Preparation of Enzymatic Layer
Transducers pH-sensitive ISFETs and pNH
4-ChemFETs used as basic structures for biosensors fabrication have been described previously [
5,
6]. The pH-sensitive Si
3N
4-gate-ISFETs, used in experiments, are characterized by the following parameters: sensitivity ca 45 mV/pH and response time 0.2 s (fabricated at the Institute of Electron Technology, Poland).
The ChemFETs with poly(hydroxyethyl methacrylate) (polyHEMA) hydrogel buffering layer and Siloprene ion selective membrane containing nonactine as ionophore, used as a basic structure for EnFETs, are characterized by the sensitivity about 55 mV/decade and a very good durability (ca 2 years).
Prior to hydration process of the silicon nitride surface, the ISFET gate area was cleaned with ethyl acetate. The glutaraldehyde layer was deposited twice from 2.5% water solution. Then, EnFETs were dried at room temperature for 1.5 hr and then washed with distilled water. Afterwards, the deposition of the GA layer was repeated. Next, the enzymatic layer was deposited by droplet coating method with urease solution (10 mg of the urease in 400 μl of the 5 mM phosphate buffer at the pH 6.0). After that, the EnFETs were dried at room temperature overnight and then non-attached enzyme molecules were washed out by vigorously stirred phosphate buffer solution. This chemical method of the urease immobilization is based on Schiff’s base formation between amino type groups on the silicon nitride surface, amino groups on the enzyme and aldehyde groups from glutaraldehyde.
The enzymatic membranes for ChemFETs were prepared in the following way: a portion of 5 µl aqueous solution of urease (concentration 10 mg/ml each) was deposited onto the ChemFET gate area covered with ion-selective membrane of Siloprene (dried at room temperature for 2-3 days) and then left under a cover for almost completely water evaporation for 2-3 hours at room temperature. Afterwards, 10 µl of 2.5% glutaraldehyde aqueous solution was placed onto the layer of enzyme and albumin mixture and left under a cover for crosslinking for 60 minutes at 4°C. The excess of glutaraldehyde was carefully washed off with deionized water and the sensor was washed for 1 hour in 0.005 M orthophosphate buffer at pH 6.
Measurements
The calibrations of the EnFETs were performed for different sodium ortophosphate (NaH2PO4) buffer solutions at the concentrations: 1, 5, 10, 25 and 100 mM and at pH 6.0, 7.0, and 8.0, containing 0.1 M NaCl. Before each calibration session, the EnFETs were conditioned in the buffer solution for 40 minutes. The subsequent additions of the standard urea solutions were added in the intervals of 10 minutes. To avoid dilution of the measuring buffer solution, the urea standards were prepared with use of the same measuring buffer.
The output signal, Ugs, (gate-source voltage) was recorded by computerized measurement setup with multi-channel amplifier system. The EnFETs were supplied with constant drain-source voltage, Uds=2.5 V and constant drain current, Id=100 μA. After each calibration session, the EnFETs were flushed with distilled water and dried at room temperature for 1 hr. Between the subsequent calibrations the EnFETs were stored dry at temperature +4°C. The calibration sessions were repeated twice or three times a week.
The urea determinations were performed in bovine blood plasma with anticoagulant and in hemodialysis fluid (concentrate of hemodialysis fluid, type D605, MTN Medizintechnik GmbH, Neubrandenburg, Germany, diluted in the ratio 1:35).
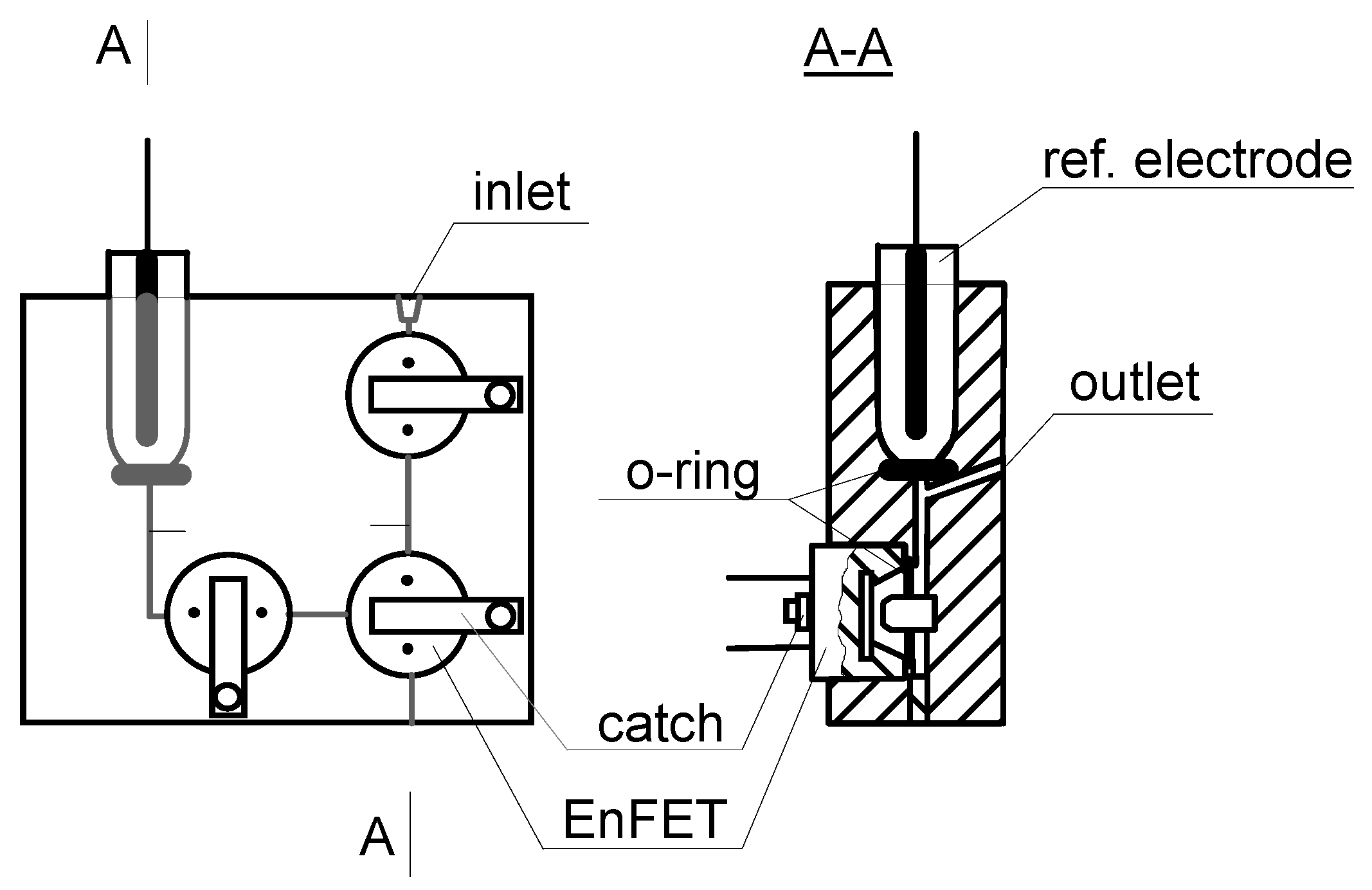
Figure 1.
Schematic diagram of the serial flow-through cells.
Figure 1.
Schematic diagram of the serial flow-through cells.
The pH-detection based EnFETs were placed in the serial flow-through cells (
Fig. 1). Based on the calibration curves taken in plasma and dialysate, pairs of calibrations points were chosen for determinations of urea in the prepared samples. After each calibration, the subsequent determinations of urea in samples were repeated 5 times. Between changes of the sample in the measuring cells, the cells were washed with phosphate buffer at pH 6.0.
The ChemFET based biosensors were immersed in the solution to be measured (0.005 M orthophosphate buffer), connected to the measurement setup and conditioned for 2 hours (connected to the power supply). The calibration curves were obtained at room temperature by means of the standard addition method. The urea standard solutions were prepared by dissolving of a weighed amount of urea in following fluids: 50 mM tris/HCl buffer solution at pH 7.5, dialysate based on D-605 hemodialysis fluid concentrate (diluted concentrate), mixture of dialysate and the buffer, mixture of blood plasma and the buffer.