Sensing Magnetic Fields with Magnetosensitive Ion Channels
Abstract
:1. Introduction
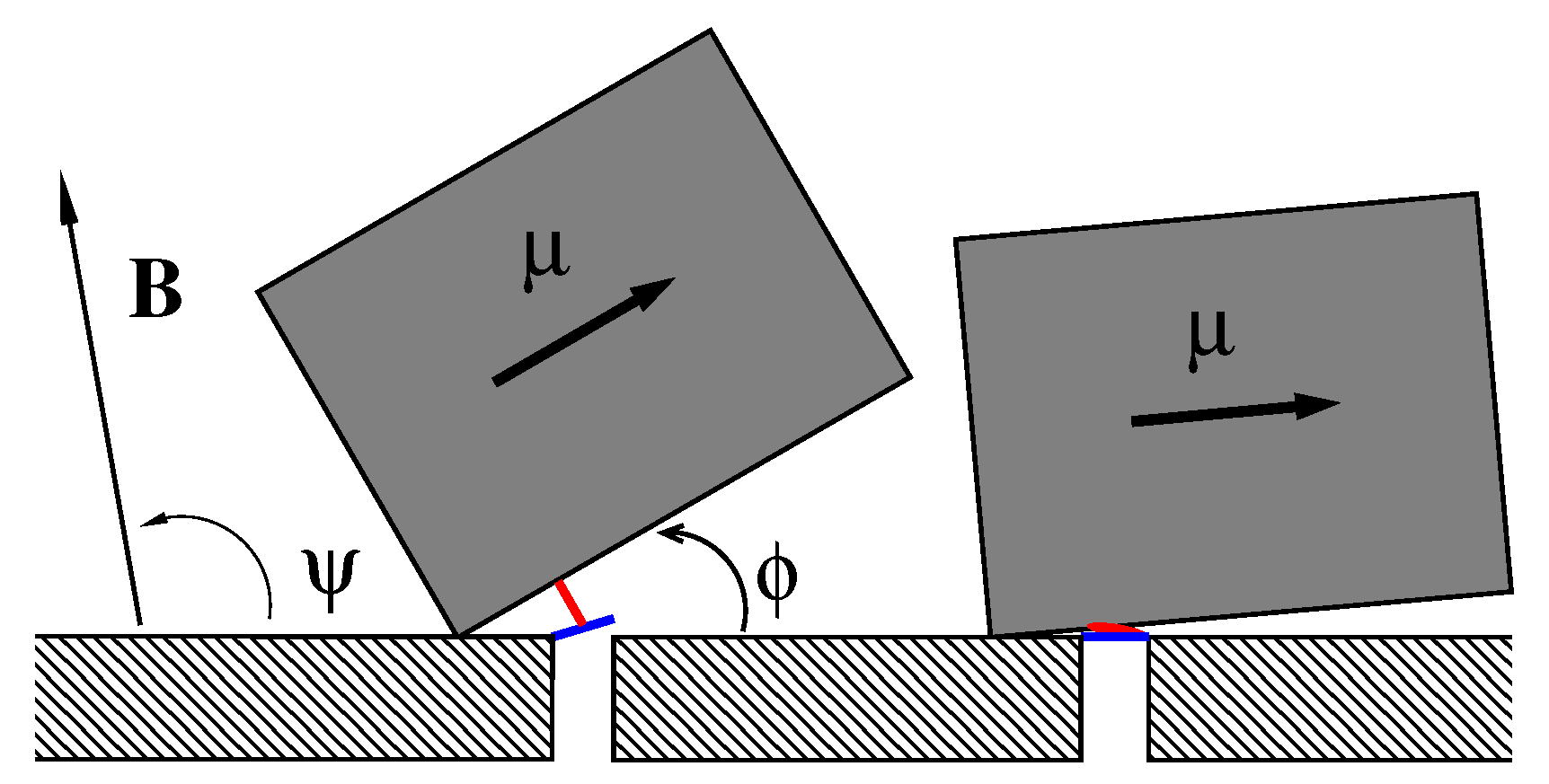
2. Model
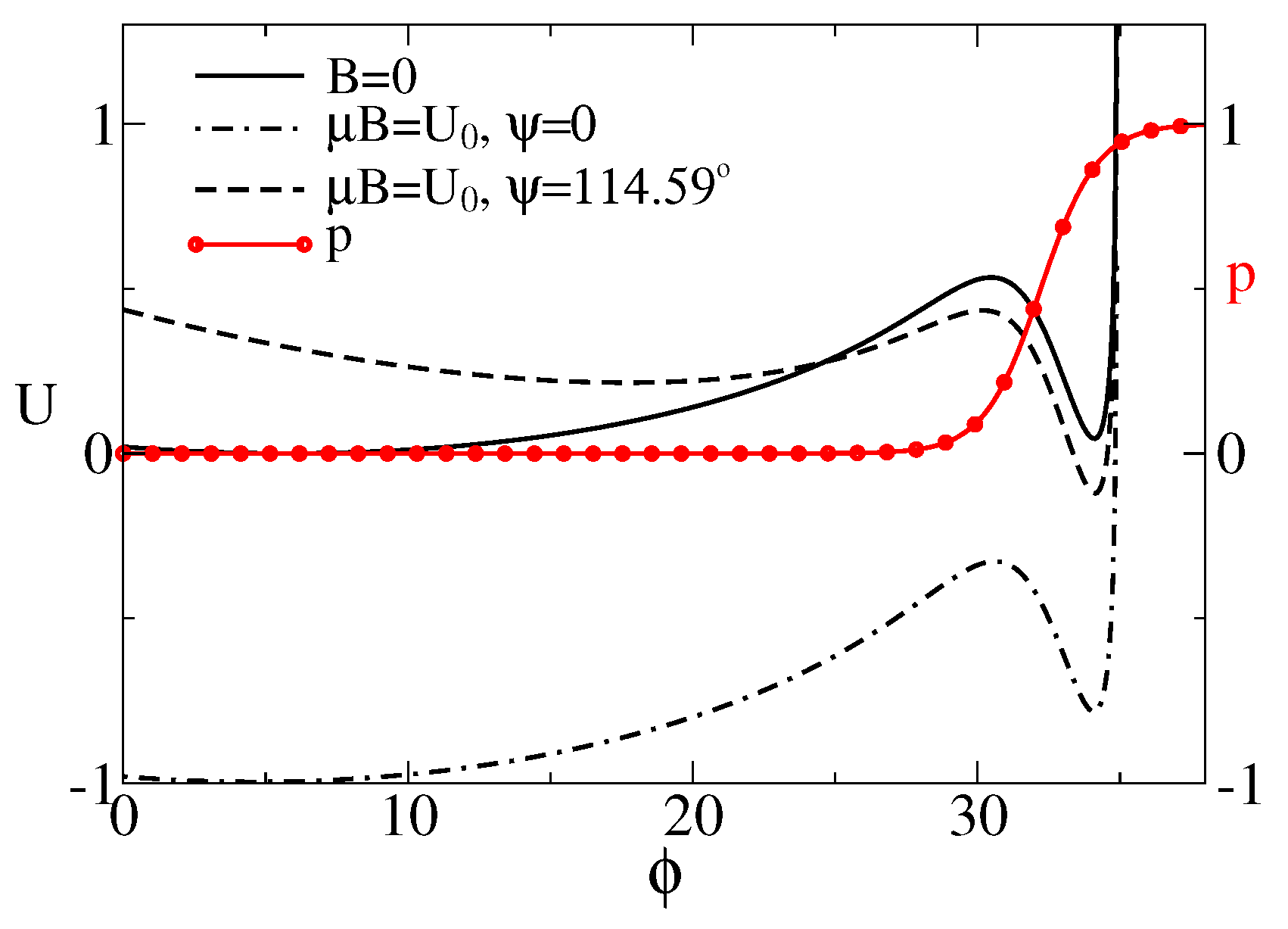
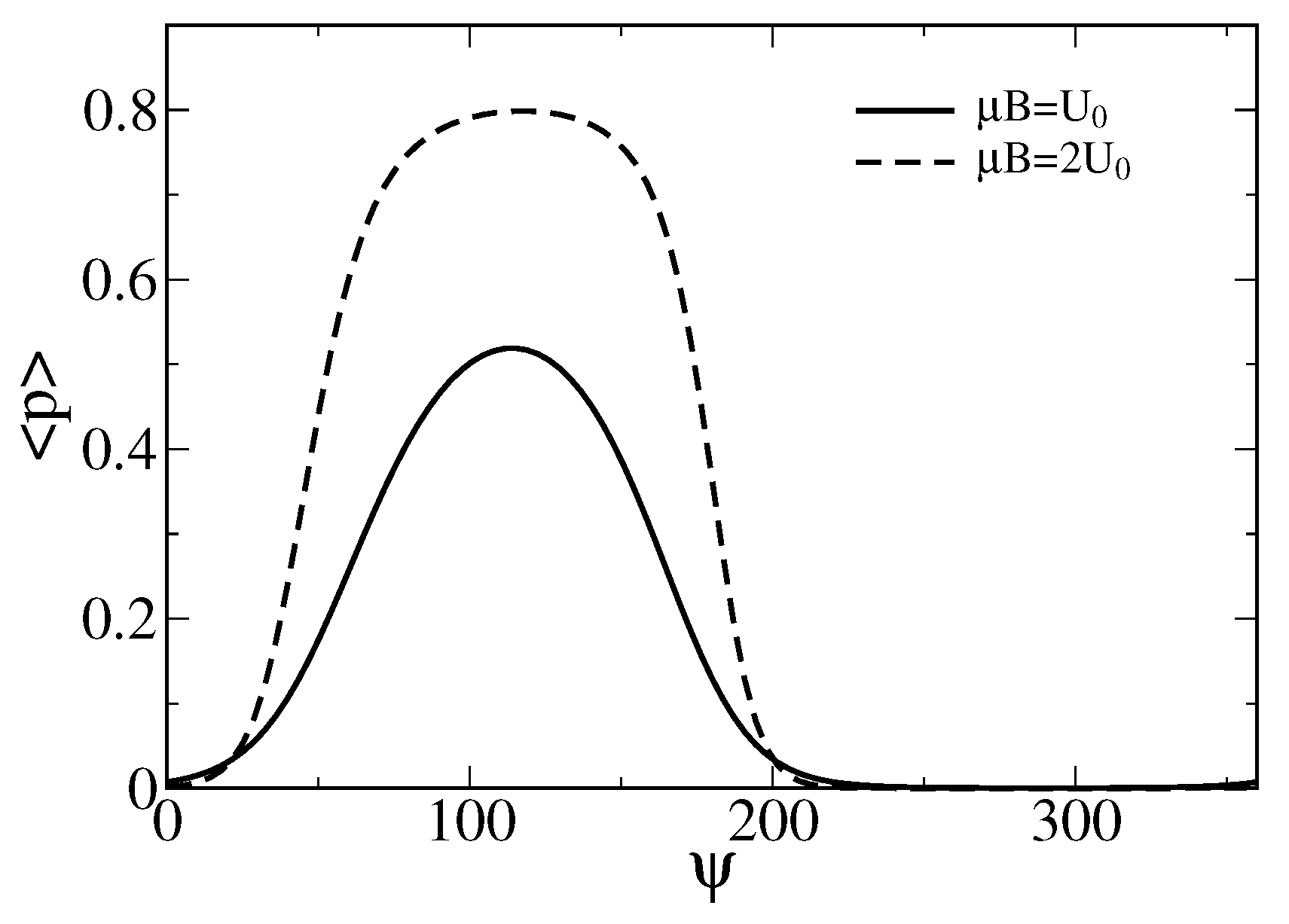
3. Theory and Results
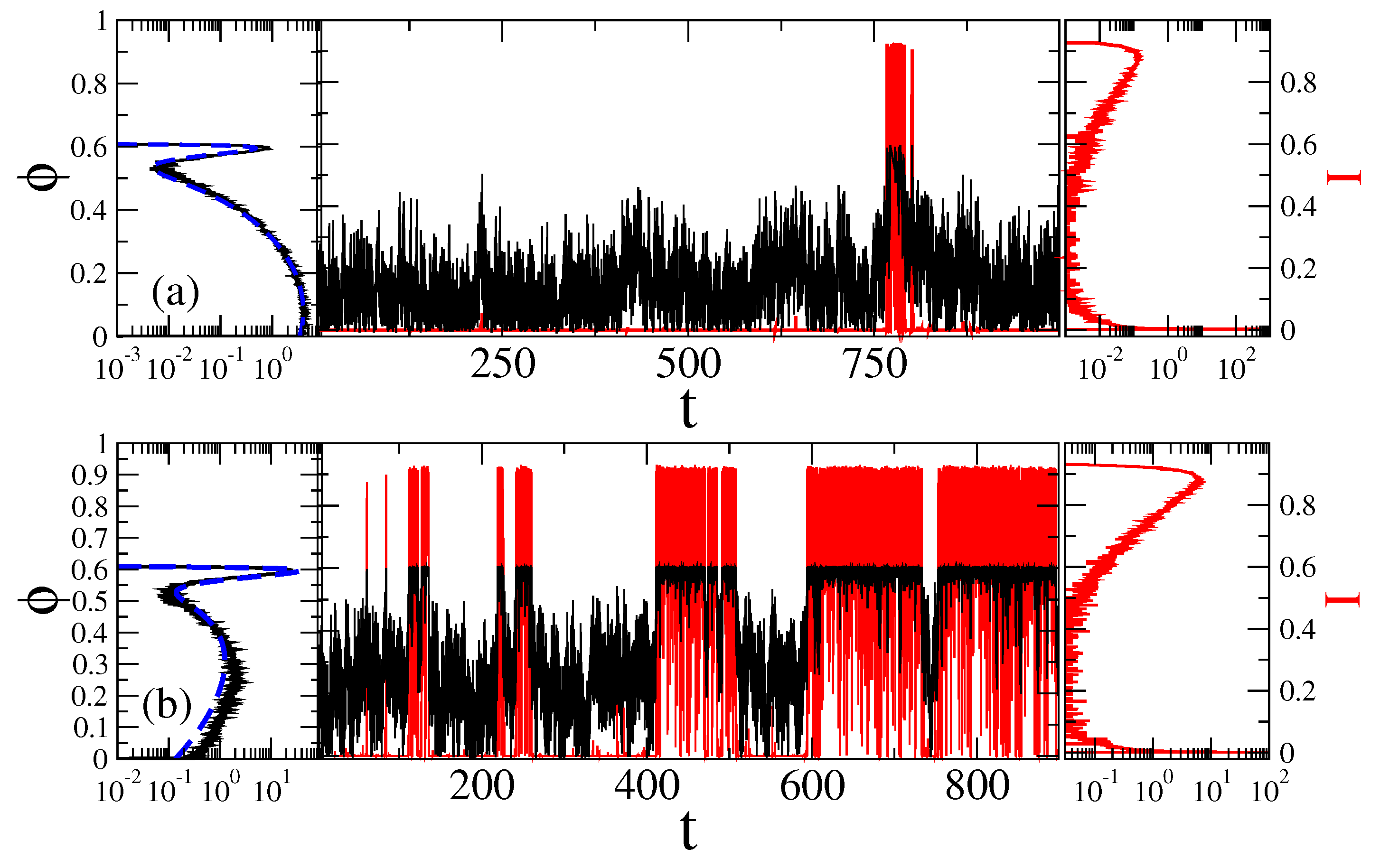
3.1. Stochastic Dynamics without Memory
Separation of Closed and Open States with a Single Threshold
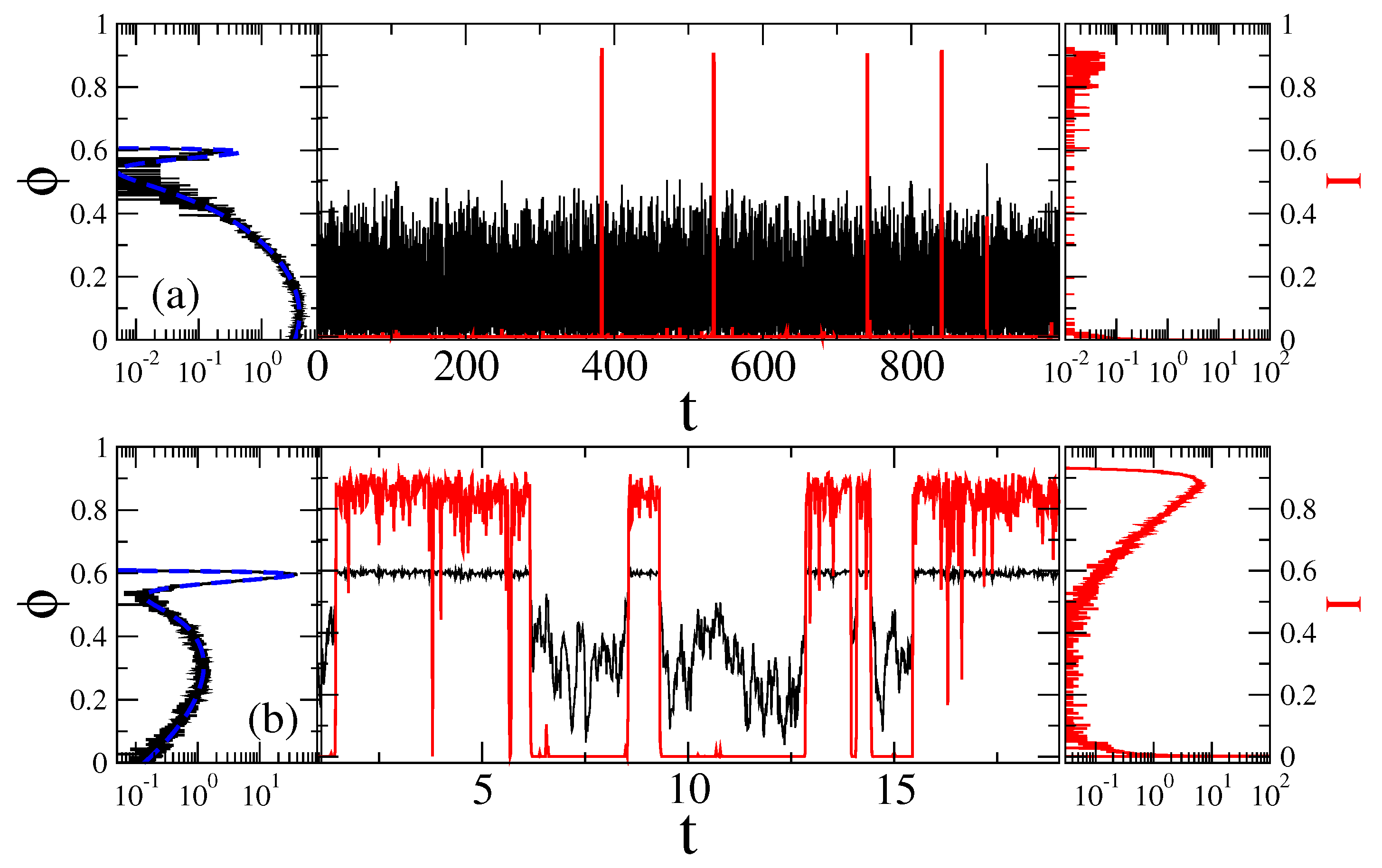
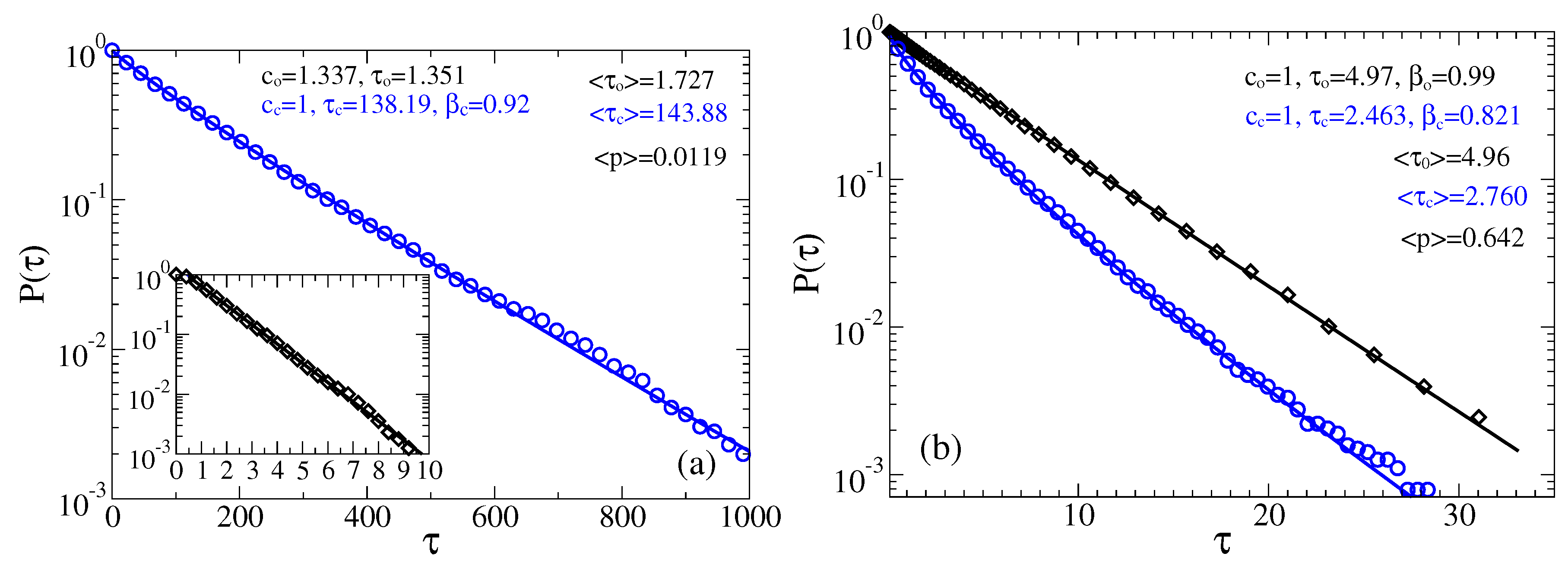
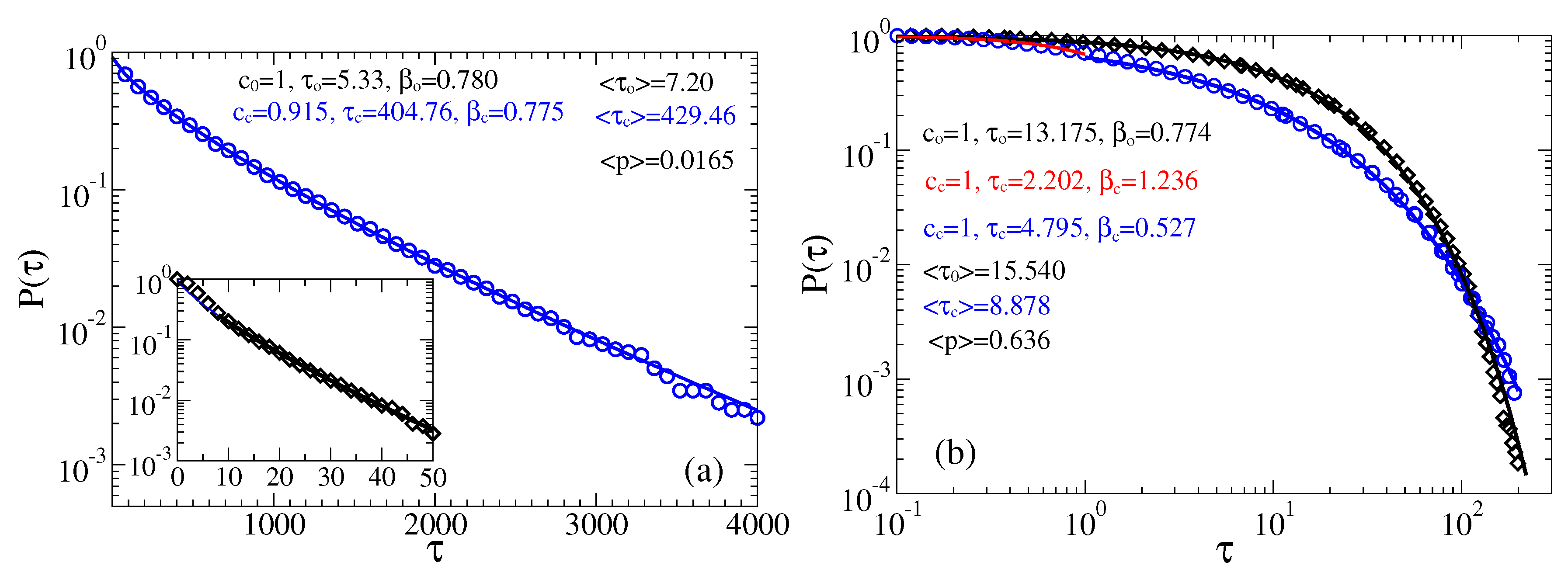
3.2. Stochastic Dynamics in Viscoelastic Environment
3.2.1. Intermediate fractional friction,
3.2.2. Strong Fractional Friction, 10
4. Discussion
5. Methods
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Webb, S.; Dodds, D. Inhibition of bacterial cell growth by 136 gc microwaves. Nature 1968, 218, 374. [Google Scholar] [CrossRef] [PubMed]
- Devyatkov, N. Effect of electromagnetic radiation of a millimeter wavelength range on biological objects. Sov. Phys. Uspekhi 1974, 16, 568. [Google Scholar] [CrossRef]
- Devyatkov, N.D.; Golant, M.B.; Betsky, O.V. Millimeter Waves and Their Role in Vital Processes; Radio and Svyaz: Moscow, Russia, 1991. [Google Scholar]
- Barnes, F.S.; Greenebaum, B. (Eds.) Handbook of Biological Effects of Electromagnetic Fields: Bioengineering and Biophysical Aspects of Electromagnetic Fields, 3rd ed.; Taylor & Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- Binhi, V.N. Magnetobiology: Underlying Physical Principles; Academic Press: San Diego, CA, USA, 2002. [Google Scholar]
- Frey, A.H. Human auditory system response to modulated electromagnetic energy. J. Appl. Physiol. 1962, 17, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.R.; Finch, E.D. Microwave hearing: Evidence for thermoacoustic auditory stimulation by pulsed microwaves. Science 1974, 185, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Sharp, J.C.; Grove, H.M.; Gandhi, O.P. Generation of acoustic signals by pulsed microwave energy. IEEE Trans. Microw. Theory Tech. 1974, 22, 582–584. [Google Scholar] [CrossRef]
- Guy, A.W.; Chou, C.K.; Lin, J.C.; Christensen, D. Microwave induced acoustic effects in mamalian auditory systems and physical materials. Ann. N. Y. Acad. Sci. 1975, 247, 194–215. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.C. On microwave-induced hearing sensation. IEEE Trans. Microw. Theory Tech. 1977, 25, 605–613. [Google Scholar] [CrossRef]
- Lin, J.C. Further studies on the microwave auditory effect. IEEE Trans. Microw. Theory Tech. 1977, 25, 938–943. [Google Scholar] [CrossRef]
- Watanabe, Y.; Tanaka, T.; Taki, M.; Watanabe, S. FDTD Analysis of microwave hearing effect. IEEE Trans. Microw. Theory Tech. 2000, 48, 2126–2132. [Google Scholar] [CrossRef]
- Elder, J.; Chou, C. Auditory response to pulsed radiofrequency energy. Bioelectromagnetics 2003, 6, S163–S173. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.R.; Glaser, R. Thermal mechanisms of interaction of radiofrequency energy with biological systems with relevance to exposure guidelines. Health Phys. 2007, 92, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.C.; Wang, Z. Hearing of microwave pulses by humans and animals: Effects, mechanism, and thresholds. Health Phys. 2007, 92, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Yitzhak, N.M.; Ruppin, R.; Hareuveny, R. Generalized model of the microwave auditory effect. Phys. Med. Biol. 2009, 54, 4037–4049. [Google Scholar] [CrossRef] [PubMed]
- Pikov, V.; Arakaki, X.; Harrington, M.; Fraser, S.E.; Siegel, P.H. Modulation of neuronal activity and plasma membrane properties with low-power millimeter waves in organotypic cortical slices. J. Neural Eng. 2010, 7, 045003. [Google Scholar] [CrossRef] [PubMed]
- Shneider, M.N.; Pekker, M. Non-thermal influence of a weak microwave on nerve fiber activity. J. Phys. Chem. Biophys. 2014, 4, 164. [Google Scholar]
- Pall, M.L. Microwave frequency electromagnetic fields (EMFs) produce widespread neuropsychiatric effects including depression. J. Chem. Neuroanat. 2016, 75, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Grissom, C.B. Magnetic Field Effects in Biology: A Survey of Possible Mechanisms with Emphasis on Radical-Pair Recombination. Chem. Rev. 1995, 95, 3–24. [Google Scholar] [CrossRef]
- Wiltschko, W.; Wiltschko, R. Magnetic orientation and magnetoreception in birds and other animals. J. Comp. Physiol. A 2005, 191, 675–693. [Google Scholar] [CrossRef] [PubMed]
- Schulten, K.; Swenberg, C.E.; Weller, A. A Biomagnetic sensory mechanism ased on magnetic field modulated coherent electron spin motion. Z. Phys. Chem. 1978, 111, 1–5. [Google Scholar] [CrossRef]
- Ritz, T.; Adem, S.; Schulten, K. A model for photoreceptor-based magnetoreception in birds. Biophys. J. 2000, 78, 707–718. [Google Scholar] [CrossRef]
- Ritz, T.; Ahmad, M.; Mouritsen, H.; Wiltschko, R.; Wiltschko, W. Photoreceptor-based magnetoreception: Optimal design of receptor molecules, cells, and neuronal processing. J. R. Soc. Interface 2010, 7, S135–S146. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, C.T.; Hore, P.J. Chemical magnetoreception in birds: The radical pair mechanism. Proc. Natl. Acad. Sci. USA 2009, 106, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Kirschvink, J.L.; Jones, D.S.; MacFadden, B.J. (Eds.) Magnetite Biomineralization and Magnetoreception in Organisms: A New Biomagnetism; Plenum Press: New York, NY, USA, 1985. [Google Scholar]
- Kirschvink, J.L.; Gould, J.L. Biogenic magnetite as a basis for magnetic field detection in animals. Biosystems 1981, 13, 181–201. [Google Scholar] [CrossRef]
- Kirschvink, J.L. Comment on ”Constraints on biological effects of weak extremely-low-frequency electromagnetic fields”. Phys. Rev. A 1992, 46, 2178–2184. [Google Scholar] [CrossRef] [PubMed]
- Eder, S.H.; Cadiou, H.; Muhamad, A.; McNaughton, P.A.; Kirschvink, J.L.; Winklhofer, M. Magnetic characterization of isolated candidate vertebrate magnetoreceptor cells. Proc. Natl. Acad. Sci. USA 2012, 109, 12022–12027. [Google Scholar] [CrossRef] [PubMed]
- Kirschvink, J.L.; Kobayashi-Kirschvink, A.; Woodford, B.J. Magnetite biomineralization in the human brain. Proc. Natl. Acad. Sci. USA 1992, 89, 7683–7687. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.; Fuller, M.; Zoeger, J.; Dobson, J.; Heller, F.; Hammann, J.; Caine, E.; Moskowitz, B.M. Magnetic material in the human hippocampus. Brain Res. Bull. 1995, 36, 149–153. [Google Scholar] [CrossRef]
- Dobson, J. Investigation of age-related variations in biogenic magnetite levels in the human hippocampus. Exp. Brain Res. 2002, 144, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Maher, B.A.; Ahmed, I.A.M.; Karloukovski, V.; MacLaren, D.A.; Foulds, P.G.; Allsop, D.; Mann, D.M.A.; Torres-Jardon, R.; Calderon-Garciduenas, L. Magnetite pollution nanoparticles in the human brain. Proc. Natl. Acad. Sci. USA 2016, 113, 10797–10801. [Google Scholar] [CrossRef] [PubMed]
- Giere, R. Magnetite in the human body: Biogenic vs. anthropogenic. Proc. Natl. Acad. Sci. USA 2016, 113, 11986–11987. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, R. Magnetotactic bacteria. Science 1975, 190, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Faivre, D.; Schüler, D. Magnetotactic Bacteria and Magnetosomes. Chem. Rev. 2008, 108, 4875–4898. [Google Scholar] [CrossRef] [PubMed]
- Abracado, L.G.; Abreu, F.; Keim, C.N.; Campos, A.P.C.; Lins, U.; Farina, M. Magnetosome chain superstructure in uncultured magnetotactic bacteria. Phys. Biol. 2010, 7, 046016. [Google Scholar] [CrossRef] [PubMed]
- Thomas-Keprta, K.L.; Bazylinski, D.A.; Kirschvink, J.L.; Clemett, S.J.; McKay, D.S.; Wentworth, S.J.; Vali, H.; Gibson, E.K.; Romanek, C.S. Elongated prismatic magnetite crystals in ALH84001 carbonate globules: Potential Martian magnetofossils. Geochim. Cosmochim. Acta 2000, 64, 4049–4081. [Google Scholar] [CrossRef]
- Adair, R.K. Constraints on biological effects of weak extremely-low-frequency electromagnetic fields. Phys. Rev. A 1991, 43, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Adair, R.K. Constraints of thermal noise on the effects of weak 60-Hz magnetic fields acting on biological magnetite. Proc. Natl. Acad. Sci. USA 1994, 91, 2925–2929. [Google Scholar] [CrossRef] [PubMed]
- Kirschvink, J.L. Microwave absorption by magnetite: A possible mechanism for coupling nonthermal levels of radiation to biological systems. Bioelectromagnetics 1996, 17, 187–194. [Google Scholar] [CrossRef]
- Belyaeva, O.Y.; Karpachev, S.N.; Zarembo, L.K. Magnetoacoustics of ferrites and magnetoacoustic resonance. Sov. Phys. Uspekhi 1992, 35, 106–122. [Google Scholar] [CrossRef]
- Kumar, C.S.S.R.; Mohammad, F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 789–808. [Google Scholar] [CrossRef] [PubMed]
- Monzel, C.; Vicario, C.; Piehler, J.; Coppey, M.; Dahan, M. Magnetic control of cellular processes using biofunctional nanoparticles. Chem. Sci. 2017, 8, 7330–7338. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.A.; Smith, C.J.; Ottolini, M.; Barker, B.S.; Purohit, A.M.; Grippo, R.M.; Gaykema, R.P.; Spano, A.J.; Beenhakker, M.P.; Kucenas, S.; et al. Genetically targeted magnetic control of the nervous system. Nat. Neurosci. 2016, 19, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Tay, A.; Kunze, A.; Murray, C.; Di Carlo, D. Induction of Calcium Influx in Cortical Neural Networks by Nanomagnetic Forces. ACS Nano 2016, 10, 2331–2341. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, J.W.; Levy, M.; Kao, A.; Noh, S.h.; Bozovic, D.; Cheon, J. Magnetic Nanoparticles for Ultrafast Mechanical Control of Inner Ear Hair Cells. ACS Nano 2014, 8, 6590–6598. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, D.; Stava, E.; Yu, M.; Blick, R.H. Creation and regulation of ion channels across reconstituted phospholipid bilayers generated by streptavidin-linked magnetite nanoparticles. Phys. Rev. E 2014, 89, 012707. [Google Scholar]
- Yorke, E.D. A possible magnetic transducer in birds. J. Theor. Biol. 1979, 77, 101–105. [Google Scholar] [CrossRef]
- Kirschvink, J.L.; Kobayashi-Kirschvink, A.; Diaz-Ricci, J.C.; Kirschvink, S.J. Magnetite in human tissues: A mechanism for the biological effects of weak ELF magnetic fields. Bioelectromagnetics 1992, 13, S101–S113. [Google Scholar] [CrossRef]
- Winklhofer, M.; Kirschvink, J.L. A quantitative assessment of torque-transducer models for magnetoreception. J. R. Soc. Interface 2010, 7, S273–S289. [Google Scholar] [CrossRef] [PubMed]
- Cadiou, H.; McNaughton, P.A. Avian magnetite-based magnetoreception: A physiologist’s perspective. J. R. Soc. Interface 2010, 7, S193–S205. [Google Scholar]
- Kubo, R. Fluctuation-Dissipation Theorem. Rep. Prog. Theor. Phys. 1966, 29, 255. [Google Scholar] [CrossRef]
- Kubo, R.; Toda, M.; Hashitsume, M. Nonequilibrium Statistical Mechanics, 2nd ed.; Springer: Berlin, Germany, 1991. [Google Scholar]
- Zwanzig, R. Nonequilibrium Statistical Mechanics; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Parry, B.R.; Surovtsev, I.V.; Cabeen, M.T.; O’Hern, C.S.; Dufresne, E.R.; Jacobs-Wagner, C. The bacterial cytoplasm has glass-like properties and is fluidized by metabolic activity. Cell 2014, 156, 183. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.; Kondev, J.; Theriot, J.; Garcia, H.G. Physical Biology of the Cell, 2nd ed.; Garland Science: London, UK; New York, NY, USA, 1991. [Google Scholar]
- Binhi, V.N.; Chernavskii, D.S. Stochastic dynamics of magnetosomes in cytoskeleton. Europhys. Lett. 2005, 70, 850–856. [Google Scholar] [CrossRef]
- Binhi, V. Stochastic dynamics of magnetosomes and a mechanism of biological orientation in the geomagnetic field. Bioelectromagnetics 2006, 27, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Binhi, V. Do naturally occurring magnetic nanoparticles in the human body mediate increased risk of childhood leukaemia with EMF exposure? Int. J. Radiat. Biol. 2008, 84, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Goychuk, I. Modeling magnetosensitive ion channels in the viscoelastic environment of living cells. Phys. Rev. E 2015, 92, 042711. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.; Hudspeth, A. Compliance of the hair bundle associated with gating of mechanoelectrical transduction channels in the Bullfrog’s saccular hair cell. Neuron 1988, 1, 189–199. [Google Scholar] [CrossRef]
- Hudspeth, A.J.; Choe, Y.; Mehta, A.D.; Martin, P. Putting ion channels to work: Mechanoelectrical transduction, adaptation, and amplification by hair cells. Proc. Natl. Acad. Sci. USA 2000, 97, 11765–11772. [Google Scholar] [CrossRef] [PubMed]
- Masaro, L.; Zhu, X. Physical models of diffusion for polymer solutions, gels and solids. Prog. Polym. Sci. 1999, 24, 731–775. [Google Scholar] [CrossRef]
- Odijk, T. Depletion Theory of Protein Transport in Semi-Dilute Polymer Solutions. Biophys. J. 2000, 79, 2314–2321. [Google Scholar] [CrossRef]
- Holyst, R.; Bielejewska, A.; Szymanski, J.; Wilk, A.; Patkowski, A.; Gapinski, J.; Zywocinski, A.; Kalwarczyk, T.; Kalwarczyk, E.; Tabaka, M.; et al. Scaling form of viscosity at all length-scales in poly(ethylene glycol) solutions studied by fluorescence correlation spectroscopy and capillary electrophoresis. Phys. Chem. Chem. Phys. 2009, 11, 9025–9032. [Google Scholar]
- Goychuk, I.; Kharchenko, V.O.; Metzler, R. How Molecular Motors Work in the Crowded Environment of Living Cells: Coexistence and Efficiency of Normal and Anomalous Transport. PLoS ONE 2014, 9, e91700. [Google Scholar] [CrossRef] [PubMed]
- Goychuk, I.; Kharchenko, V.O.; Metzler, R. Molecular motors pulling cargos in the viscoelastic cytosol: How power strokes beat subdiffusion. Phys. Chem. Chem. Phys. 2014, 16, 16524. [Google Scholar] [CrossRef] [PubMed]
- Goychuk, I. Anomalous transport of subdiffusing cargos by single kinesin motors: The role of mechanochemical coupling and anharmonicity of tether. Phys. Biol. 2015, 12, 016013. [Google Scholar]
- Larson, R.G. The Structure and Rheology of Complex Fluids; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Waigh, T.A. Microrheology of complex fluids. Rep. Prog. Phys. 2005, 68, 685. [Google Scholar] [CrossRef]
- Amblard, F.; Maggs, A.C.; Yurke, B.; Pargellis, A.N.; Leibler, S. Subdiffusion and Anomalous Local Viscoelasticity in Actin Networks. Phys. Rev. Lett. 1996, 77, 4470–4473. [Google Scholar] [CrossRef] [PubMed]
- Caspi, A.; Granek, R.; Elbaum, M. Diffusion and directed motion in cellular transport. Phys. Rev. E 2002, 66, 011916. [Google Scholar] [CrossRef] [PubMed]
- Guigas, G.; Kalla, C.; Weiss, M. Probing the nanoscale viscoelasticity of intracellular fluids in living cells. Biophys. J. 2007, 93, 316. [Google Scholar] [CrossRef] [PubMed]
- Luby-Phelps, K. The physical chemistry of cytoplasm and its influence on cell function: An update. Mol. Biol. Cell 2013, 24, 2593–2596. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.C.; Spakowitz, A.J.; Theriot, J.A. Bacterial Chromosomal Loci Move Subdiffusively through a Viscoelastic Cytoplasm. Phys. Rev. Lett. 2010, 104, 238102. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Filobelo, L.; Pham, N.D.Q.; Galkin, O.; Uzunova, V.V.; Vekilov, P.G. Viscoelasticity in Homogeneous Protein Solutions. Phys. Rev. Lett. 2009, 102, 058101. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, J.; Weiss, M. Elucidating the Origin of Anomalous Diffusion in Crowded Fluids. Phys. Rev. Lett. 2009, 103, 038102. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.W.; Kenwright, D.A.; Waigh, T.A.; Woodman, P.G.; Allan, V.J. Modes of correlated angular motion in live cells across three distinct time scales. Phys. Biol. 2013, 10, 036002. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, D.; Tardin, C.; Schmidt, C.F.; MacKintosh, F.C. Nonequilibrium Mechanics of Active Cytoskeletal Networks. Science 2007, 315, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Robert, D.; Nguyen, T.H.; Gallet, F.; Wilhelm, C. Diffusion and directed motion in cellular transport. PLoS ONE 2010, 4, e10046. [Google Scholar]
- Weiss, M. Single-particle tracking data reveal anticorrelated fractional Brownian motion in crowded fluids. Phys. Rev. E 2013, 88, 010101. [Google Scholar] [CrossRef] [PubMed]
- Goychuk, I. Fractional-time random walk subdiffusion and anomalous transport with finite mean residence times: Faster, not slower. Phys. Rev. E 2012, 86, 021113. [Google Scholar] [CrossRef] [PubMed]
- Goychuk, I. Is subdiffusional transport slower than normal? Fluct. Noise Lett. 2012, 11, 1240009. [Google Scholar] [CrossRef]
- Santamaria-Holek, I.; Rubi, J.M.; Gadomski, A. Thermokinetic Approach of Single Particles and Clusters Involving Anomalous Diffusion under Viscoelastic Response. J. Phys. Chem. B 2007, 111, 2293–2298. [Google Scholar] [CrossRef] [PubMed]
- Mason, T.G.; Weitz, D.A. Optical Measurements of Frequency-Dependent Linear Viscoelastic Moduli of Complex Fluids. Phys. Rev. Lett. 1995, 74, 1250–1253. [Google Scholar] [CrossRef] [PubMed]
- Goychuk, I. Viscoelastic Subdiffusion: Generalized Langevin Equation Approach. Adv. Chem. Phys. 2012, 50, 187–253. [Google Scholar]
- Saxton, M.J.; Jacobsen, K. Single-particle tracking: Application to membrane dynamics. Ann. Rev. Biophys. Biomol. Struct. 1997, 26, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Seisenberger, G.; Ried, M.U.; Endress, T.; Büning, H.; Hallek, M.; Bräuchle, C. Real-time single-molecule imaging of the infection pathway of an adeno-associated virus. Science 2001, 294, 1929–1932. [Google Scholar] [CrossRef] [PubMed]
- Banks, D.S.; Fradin, C. Anomalous Diffusion of Proteins Due to Molecular Crowding. Biophys. J. 2005, 89, 2960–2971. [Google Scholar] [CrossRef] [PubMed]
- Tolic-Norrelykke, I.M.; Munteanu, E.L.; Thon, G.; Oddershede, L.; Berg-Sorensen, K. Anomalous diffusion in living yeast cells. Phys. Rev. Lett. 2004, 93, 078102. [Google Scholar] [CrossRef] [PubMed]
- Golding, I.; Cox, E.C. Physical nature of bacterial cytoplasm. Phys. Rev. Lett. 2006, 96, 098102. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Elsner, M.; Kartberg, F.; Nilsson, T. Anomalous Subdiffusion is a Measure for Cytoplasmic Crowding in Living Cells. Biophys. J. 2004, 87, 3518–3524. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.H.; Tejedor, V.; Burov, S.; Barkai, E.; Selhuber-Unkel, C.; Berg-Sørensen, K.; Oddershede, L.; Metzler, R. In vivo anomalous diffusion and weak ergodicity breaking of lipid granules. Phys. Rev. Lett. 2011, 106, 048103. [Google Scholar] [CrossRef] [PubMed]
- Bruno, L.; Salierno, M.; Wetzler, D.E.; Desposito, M.A.; Levi, V. Mechanical properties of organelles driven by microtubuli-dependent molecular motors in living cells. PLoS ONE 2011, 6, e18332. [Google Scholar] [CrossRef] [PubMed]
- Tabei, S.M.A.; Burov, S.; Kima, H.Y.; Kuznetsov, A.; Huynha, T.; Jureller, J.; Philipson, L.H.; Dinner, A.R.; Scherer, N.F. Intracellular transport of insulin granules is a subordinated random walk. Proc. Natl. Acad. Sci. USA 2013, 110, 4911–4916. [Google Scholar] [CrossRef] [PubMed]
- Weigel, A.V.; Simon, B.; Tamkun, M.M.; Krapf, D. Ergodic and nonergodic processes coexist in the plasma membrane as observed by single-molecule tracking. Proc. Natl. Acad. Sci. USA 2011, 108, 6438–6443. [Google Scholar] [CrossRef] [PubMed]
- Bertseva, E.; Grebenkov, D.; Schmidhauser, P.; Gribkova, S.; Jeney, S.; Forro, L. Optical trapping microrheology in cultured human cells. Eur. Phys. J. E 2012, 35, 63. [Google Scholar] [CrossRef] [PubMed]
- Höfling, F.; Franosch, T. Anomalous transport in the crowded world of biological cells. Rep. Prog. Phys. 2013, 76, 046602. [Google Scholar] [CrossRef] [PubMed]
- Goychuk, I. Viscoelastic subdiffusion: From anomalous to normal. Phys. Rev. E 2009, 80, 046125. [Google Scholar] [CrossRef] [PubMed]
- Grote, R.F.; Hynes, J.T. The stable states picture of chemical reactions. II. Rate constants for condensed and gas phase reaction models. J. Chem. Phys. 1980, 73, 2715–2732. [Google Scholar] [CrossRef]
- Hanggi, P.; Mojtabai, F. Thermally activated escape rate in presence of long-time memory. Phys. Rev. A 1982, 26, 1168–1170. [Google Scholar] [CrossRef]
- Carmeli, B.; Nitzan, A. Non-Markovian theory of activated rate processes. I. Formalism. J. Chem. Phys. 1983, 79, 393–404. [Google Scholar] [CrossRef]
- Pollak, E.; Grabert, H.; Hänggi, P. Theory of activated rate processes for arbitrary frequency dependent friction: Solution of the turnover problem. J. Chem. Phys. 1989, 91, 4073–4087. [Google Scholar] [CrossRef]
- Hänggi, P.; Talkner, P.; Borkovec, M. Reaction-rate theory: Fifty years after Kramers. Rev. Mod. Phys. 1990, 62, 251–341. [Google Scholar] [CrossRef]
- Liebovitch, L.; Sullivan, J. Fractal analysis of a voltage-dependent potassium channel from cultured mouse hippocampal neurons. Biophys. J. 1987, 52, 979–988. [Google Scholar] [CrossRef]
- Läuger, P. Internal motions in proteins and gating kinetics of ionic channels. Biophys. J. 1988, 53, 877–884. [Google Scholar] [CrossRef]
- Sansom, M.; Ball, F.; Kerry, C.; McGee, R.; Ramsey, R.; Usherwood, P. Markov, fractal, diffusion, and related models of ion channel gating. A comparison with experimental data from two ion channels. Biophys. J. 1989, 56, 1229–1243. [Google Scholar] [CrossRef]
- Bezrukov, S.M.; Winterhalter, M. Examining Noise Sources at the Single-Molecule Level: 1/f Noise of an Open Maltoporin Channel. Phys. Rev. Lett. 2000, 85, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Millhauser, G.L.; Salpeter, E.E.; Oswald, R.E. Diffusion models of ion-channel gating and the origin of power-law distributions from single-channel recording. Proc. Natl. Acad. Sci. USA 1988, 85, 1503–1507. [Google Scholar] [CrossRef] [PubMed]
- Croxton, T.L. A model of the gating of ion channels. Biochim. Biophys. Acta Biomembr. 1988, 946, 19–24. [Google Scholar] [CrossRef]
- Frauenfelder, H.; Sligar, S.; Wolynes, P. The energy landscapes and motions of proteins. Science 1991, 254, 1598–1603. [Google Scholar] [CrossRef] [PubMed]
- Frauenfelder, H.; Fenimore, P.W.; Chen, G.; McMahon, B.H. Protein folding is slaved to solvent motions. Proc. Natl. Acad. Sci. USA 2006, 103, 15469–15472. [Google Scholar] [CrossRef] [PubMed]
- Frauenfelder, H.; Chen, G.; Berendzen, J.; Fenimore, P.W.; Jansson, H.; McMahon, B.H.; Stroe, I.R.; Swenson, J.; Young, R.D. A unified model of protein dynamics. Proc. Natl. Acad. Sci. USA 2009, 106, 5129–5134. [Google Scholar] [CrossRef] [PubMed]
- Goychuk, I.; Hänggi, P. Ion channel gating: A first-passage time analysis of the Kramers type. Proc. Natl. Acad. Sci. USA 2002, 99, 3552–3556. [Google Scholar] [CrossRef] [PubMed]
- Goychuk, I.; Hänggi, P. Fractional diffusion modeling of ion channel gating. Phys. Rev. E 2004, 70, 051915. [Google Scholar] [CrossRef] [PubMed]
- Herrchen, M.; Öttinger, H.C. A detailed comparison of various FENE dumbbell models. J. Non-Newtonian Fluid Mech. 1997, 68, 17–42. [Google Scholar] [CrossRef]
- Coffey, W.T.; Kalmykov, Y.P. The Langevin Equation with Applications to Stochastic Problems in Physics, Chemistry and Electrical Engineering, 3rd ed.; World Scientific: Hackensack, NJ, USA, 2012. [Google Scholar]
- De la Torre, J.G.; Bloomfield, V.A. Hydrodynamic properties of complex, rigid, biological macromolecules: Theory and applications. Q. Rev. Biophys. 1981, 14, 81–139. [Google Scholar] [CrossRef]
- Gard, T.C. Introduction to Stochastic Differential Equations; Dekker: New York, NY, USA, 1988. [Google Scholar]
- Haase, M.; Hübner, C.G.; Reuther, E.; Herrmann, A.; Müllen, K.; Basché, T. Exponential and Power-Law Kinetics in Single-Molecule Fluorescence Intermittency. J. Phys. Chem. B 2004, 108, 10445–10450. [Google Scholar] [CrossRef]
- Lippitz, M.; Kulzer, F.; Orrit, M. Statistical Evaluation of Single Nano-Object Fluorescence. ChemPhysChem 2005, 6, 770–789. [Google Scholar] [CrossRef] [PubMed]
- Stefani, F.D.; Zhong, X.; Knoll, W.; Han, M.; Kreiter, M. Memory in quantum-dot photoluminescence blinking. New J. Phys. 2005, 7, 197. [Google Scholar] [CrossRef]
- Hoogenboom, J.P.; Hernando, J.; van Dijk, E.M.H.P.; van Hulst, N.F.; García-Parajó, M.F. Power-Law Blinking in the Fluorescence of Single Organic Molecules. ChemPhysChem 2007, 8, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Gemant, A. A Method of Analyzing Experimental Results Obtained from Elasto-Viscous Bodies. Physics 1936, 7, 311–317. [Google Scholar] [CrossRef]
- Goychuk, I. Anomalous relaxation and dielectric response. Phys. Rev. E 2007, 76, 040102(R). [Google Scholar]
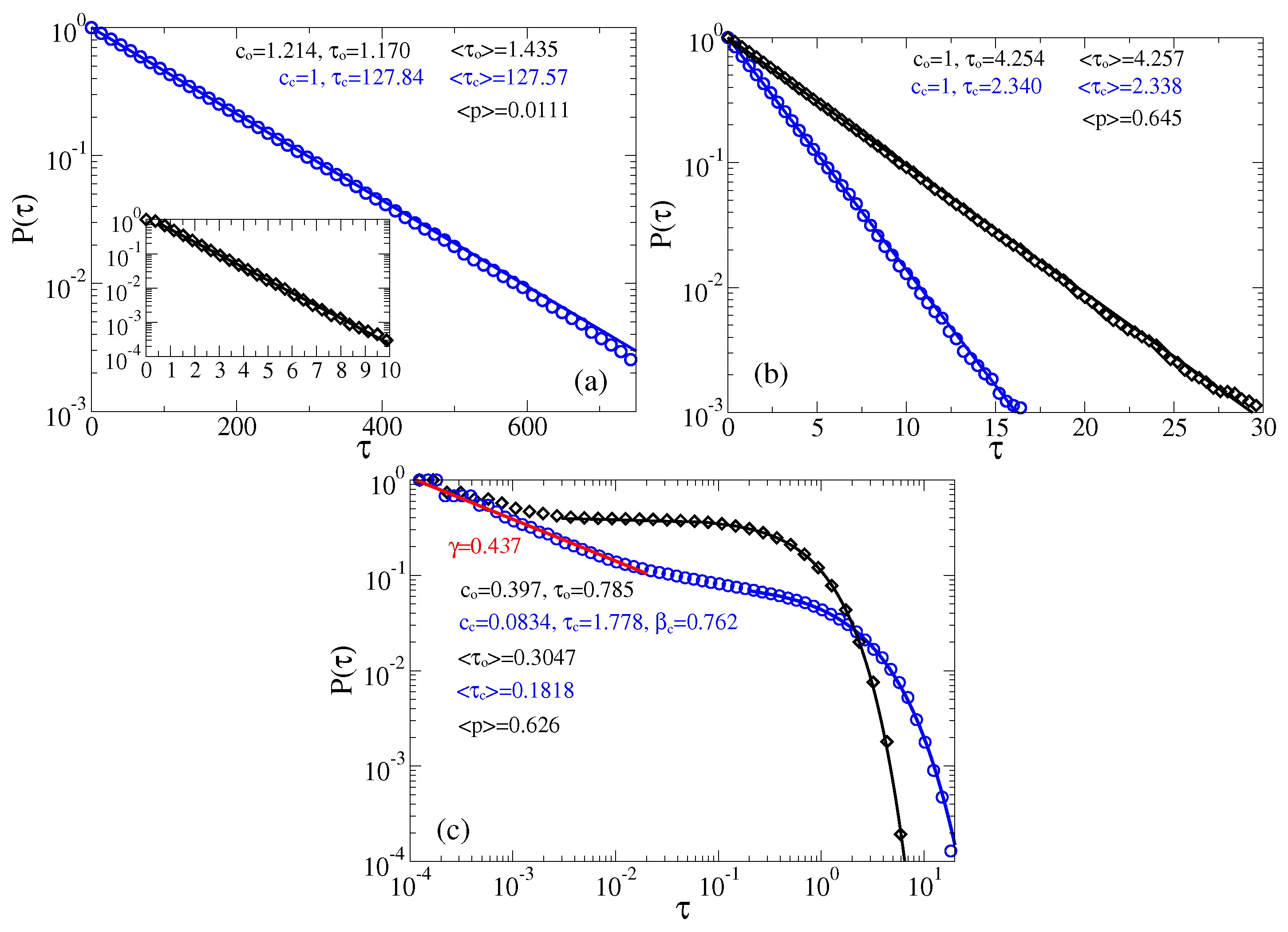
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goychuk, I. Sensing Magnetic Fields with Magnetosensitive Ion Channels. Sensors 2018, 18, 728. https://doi.org/10.3390/s18030728
Goychuk I. Sensing Magnetic Fields with Magnetosensitive Ion Channels. Sensors. 2018; 18(3):728. https://doi.org/10.3390/s18030728
Chicago/Turabian StyleGoychuk, Igor. 2018. "Sensing Magnetic Fields with Magnetosensitive Ion Channels" Sensors 18, no. 3: 728. https://doi.org/10.3390/s18030728




