Electrochemical Sensor for Tryptophan Determination Based on Copper-cobalt Hexacyanoferrate Film Modified Graphite Electrode
Abstract
:1. Introduction
2. Experimental
2.1 Reagents and apparatus
2.2 Preparation of CuCoHCF film
3. Results and Discussions
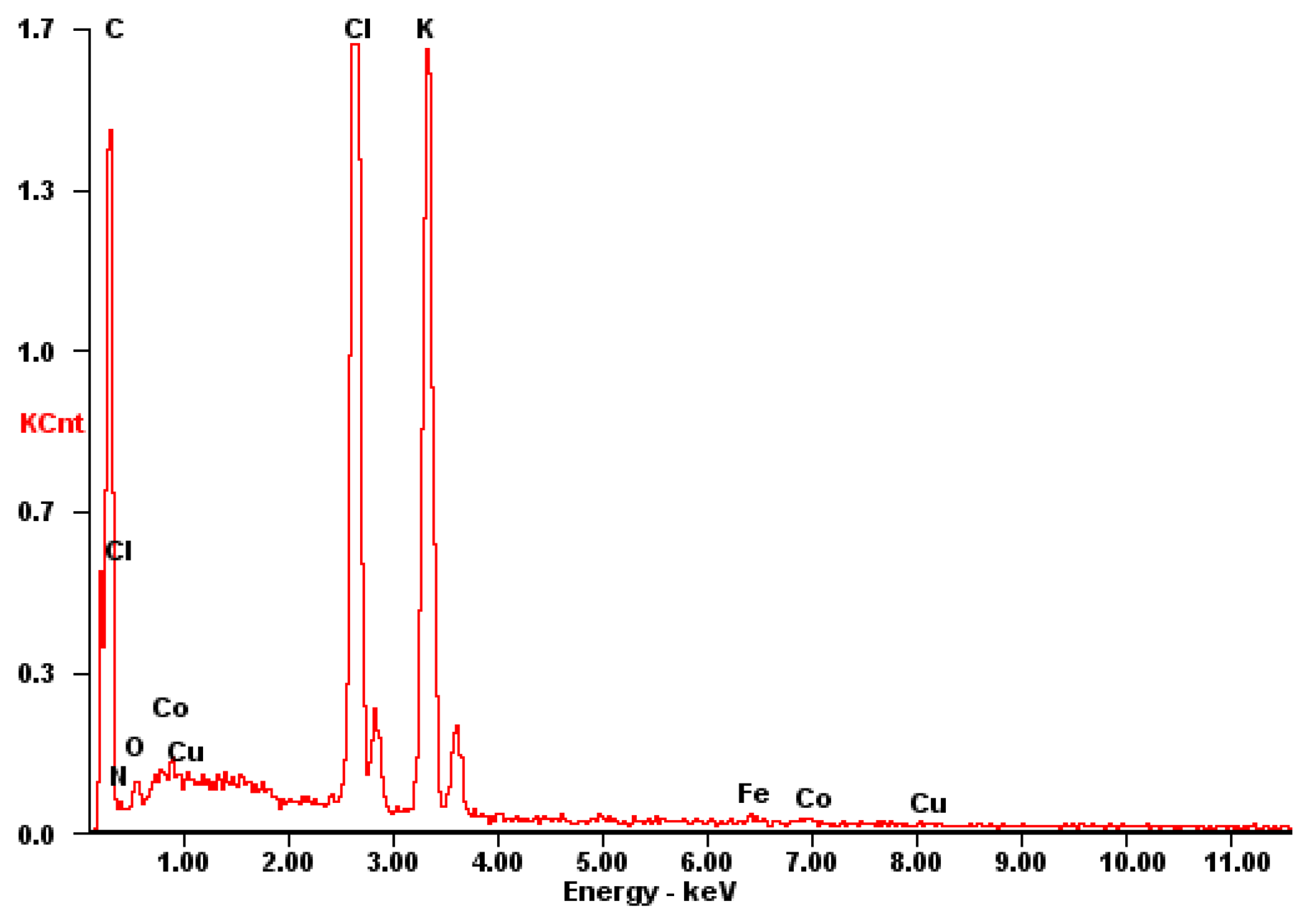
3.1 Electrochemical characterization of CuCoHCF modified graphite electrode
3.2 Electrocatalytic oxidation of tryptophan at the surface of CuCoHCF-modified graphite electrode
3.3 Optimization of experimental variables for electrocatalytic ability on tryptophan
3.3.1 Effect of scan cycles in electrodeposition
3.3.2 Effect of the ratio of Cu(II) and Co(II) in the solution
3.3.3 Effect of pH value
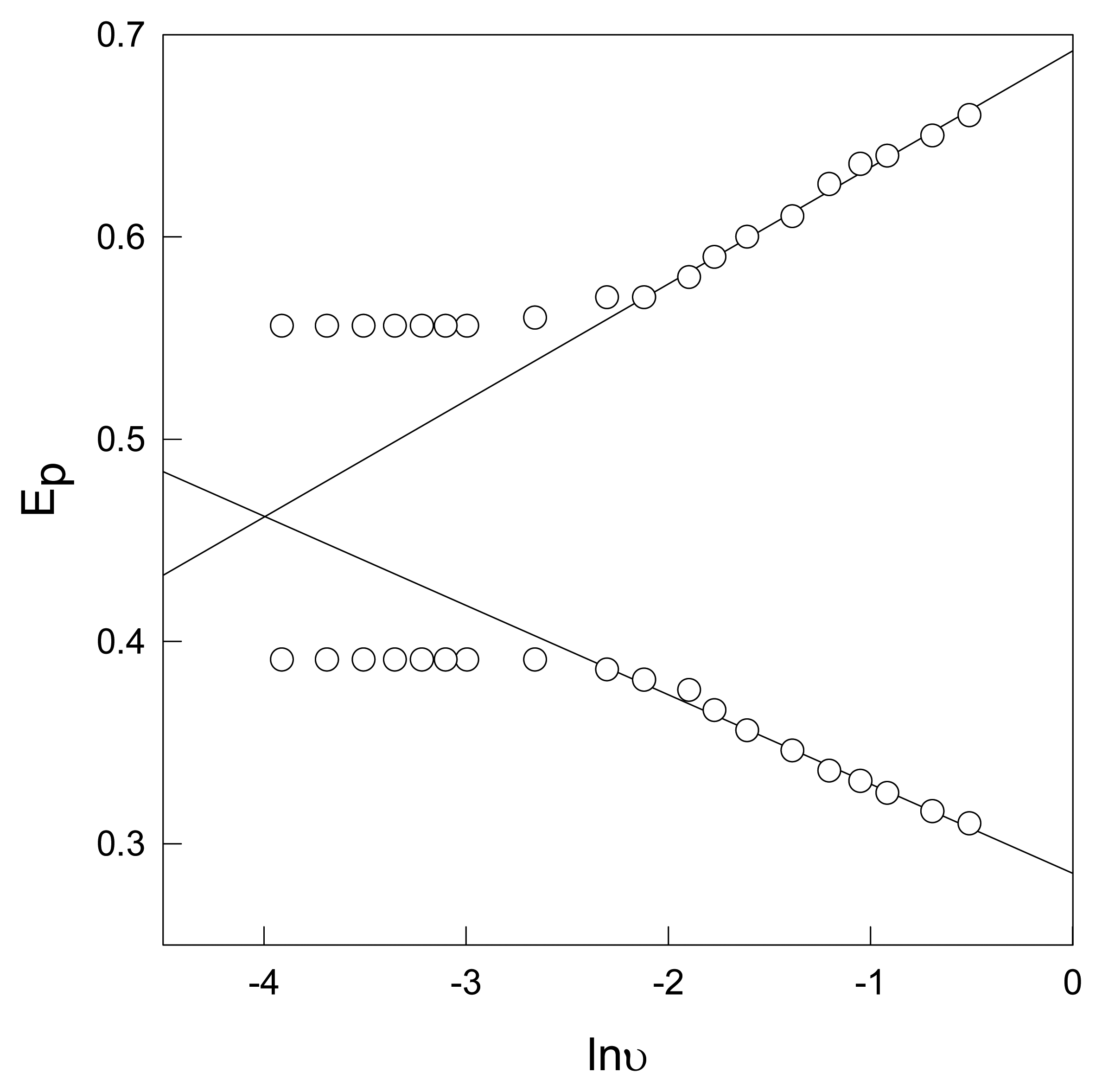
3.3.4 Effect of applied voltage
3.4 Analytical properties
3.5 Determination of tryptophan in milk
4. Conclusion
Acknowledgments
References
- Amelung, W.; Zhang, X.; Flach, K.W. Amino acids in grassland soils: climatic effects on concentrations and chirality. Geoderma 2006, 130, 207–217. [Google Scholar]
- Silván, J. M.; van de Lagemaat, J.; Olano, A.; del Castillo, M. D. Analysis and biological properties of amino acid derivates formed by Maillard reaction in foods. J. Pharmaceu. Biomed. Anal. 2006, 41, 1543–1551. [Google Scholar]
- Ali, H.; Pätzold, R.; Brückner, H. Determination of l- and d-amino acids in smokeless tobacco products and tobacco. Food Chem. 2006, 99, 803–812. [Google Scholar]
- Ensafi, A.A.; Hajian, R. Determination of tryptophan and histidine by adsorptive cathodic stripping voltammetry using H-point standard addition method. Anal. Chim. Acta 2006, 580, 236–243. [Google Scholar]
- Delgado-Andrade, C.; Rufián-Hanares, J.A.; Jiménez-Pérez, S.; Morales, F.J. Tryptophan determination in milk-based ingredients and dried sport supplements by liquid chromatography with fluorescence detection. Food Chem. 2006, 98, 580–585. [Google Scholar]
- Bo, T.; Pawliszyn, J. Characterization of bovine serum albumin–tryptophan interaction by capillary isoelectric focusing with whole column imaging detection. J. Chromatogra. A 2006, 1105, 25–32. [Google Scholar]
- Yust, M.M.; Pedroche, J.; Calle, J.G.; Vioque, J.; Millán, F.; Alaiz, M. Determination of tryptophan by high-performance liquid chromatography of alkaline hydrolysates with spectrophotometric detection. Food Chem. 2004, 85, 317–320. [Google Scholar]
- Macdonald, S.M.; Roscoe, S.G. Electrochemical oxidation reactions of tyrosine, tryptophan and related dipeptides. Electrochim. Acta 1997, 42, 1189–1200. [Google Scholar]
- Zhao, Y.D.; Zhang, W.D.; Chen, H.; Luo, Q.M. Electrocatlytic oxidation of cysteine at carbon nanotube powder microelectrode and its detection. Sens. Actuators B 2003, 92, 279–285. [Google Scholar]
- Fei, S.D.; Chen, J.H.; Yao, S.Z.; Deng, G.H.; He, D.L.; Kuang, Y.F. Electrochemical behavior of L-cysteine and its detection at carbon nanotube electrode modified with platinum. Anal. Biochem. 2005, 339, 29–35. [Google Scholar]
- Ricci, F.; Palleschi, G. Sensor and biosensor preparation, optimization and application of Prussian Blue modified electrodes. Biosens. Bioelectron 2005, 21, 389–407. [Google Scholar]
- Li, C.M.; Sun, C.Q.; Chen, W.; Pan, L. Electrochemical thin film deposition of polypyrrole on different substrates. Surf. Coatings Technol. 2005, 198, 474–477. [Google Scholar]
- Ionescu, R.E.; Abu-Rabeah, K.; Cosnier, S.; Marks, R.S. Improved enzyme retention from an electropolymerized polypyrrole-alginate matrix in the development of biosensors. Electrochem. Commun. 2005, 7, 1277–1282. [Google Scholar]
- Sharpe, A.G. The chemistry of cyano complexes of the transition metals; Academic Press: New York, 1976. [Google Scholar]
- Razmi, H.; Azadbakht, A. Electrochemical characteristics of dopamine oxidation at palladium hexacyanoferrate film, electroless plated on aluminum electrode. Electrochim. Acta 2005, 50, 2193–2201. [Google Scholar]
- Tao, W.Y.; Pan, D.W.; Liu, Y.J.; Nie, L.H.; Yao, S.Z. Characterization and electrocatalytic properties of cobalt hexacyanoferrate films immobilized on Au-colloid modified gold electrodes. J. Electroanal. Chem. 2004, 572, 109–117. [Google Scholar]
- García, T.; Casero, E.; Lorenzo, E.; Pariente, F. Electrochemical sensor for sulfite determination based on iron hexacyanoferrate film modified electrodes. Sens. Actuators B 2005, 106, 803–809. [Google Scholar]
- Zen, J.M.; Chen, H.W.; Kumar, A.S. Electrochemical behavior of stable cinder/Prussian Blue analogue and its mediated nitrite oxidation. Electroanalysis 2001, 13, 1171–1178. [Google Scholar]
- Abbaspour, A.; Mehrgardi, M.A. Electrocatalytic oxidation of guanine and DNA on a carbon paste electrode modified by cobalt hexacyanoferrate films. Anal. Chem. 2004, 76, 5690–5696. [Google Scholar]
- Abbaspour, A.; Mehrgardi, M.A.; Kia, R. Electrocatalytic oxidation of guanine and ss-DNA at a cobalt (II) phthalocyanine modified carbon paste electrode. J. Electroanal. Chem. 2004, 568, 261–266. [Google Scholar]
- Kukulka-Walkiewicz, J.; Stroka, J.; Malik, M.A.; Kulesza, P.J.; Galus, Z. Films of mixed nickel(II) and thallium(I) hexacyanoferrates(III,II): voltammetric preparation and characterization. Electrochim. Acta 2001, 46, 4057–4063. [Google Scholar]
- Joseph, J.; Gomathi, H.; Rao, G.P. Modification of carbon electrodes with zinc hexacyanoferrate. J. Electroanal. Chem. 1997, 431, 231–235. [Google Scholar]
- Tao, W.Y.; Pan, D.W.; Liu, Y.J.; Nie, L.H.; Yao, S.Z. An amperometric hydrogen peroxide sensor based on immobilization of hemoglobin in poly(o-aminophenol) film at iron-cobalt hexacyanoferrate-modified gold electrode. Anal. Biochem. 2005, 338, 332–340. [Google Scholar]
- Cui, X.P.; Hong, L.; Lin, X.Q. Electrochemical preparation, characterization and application of electrodes modified with hybrid hexacyanoferrates of copper and cobalt. J. Electroanal.Chem. 2002, 526, 115–124. [Google Scholar]
- Laviron, E. Adsorption autoinhibition and autocatalysis in polarography and linear potential sweep voltammetry. J. Electroanal. Chem. 1974, 52, 355–393. [Google Scholar]
- Ma, H.Y.; Hu, N.F.; Rusling, J.F. Electroactive mMyoglobin films grown layer-by-layer with poly(styrenesulfonate) on pyrolytic graphite electrodes. Langmuir 2000, 16, 4969–4975. [Google Scholar]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar]
- García-Jareño, J.J.; Sanmatías, A.; Navarro-Laboulais, J.; Vicente, F. Chronoamperometry of prussian blue films on ITO electrodes: ohmic drop and film thickness effect. Electrochim. Acta 1999, 44, 4753–4762. [Google Scholar]
- Kasem, K.K.; Huddleston, L. Iridium-based hexacyanometallate thin films in aqueous electrolytes. Platinum Metals Rev. 2004, 48, 159–167. [Google Scholar]
- Chen, G.N.; Zhao, Z.F.; Wang, X.L.; Duan, J.P.; Chen, H.Q. Electrochemical behavior of tryptophan and its derivatives at a glassy carbon electrode modified with hemin. Anal. Chim. Acta 2002, 452, 245–254. [Google Scholar]







| Added tryptophan (10-4 M) | Detected tryptophana (10-4 M) | Tryptophan in milk (10-4 M) | Average (10-4 M) | SD | |
|---|---|---|---|---|---|
| 1 | 0.5 | 2.4±0.2 | 1.9±0.2 | 1.8 | 0.15 |
| 2 | 1.0 | 2.7±0.3 | 1.7±0.4 | ||
| 3 | 1.5 | 3.3±0.3 | 1.8±0.3 | ||
| 4 | 2.0 | 3.8±0.2 | 1.8±0.2 | ||
| 5 | 2.5 | 4.5±0.1 | 2.0±0.1 |
© 2007 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Liu, Y.; Xu, L. Electrochemical Sensor for Tryptophan Determination Based on Copper-cobalt Hexacyanoferrate Film Modified Graphite Electrode. Sensors 2007, 7, 2446-2457. https://doi.org/10.3390/s7102446
Liu Y, Xu L. Electrochemical Sensor for Tryptophan Determination Based on Copper-cobalt Hexacyanoferrate Film Modified Graphite Electrode. Sensors. 2007; 7(10):2446-2457. https://doi.org/10.3390/s7102446
Chicago/Turabian StyleLiu, Yingju, and Li Xu. 2007. "Electrochemical Sensor for Tryptophan Determination Based on Copper-cobalt Hexacyanoferrate Film Modified Graphite Electrode" Sensors 7, no. 10: 2446-2457. https://doi.org/10.3390/s7102446




