fluidity computation [3,41,62,64] | Bradykinesia | Window output |
| SVM—FoG yes-no [60,63] | FoG | Window output |
| Decision tree based classifier for strides [3,41,62,64] | Bradykinesia | Minute output |
| Dyskinesia 1 min [3,41] | Dyskinesia | Minute output |
| Bradykinesia 1 min [3,41,64] | Bradykinesia | Minute output |
| Cadence Estimation 1 min [3,41] | Gait | Minute output |
| Step length 1 min [3,41] | Gait | Minute output |
| Step velocity 1 min [3,41] | Gait | Minute output |
| Tree-based classifier for ON/OFF state [3,41] | ON/OFF | 10-min output |
| Patients | Gender | H&Y (ON) | H&Y (OFF) | Age | UPDRS III (OFF) | UPDRS III (ON) |
|---|---|---|---|---|---|---|
| Patient 1 | Male | 2.5 | 3 | 62 | 5 | 10 |
| Patient 2 | Male | 2.5 | 3 | 69 | 18 | 27 |
| Patient 3 | Male | 2 | 3 | 70 | 7 | 24 |
| Patient 4 | Male | 2.5 | 3 | 54 | 21 | 35 |
| Patient 5 | Male | 2.5 | 3 | 61 | 8 | 40 |
| Patient 6 | Female | 2 | 3 | 59 | 11 | 20 |
| Patient 7 | Male | 2.5 | 3 | 76 | 42 | 45 |
| Patient 8 | Female | 2.5 | 3 | 71 | 11 | 24 |
| Patient 9 | Female | 2.5 | 3 | 66 | 4 | 12 |
| Patient 10 | Male | 2.5 | 3 | 66 | 24 | 35 |
| Patient 11 | Male | 2 | 2.5 | 61 | 17 | 32 |
| Patient 12 | Female | 2.5 | 3 | 71 | 6 | 17 |
| Name | 9 × 3 |
| Manufacturer | UPC-CETpD |
| Sample Frequency (Hz) | 1 to 1600 |
| Autonomy when sampling at 50 Hz | 23.09 days at waking hours (Algorithms) |
| 9.6 days continuously (Algorithms) | |
| 3.81 days continuously (Data Capture) | |
| 9.14 days at waking hours (Data Capture) | |
| Size (mm3) | 99 × 53 × 19 |
| Weight (g) | 83 |
| Storage Unit | Yes |
| Wireless | Yes |
| Accelerometer | Yes |
| Gyroscope | Yes |
| Magnetometer | Yes |
| Barometric Pressure | Yes |
| GPS | No |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Martín, D.; Pérez-López, C.; Samà, A.; Català, A.; Moreno Arostegui, J.M.; Cabestany, J.; Mestre, B.; Alcaine, S.; Prats, A.; Cruz Crespo, M.D.l.; et al. A Waist-Worn Inertial Measurement Unit for Long-Term Monitoring of Parkinson’s Disease Patients. Sensors 2017, 17, 827. https://doi.org/10.3390/s17040827
Rodríguez-Martín D, Pérez-López C, Samà A, Català A, Moreno Arostegui JM, Cabestany J, Mestre B, Alcaine S, Prats A, Cruz Crespo MDl, et al. A Waist-Worn Inertial Measurement Unit for Long-Term Monitoring of Parkinson’s Disease Patients. Sensors. 2017; 17(4):827. https://doi.org/10.3390/s17040827
Chicago/Turabian StyleRodríguez-Martín, Daniel, Carlos Pérez-López, Albert Samà, Andreu Català, Joan Manuel Moreno Arostegui, Joan Cabestany, Berta Mestre, Sheila Alcaine, Anna Prats, María De la Cruz Crespo, and et al. 2017. "A Waist-Worn Inertial Measurement Unit for Long-Term Monitoring of Parkinson’s Disease Patients" Sensors 17, no. 4: 827. https://doi.org/10.3390/s17040827






