Ppbv-Level Ethane Detection Using Quartz-Enhanced Photoacoustic Spectroscopy with a Continuous-Wave, Room Temperature Interband Cascade Laser
Abstract
:1. Introduction
2. Experimental Setup
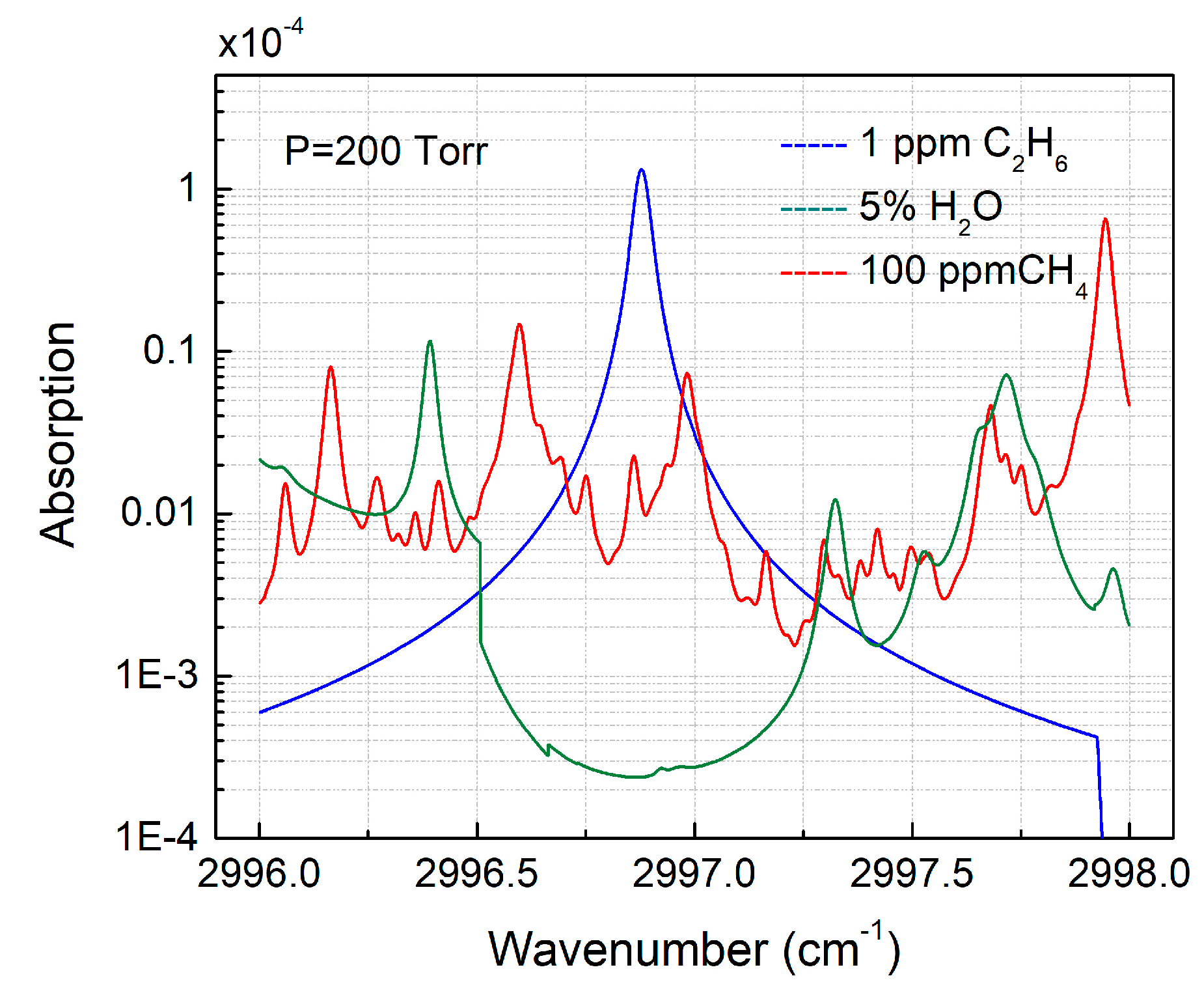
2.1. Absorption Line Selection
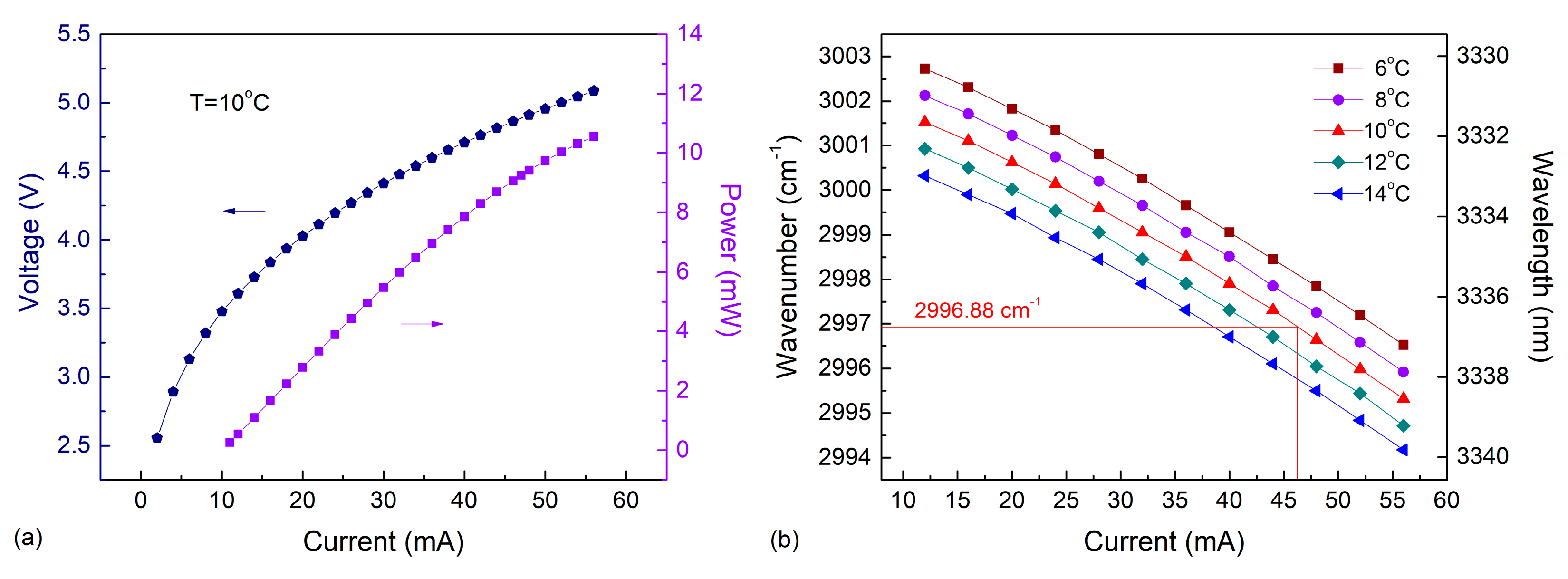
2.2. ICL Characteristics
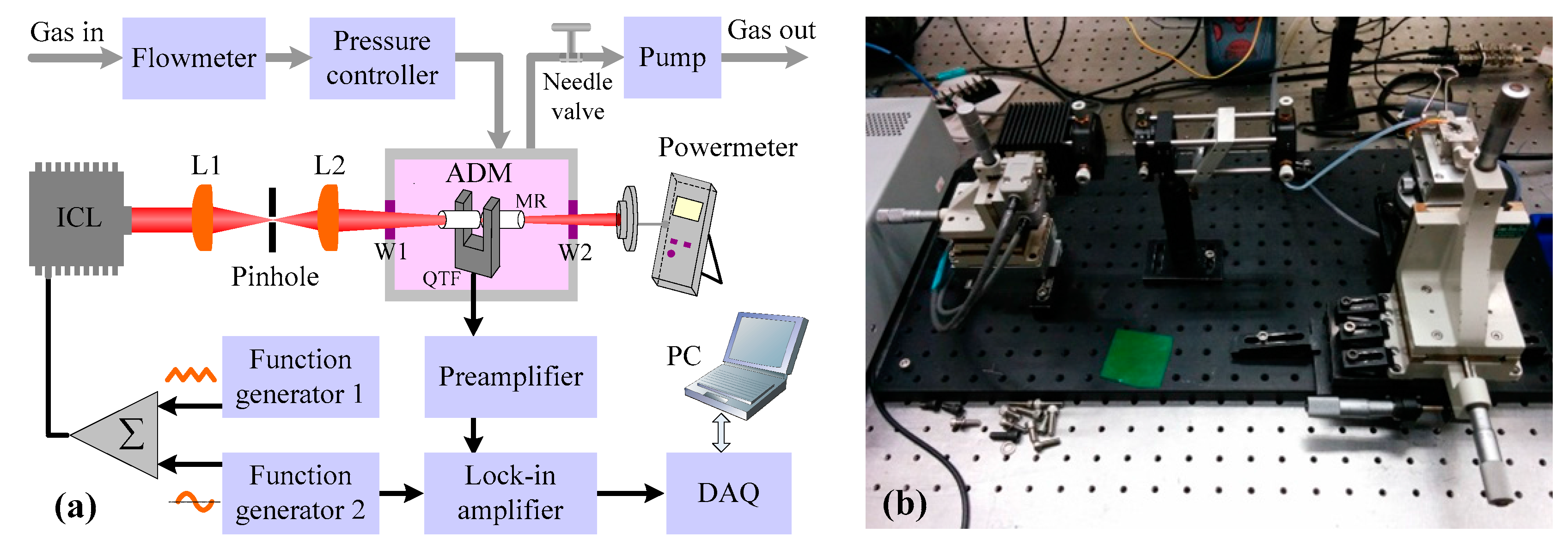
2.3. Sensor Architecture
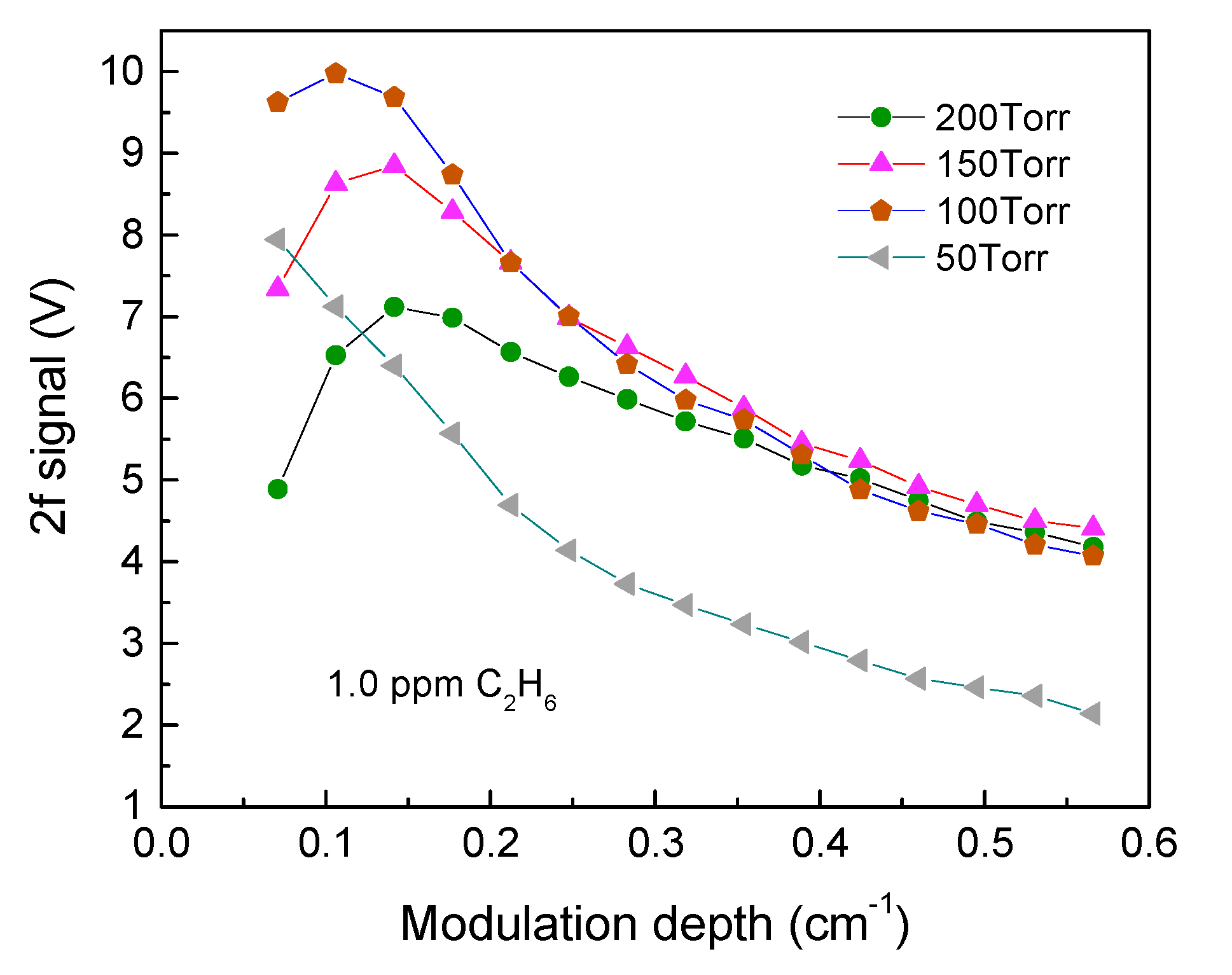
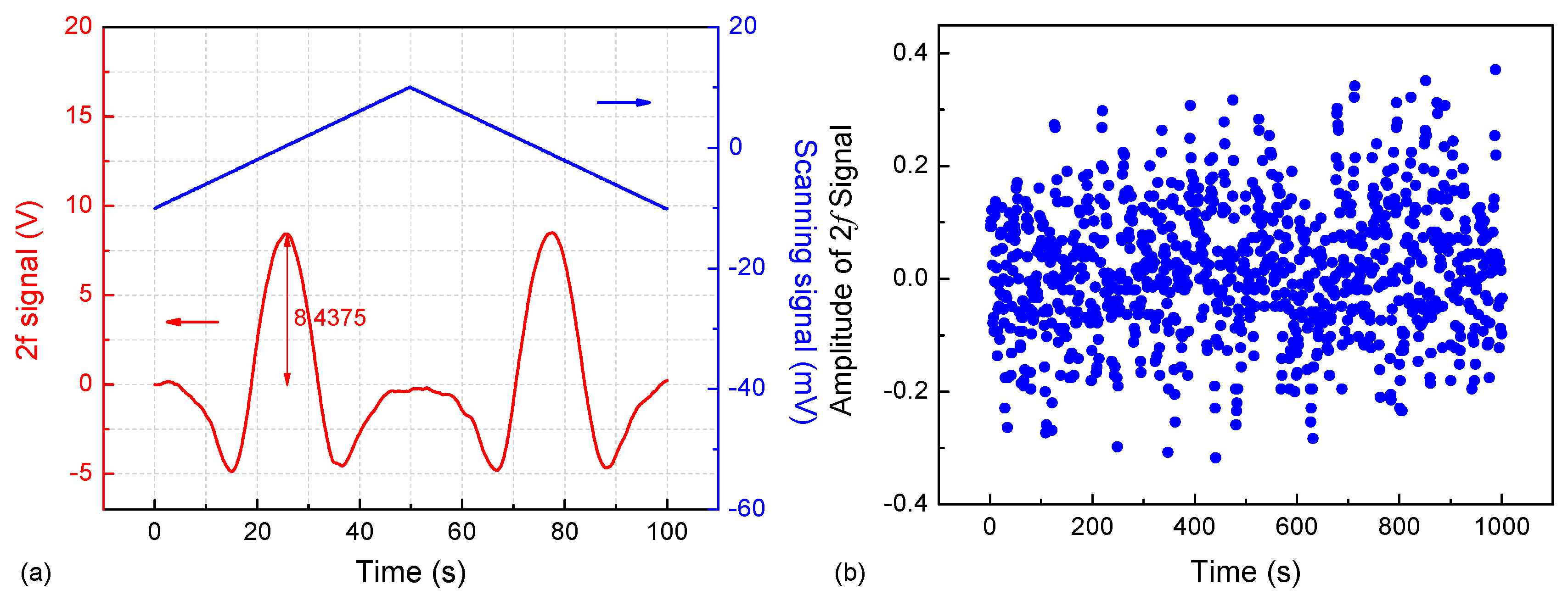
2.4. Optimization of Modulation Depth
3. Sensor Performance and Discussion
3.1. Estimation on SNR
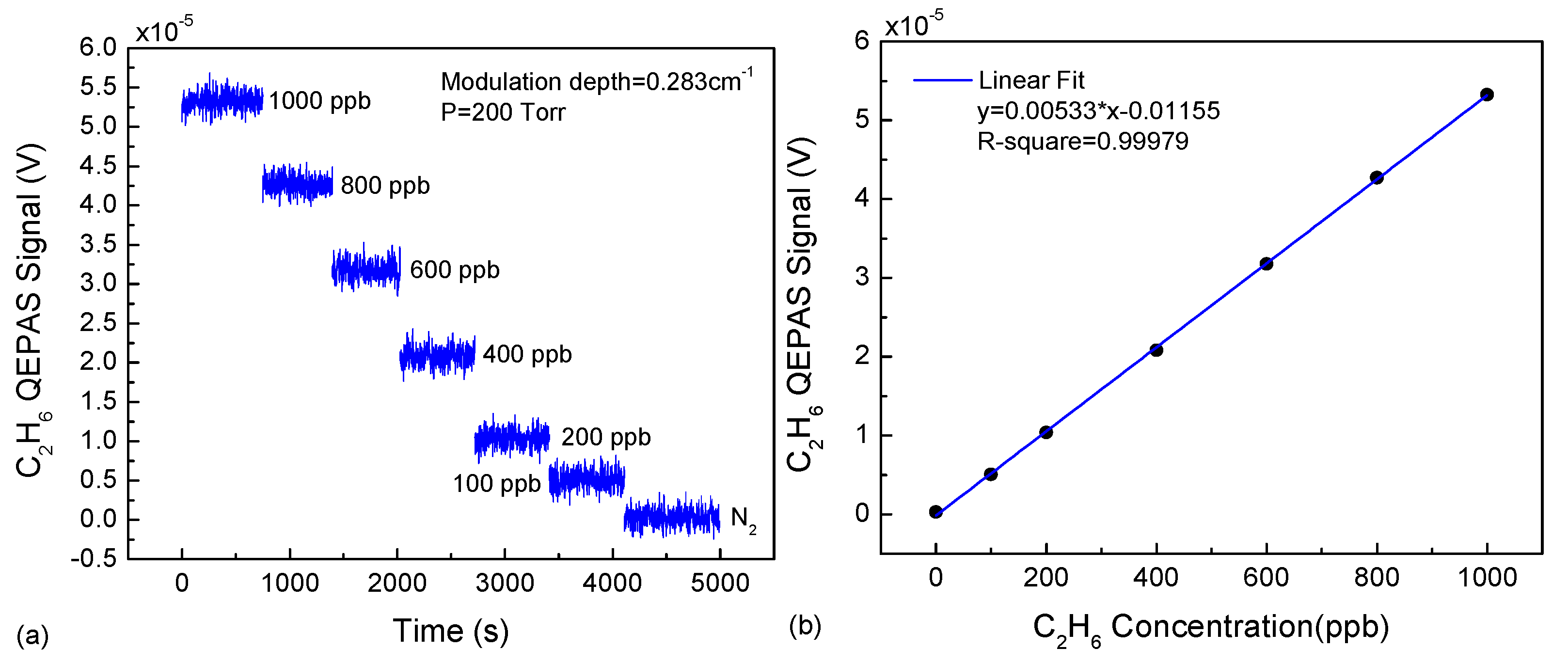
3.2. Experiment and Results
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Simpson, I.J.; Rowland, F.S.; Meinardi, S.; Blake, D.R. Influence of biomass burning during recent fluctuations in the slow growth of global tropospheric methane. Geophys. Res. Lett. 2006, 33, L22808. [Google Scholar] [CrossRef]
- Xiao, Y.; Logan, J.A.; Jacob, D.J.; Hudman, R.C.; Yantosca, R.; Blake, D.R. Global budget of ethane and regional constraints on U.S. sources. J. Geophys. Res. 2008, 113, D21306. [Google Scholar] [CrossRef]
- Thomson, L.C.; Hirst, B.; Gibson, G.; Gillespie, S.; Jonathan, P.; Skeldon, K.D.; Padgett, M.J. An improved algorithm for locating a gas source using inverse methods. Atmos. Environ. 2007, 41, 1128–1134. [Google Scholar] [CrossRef]
- Etiope, G.; Ciccioli, P. Earth’s degassing: A missing ethane and propane source. Science 2009, 323, 478. [Google Scholar] [CrossRef] [PubMed]
- Paredi, P.; Kharitonov, S.A.; Barnes, P.J. Elevation of exhaled ethane concentration in asthma. Am. J. Respir. Crit. Care Med. 2000, 162, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Puri, B.K.; Ross, B.M.; Treasaden, I.H. Increased levels of ethane, a non-invasive, quantitative, direct marker of n-3 lipid peroxidation, in the breath of patients with schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Skeldon, K.D.; McMillan, L.C.; Wyse, C.A.; Monk, S.D.; Gibson, G.; Patterson, C.; France, T.; Longbottom, C.; Padgett, M.J. Application of laser spectroscopy for measurement of exhaled ethane in patients with lung cancer. Respir. Med. 2006, 100, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Lackner, M. Tunable Diode Laser Absorption Spectroscopy (TDLAS) in the Process Industries: A Review. Rev. Chem. Eng. 2007, 23, 65–147. [Google Scholar] [CrossRef]
- Varga, A.; Bozoki, Z.; Szakall, M.; Szabó, G. Photoacoustic system for on-line process monitoring of hydrogen sulfide (H2S) concentration in natural gas streams. Appl. Phys. B. 2006, 85, 315–321. [Google Scholar] [CrossRef]
- Martin-Mateos, P.; Hayden, J.; Acedo, P.; Lendl, B. Heterodyne Phase-Sensitive Dispersion Spectroscopy in the Mid-Infrared with a Quantum Cascade Laser. Anal. Chem. 2017, 89, 5916–5922. [Google Scholar] [CrossRef] [PubMed]
- Waclawek, J.P.; Bauer, V.C.; Moser, H.; Lendl, B. 2f-wavelength modulation Fabry-Perot photothermal interferometry. Opt. Express 2016, 24, 28958–28967. [Google Scholar] [CrossRef] [PubMed]
- Waclawek, J.P.; Lewicki, R.; Moser, H.; Brandstetter, M.; Tittel, F.K.; Lendl, B. Quartz-enhanced photoacoustic spectroscopy-based sensor system for sulfur dioxide detection using a CW DFB-QCL. Appl. Phys. B Lasers Opt. 2014, 117, 113–120. [Google Scholar] [CrossRef]
- Patimisco, P.; Scamarcio, G.; Tittel, F.K.; Spagnolo, V. Quartz-Enhanced Photoacoustic Spectroscopy. Sensors 2014, 14, 6165–6206. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Liu, K.; Chen, W.; Tan, T.; Wang, L.; Gao, X. Application of a broadband blue laser diode to trace NO2 detection using off-beam quartz-enhanced photoacoustic spectroscopy. Opt. Lett. 2011, 36, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Lewicki, R.; Waclawek, J.; Jahjah, M.; Ma, Y.; Chrysostom, E.; Lendl, B.; Tittel, F.K. A sensitive CW DFB quantum cascade laser based QEPAS sensor for detection of SO2. In Proceedings of the 2012 Conference on Lasers and Electro-Optics, San Jose, CA, USA, 6–11 May 2012. [Google Scholar]
- Gray, S.; Liu, A.; Xie, F.; Zah, C.E. Detection of nitric oxide in air with a 5.2 μm distributed-feedback quantum cascade laser using quartz-enhanced photoacoustic spectroscopy. Opt. Express 2010, 18, 23353–23357. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Wu, H.; Zheng, H.; Liu, Y.; Jiang, W.; Zhang, L.; Ma, W.; Ren, W.; Yin, W.; Jia, S.; et al. Double acoustic microresonator quartz-enhanced photoacoustic spectroscopy. Opt. Lett. 2014, 39, 2479–2482. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Sampaolo, A.; Dong, L.; Patimisco, P.; Liu, X.; Zheng, H.; Yin, X.; Ma, W.; Zhang, L.; Yin, W.; et al. Fiber-amplifier-enhanced QEPAS sensor using a custom tuning fork with a large prong space. Appl. Phys. Lett. 2015, 107, 111104. [Google Scholar] [CrossRef]
- Waclawek, J.P.; Moser, H.; Lendl, B. Compact quantum cascade laser based quartz-enhanced photoacoustic spectroscopy sensor system for detection of carbon disulfide. Opt. Express 2016, 24, 6559–6571. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Dong, L.; Yin, X.; Liu, X.; Wu, H.; Zhang, L.; Ma, W.; Yin, W.; Jia, S. Ppbv-level QEPAS NO2 sensor by use of electrical modulation cancellation method with a high power blue LED. Sens. Actuators B Chem. 2015, 208, 173–179. [Google Scholar] [CrossRef]
- Spagnolo, V.; Patimisco, P.; Borri, S.; Scamarcio, G.; Bernacki, B.E.; Kriesel, J. Part-per-trillion level SF6 detection using a quartz enhanced photoacoustic spectroscopy-based sensor with single-mode fiber-coupled quantum cascade laser excitation. Opt. Lett. 2012, 37, 4461–4463. [Google Scholar] [CrossRef] [PubMed]
- Triki, M.; Nguyen Ba, T.; Vicet, A. Compact sensor for methane detection in the mid infrared region based on Quartz Enhanced Photoacoustic Spectroscopy. Infrared Phys. Technol. 2015, 69, 74–80. [Google Scholar] [CrossRef]
- Spagnolo, V.; Patimisco, P.; Pennetta, R.; Sampaolo, A.; Scamarcio, G.; Vitiello, M.S.; Tittel, F.K. THz Quartz-enhanced photoacoustic sensor for H2S trace gas detection. Opt. Express 2015, 23, 7574–7582. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Dallner, M.; Kamp, M.; Höfling, S.; Worschech, L.; Forchel, A. Shortened injector interband cascade lasers for 3.3 to 3.6 µm emission. Opt. Eng. 2010, 49, 111117. [Google Scholar] [CrossRef]
- Dallner, M.; Höfling, S.; Kamp, M. Room-temperature operation of InAs-based interband-cascade-lasers beyond 6µm. Electron. Lett. 2013, 49, 286–287. [Google Scholar] [CrossRef]
- Spagnolo, V.; Kosterev, A.A.; Dong, L.; Lewicki, R.; Tittel, F.K. NO trace gas sensor based on quartz-enhanced photoacoustic spectroscopy and external cavity quantum cascade laser. Appl. Phys. B 2010, 100, 125–130. [Google Scholar] [CrossRef]
- Dong, L.; Spagnolo, V.; Lewicki, R.; Tittel, F.K. Ppbv-level detection of nitric oxide using an external cavity quantum cascade laser based QEPAS sensor. Opt. Express 2011, 19, 24037–24045. [Google Scholar] [CrossRef] [PubMed]
- Werle, P. A review of recent advances in semiconductor laser based gas monitors. Spectrochim. Acta A 1998, 54, 197–236. [Google Scholar] [CrossRef]
- Schilt, S.; Thévenaz, L.; Robert, P. Wavelength modulation spectroscopy: Combined frequency and intensity laser modulation. Appl. Opt. 2003, 42, 6728–6738. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Dong, L.; Zheng, C.; Lin, J.; Wang, Y.; Tittel, F.K. Ppbv-Level Ethane Detection Using Quartz-Enhanced Photoacoustic Spectroscopy with a Continuous-Wave, Room Temperature Interband Cascade Laser. Sensors 2018, 18, 723. https://doi.org/10.3390/s18030723
Li C, Dong L, Zheng C, Lin J, Wang Y, Tittel FK. Ppbv-Level Ethane Detection Using Quartz-Enhanced Photoacoustic Spectroscopy with a Continuous-Wave, Room Temperature Interband Cascade Laser. Sensors. 2018; 18(3):723. https://doi.org/10.3390/s18030723
Chicago/Turabian StyleLi, Chunguang, Lei Dong, Chuantao Zheng, Jun Lin, Yiding Wang, and Frank K. Tittel. 2018. "Ppbv-Level Ethane Detection Using Quartz-Enhanced Photoacoustic Spectroscopy with a Continuous-Wave, Room Temperature Interband Cascade Laser" Sensors 18, no. 3: 723. https://doi.org/10.3390/s18030723





