Dinophysis Toxins: Causative Organisms, Distribution and Fate in Shellfish
Abstract
:1. Introduction

| Species | Origin and Reference |
|---|---|
| Dinophysis acuminata | South Korea [33]; Northeast Japan [34]; Northwest Denmark [35]; Northeast US [36,37]; Northeast Spain [38]. |
| D. acuta | Southwest and Northwest Spain [39,40]; Denmark [41] |
| D. caudata | South Korea [42]; Southeast Japan [43]; Northwest Spain [40] |
| D. fortii | Southeast Japan [44] |
| D. infundibula | Southwest Japan [45] |
| D. cf ovum | South Brazil [46] |
| D. sacculus | Northwest Spain [47] |
| D. tripos | Northwest Spain [48] |
2. Historic Overview
3. Toxin-Containing Species of Dinophysis/Phalacroma: Toxin Profile and Contribution to DSP Events
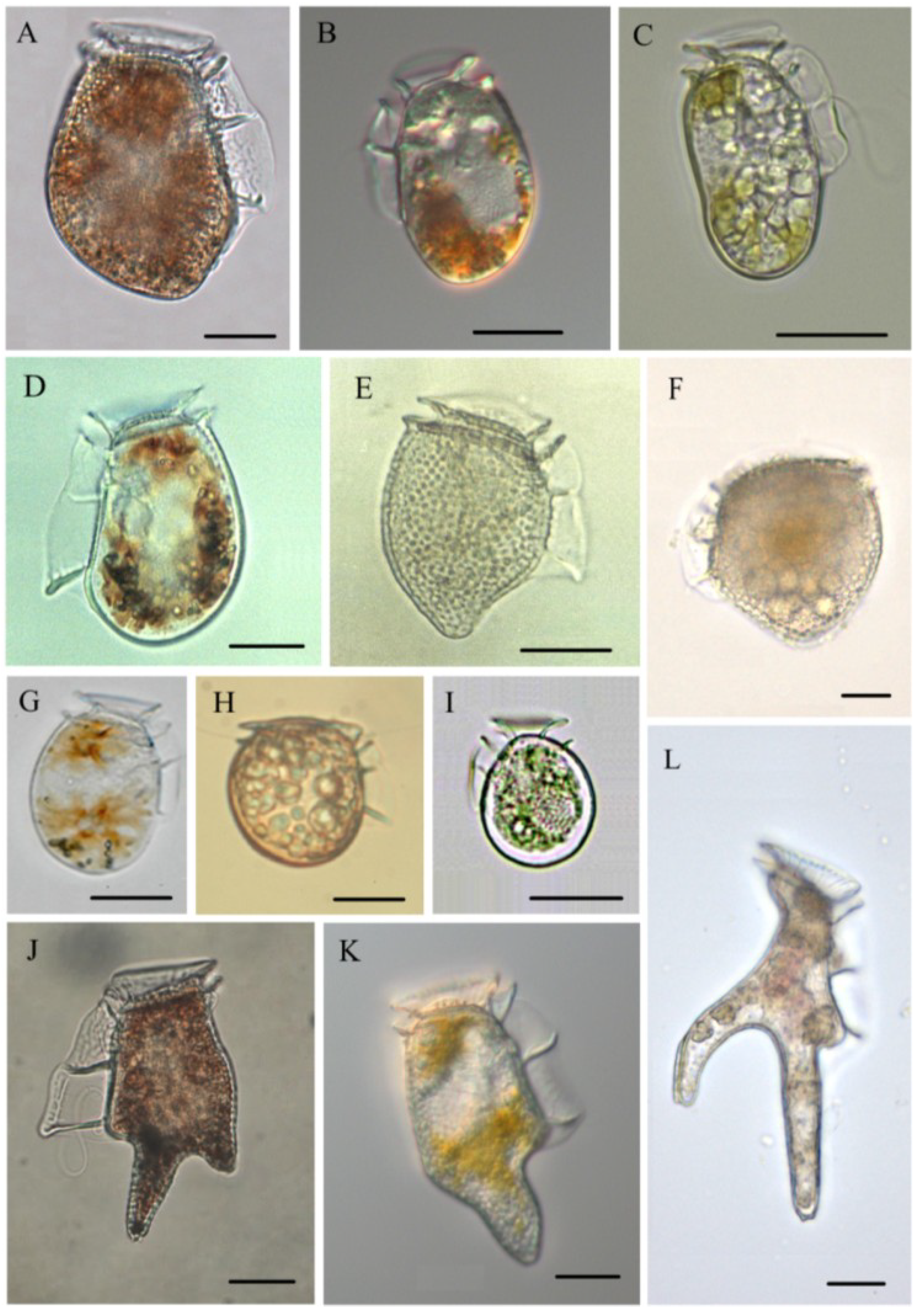
3.1. Dinophysis acuminata (Figure 2B)
3.2. Dinophysis acuta (Figure 2A)
3.3. Dinophysis caudata (Figure 2K)
3.4. Dinophysis fortii (Figure 2D)
3.5. Dinophysis infundibula (Figure 2I)
3.6. Dinophysis miles (Figure 2L)
3.7. Dinophysis norvegica (Figure 2E)
3.8. Dinophysis ovum (Figure 2G)
3.9. Dinophysis sacculus (Figure 2C)
3.10. Dinophysis tripos (Figure 2J)
3.11. Phalacroma mitra (Figure 2F)
3.12. Phalacroma rotundatum (Figure 2H)
4. Worldwide Distribution of DsT Reports Associated with Dinophysis Occurrence
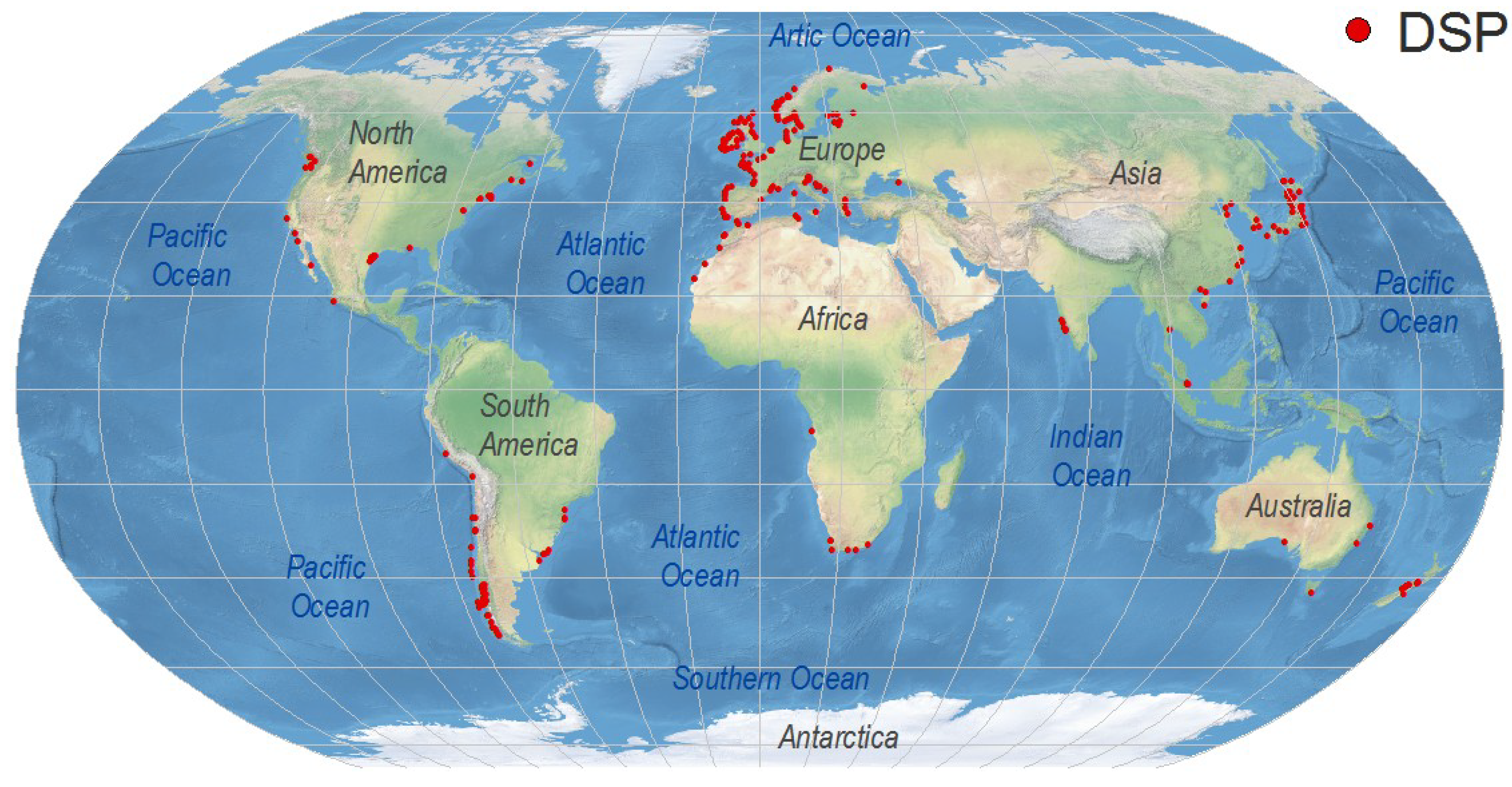
4.1. Europe
4.1.1. Atlantic Coasts and Adjacent Seas
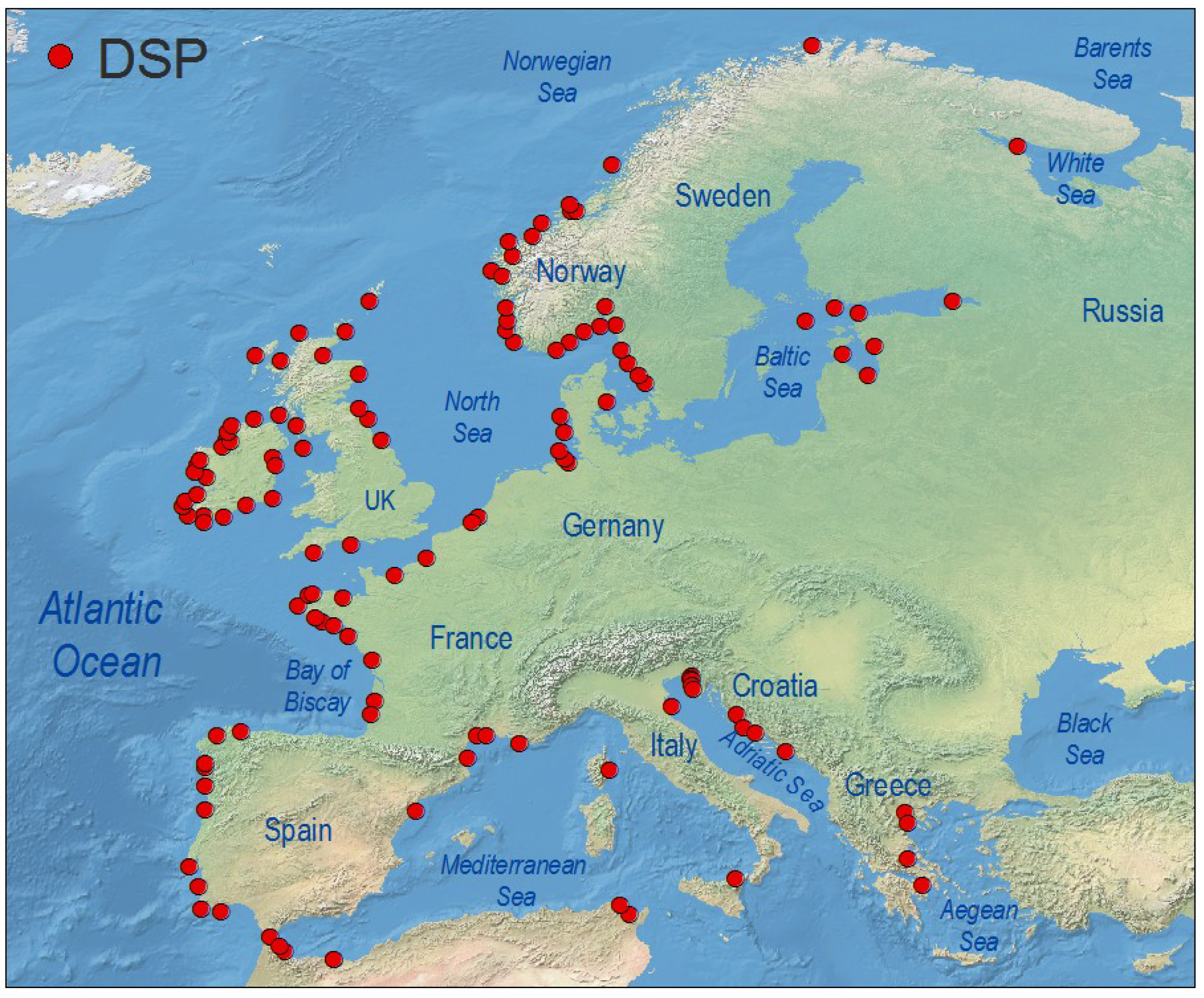
4.1.2. Arctic Ocean, Baltic Sea
4.1.3. Mediterranean Sea
4.2. Africa
4.2.1. Atlantic Coasts
4.2.2. Mediterranean Coasts
4.3. West Pacific and Indian Ocean
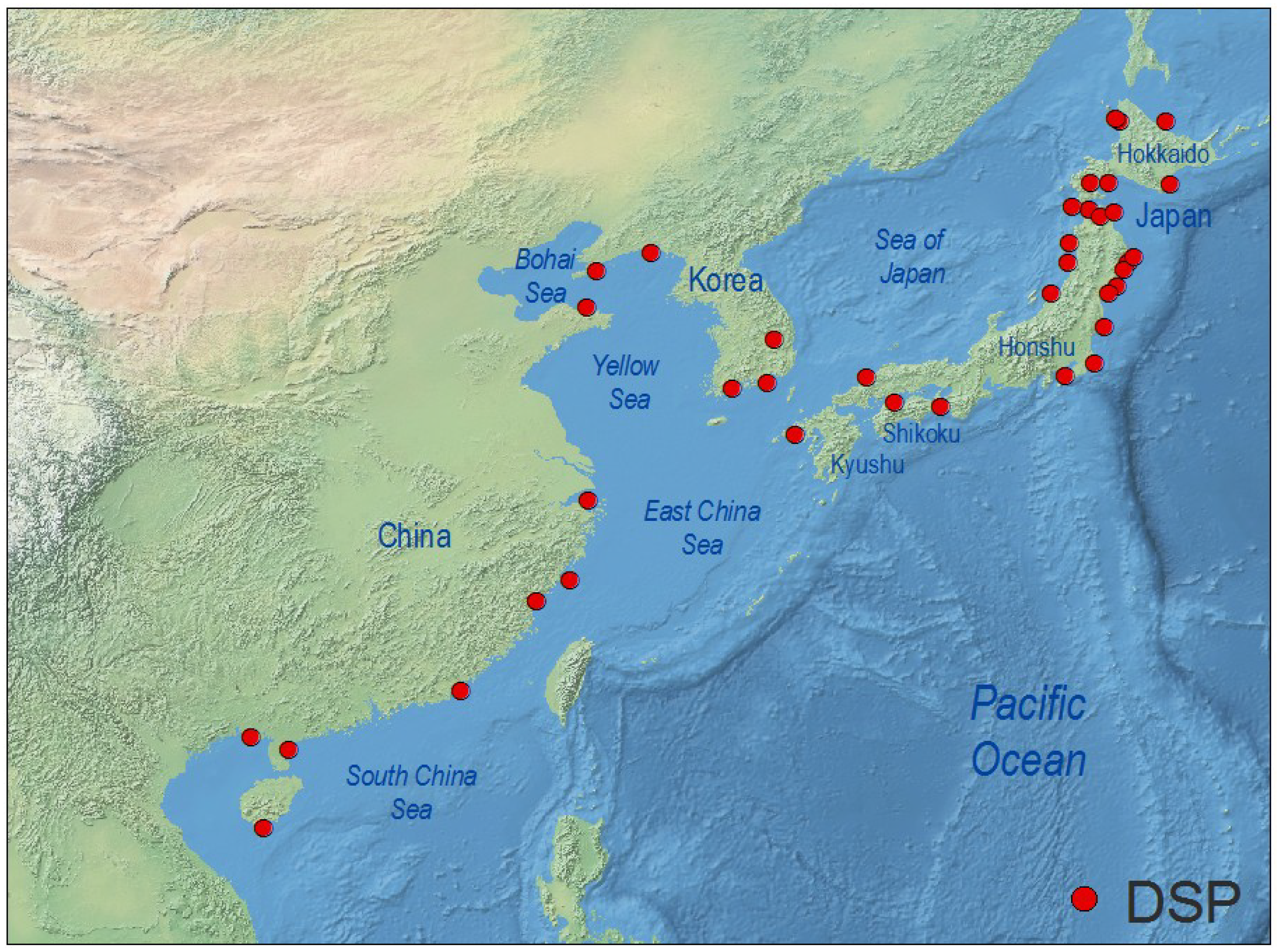
4.4. North America
4.4.1. Eastern North America
4.4.2. Northern Gulf of Mexico
4.4.3. Western North America
4.5. Central America
4.6. South America
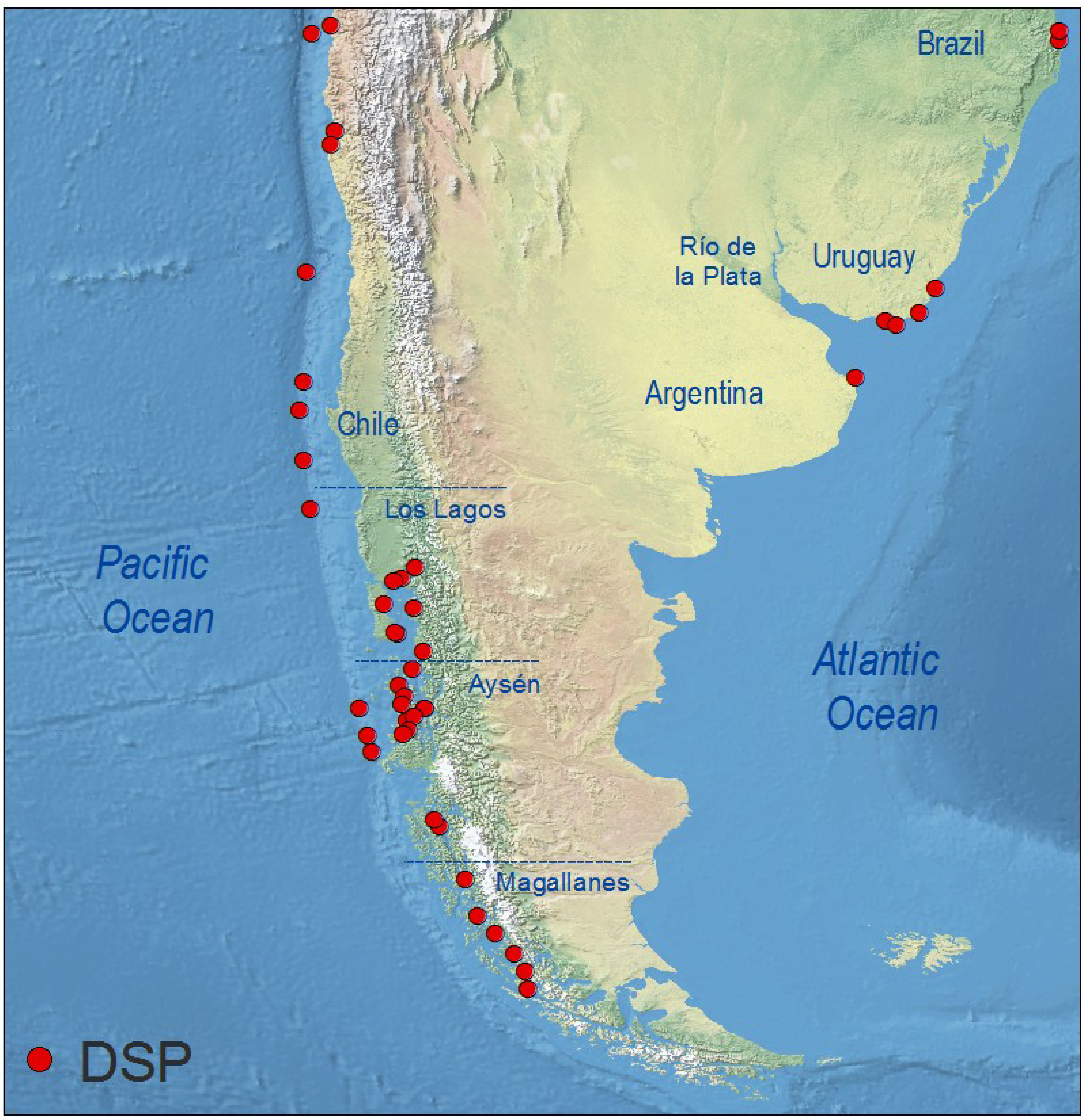
4.6.1. Pacific Coast
4.6.2. Atlantic Coast
4.7. Australia and New Zealand
5. Dynamics of Toxin Production and Accumulation in Natural Populations and in Cultures of Dinophysis Species
5.1. Observations on Field Populations of Dinophysis
5.1.1. Diurnal Variability in Toxin Content Per Cell
5.1.2. Spatial and Seasonal Variability in Toxin Content Per Cell
5.2. Observations in Dinophysis Cultures
6. Uptake, Accumulation, Detoxification, and Enzymatic Transformation of DST in Bivalves
6.1. Toxin Uptake
6.2. Balance between Uptake and Elimination
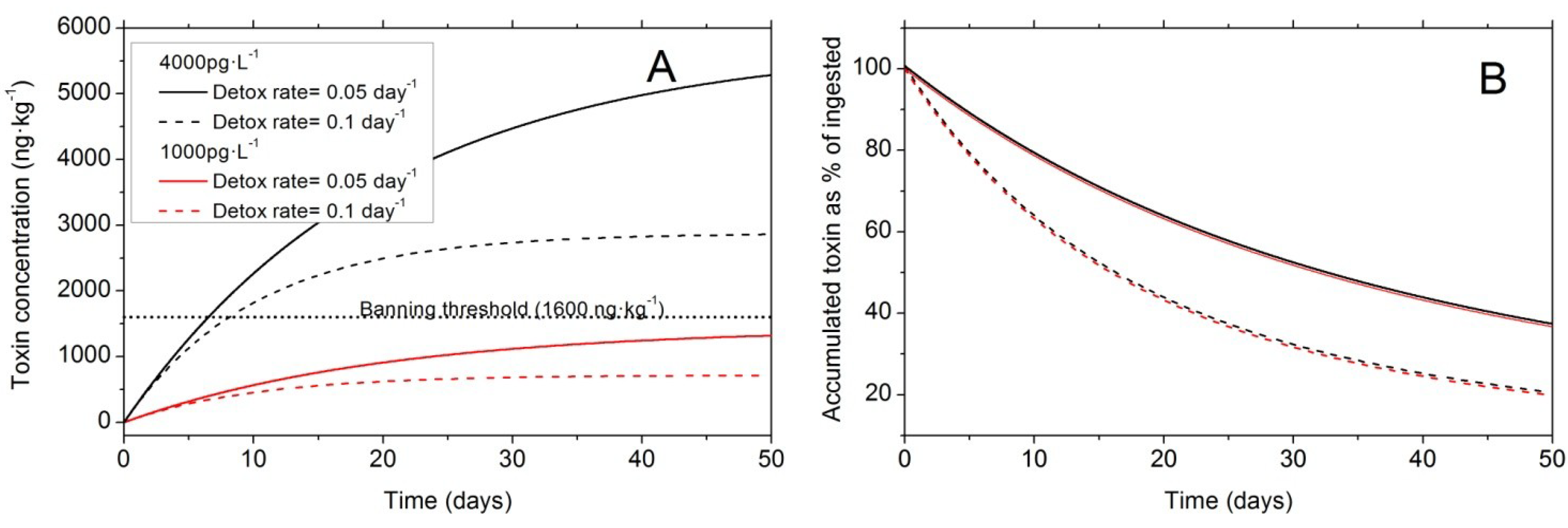
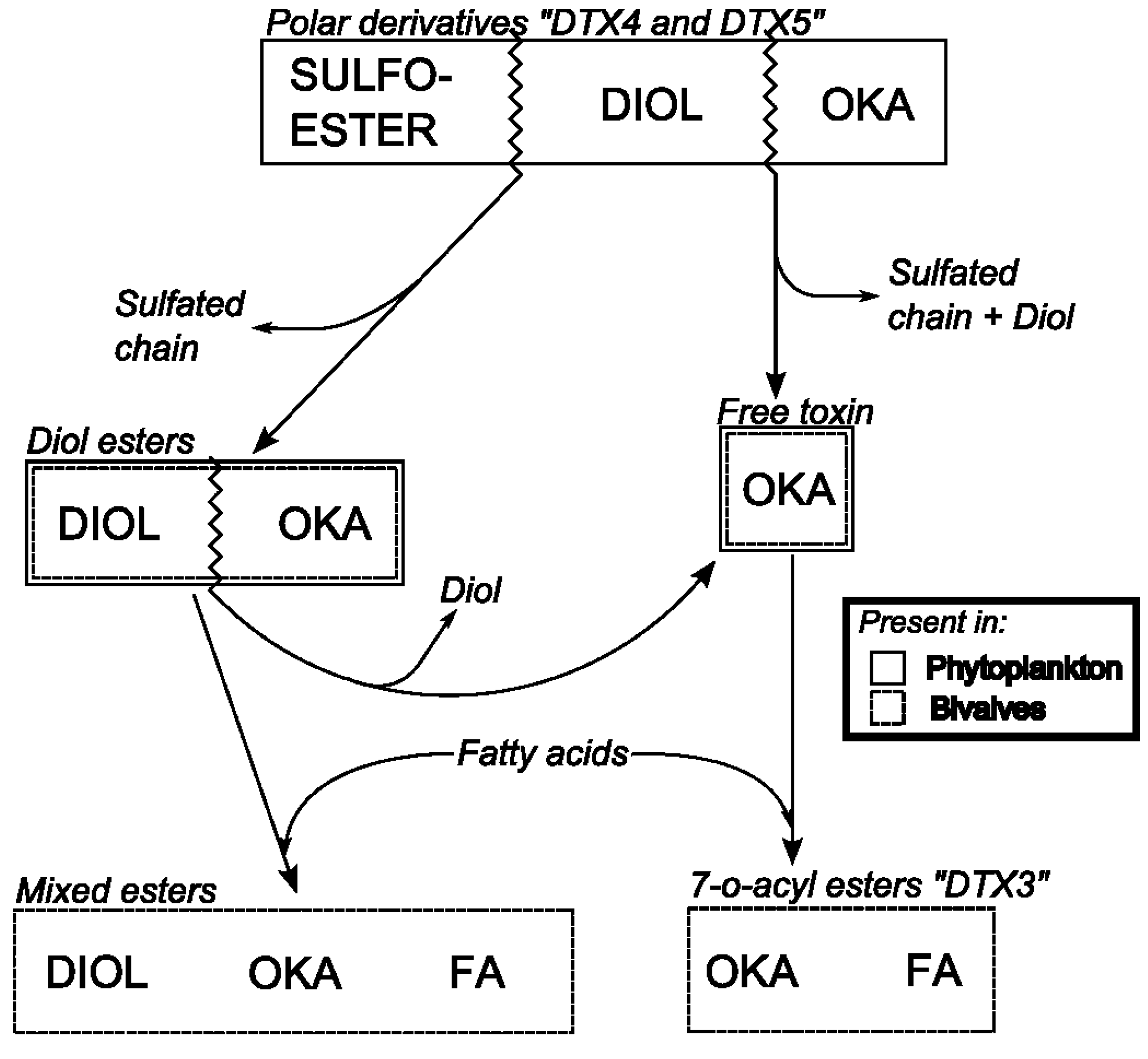
6.3. Biotransformation of Dinophysis Toxins and Derivatives
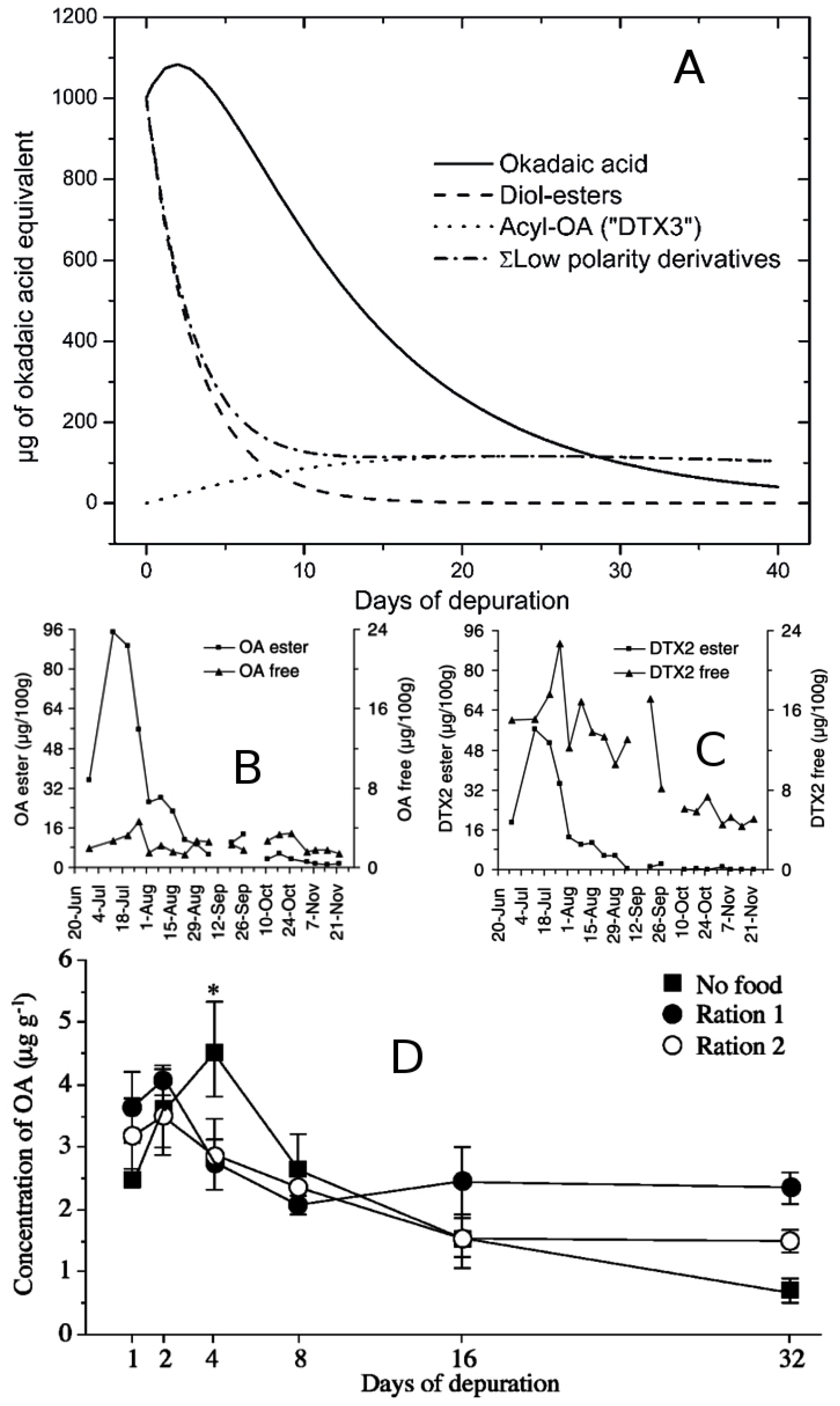
6.4. Changes in Toxin Concentration and Toxicity due to Allometric Processes
7. Assessment of Sample Collection Procedures and Available Methods for Analyses of Dinophysis Toxins
7.1.Collection Procedures
7.1.1. Individually Picked Cells
7.1.2. Net-Hauls and Plankton Concentrates
7.1.3. Toxins in Seawater
7.1.4. Dinophysis Cultures
7.1.5. Shellfish
7.2. DsT Determination Methods
7.2.1. Biological Assays
7.2.1.1. Bioassays
7.2.1.2. Phosphatase Inhibition Assay
7.2.1.3. Cytotoxicity Assays
7.2.1.4. Inmunoassays
7.2.2. Analytical Methods
7.2.2.1. Liquid Chromatography-Fluorescence Detection (LC-FLD)
7.2.2.2. Liquid Chromatography-Mass Spectrometry (LC-MS)
8. Conclusions
Acknowledgments
Conflicts of Interest
References
- Yasumoto, T.; Murata, M.; Oshima, Y.; Sano, M.; Matsumoto, G.; Clardy, J. Diarrhetic shellfish toxins. Tetrahedron 1985, 41, 1019–1025. [Google Scholar] [CrossRef]
- Domínguez, H.J.; Paz, B.; Daranas, A.H.; Norte, M.; Franco, J.M.; Fernández, J.J. Dinoflagellate polyether within the yessotoxin, pectenotoxin and okadaic acid toxin groups: Characterization, analysis and human health implications. Toxicon 2010, 56, 191–217. [Google Scholar] [CrossRef]
- Suzuki, T.; Mackenzie, L.; Stirling, D.; Adamson, J. Conversion of pectenotoxin-2 to pectenotoxin-2 seco acid in the New Zealand scallop, Pecten novaezelandiae. Fish. Sci. 2001, 67, 506–510. [Google Scholar] [CrossRef]
- Suzuki, T.; MacKenzie, L.; Stirling, D.; Adamson, J. Pectenotoxin-2 seco acid: A toxin converted from pectenotoxin-2 by the New Zealand Greenshell mussels, Perna canaliculatus. Toxicon 2001, 39, 507–514. [Google Scholar] [CrossRef]
- García, C.; Truan, D.; Lagos, M.; Santibáñez, A.; Díaz, J.C.; Lagos, N. Metabolic transformation of dinophysistoxin-3 into dinophysistoxin-1 causes human intoxication by consumption of O-acil-derivates dinophysistoxins contaminated shellfish. J. Toxicol. Sci. 2005, 30, 287–296. [Google Scholar] [CrossRef]
- Yasumoto, T.; Oshima, Y.; Yamaguchi, M. Occurrence of a new type of shellfish poisoning in the Tohoku district. Bull. Jpn. Soc. Sci. Fish. 1978, 44, 1249–1255. [Google Scholar] [CrossRef]
- Cohen, P.; Holmes, C.F.B.; Tsukitani, Y. Okadaic acid: A new probe for the study of cellular regulation. Trends Biochem. Sci. 1990, 15, 98–102. [Google Scholar] [CrossRef]
- Fujiki, H.; Suganuma, M. Unique features of the okadaic acid activity class of tumor promoters. J. Carcer Res. Clin. Oncol. 1999, 125, 150–155. [Google Scholar] [CrossRef]
- Burgess, V.; Shaw, G. Pectenotoxins—An issue for public health. A review of their comparative toxicology and metabolism. Environ. Int. 2001, 27, 275–283. [Google Scholar] [CrossRef]
- Quilliam, M.A. Chemical Method for Lipophilic Shellfish Toxins. In Manual on Harmful Marine Microalgae; Hallegraeff, G.M., Anderson, D.M., Cembella, A.D., Eds.; UNESCO Publishing: Paris, France, 2003; pp. 211–245. [Google Scholar]
- Fernández, M.L.; Míguez, A.; Cacho, E.; Martinez, A.; Diogene, J.; Yasumoto, T. Bioensayos con Mamíferos y Ensayos Bioquímicos y Celulares Para la Detección de Ficotoxinas. In Floraciones Algales Nocivas en el Cono Sur Americano; Sar, E.A., Ferrario, M., Reguera, B., Eds.; Instituto Español de Oceanografía: Madrid, Spain, 2002; pp. 79–120. [Google Scholar]
- Blanco, J.; Moroño, A.; Fernández, M.L. Toxic episodes in shellfish, produced by lipophilic phycotoxins: An overview. Revista Galega dos Recursos Mariños 2005, 1, 1–70. [Google Scholar]
- Report of the Joint FAO/IOC/WHO ad hoc Expert Consultation on Biotoxins in Bivalve Molluscs. Available online: http://unesdoc.unesco.org/images/0013/001394/139421e.pdf (accessed on 13 January 2014).
- Aune, T.; Sorby, R.; Yasumoto, T.; Ramstad, H.; Landsverk, T. Comparison of oral and intraperitoneal toxicity of yessotoxin towards mice. Toxicon 2002, 40, 77–82. [Google Scholar] [CrossRef]
- Miles, C.O.; Wilkins, A.L.; Munday, R.; Dines, M.H.; Hawkes, A.D.; Briggs, L.R.; Sandvik, M.; Jensen, D.J.; Cooney, J.M.; Holland, P.T.; et al. Isolation of pectenotoxin-2 from Dinophysis acuta and its conversion to pectenotoxin-2 seco acid, and preliminary assessment of their acute toxicities. Toxicon 2004, 43, 1–9. [Google Scholar] [CrossRef]
- Anonymous. Commission regulation (EC) No. 2074/2005 of the European parliament and of the council of 5 December 2005. Off. J. Eur. Commun. 2005, L338, 27–59. [Google Scholar]
- Codex Alimentarius Commission, Joint FAO/WHO Food Standards Programme. Report of the Twenty-Eighth Session of the Codex Committee on Fish and Fishery Products, Beijing, China, 18–22 September 2006. Circular Letter CL 2006/45-FFP; FAO: Rome, Italy, 2006. [Google Scholar]
- Lawrence, J.E.; Grant, J.; Quilliam, M.A.; Bauder, A.G.; Cembella, A.D. Colonization and growth of the toxic dinoflagellate Prorocentrum lima and associated fouling macroalgae on mussels in suspensed culture. Mar. Ecol. Prog. Ser. 2000, 201, 147–154. [Google Scholar] [CrossRef]
- Maranda, L.; Corwin, S.; Dover, S.; Morton, S.L. Prorocentrum lima (Dinophyceae) in northeastern USA coastal waters II: Toxin load in the epibiota and in shellfish. Harmful Algae 2007, 6, 632–641. [Google Scholar] [CrossRef]
- Gayoso, A.M.; Dover, S.; Morton, S.L.; Busman, M.; Moeller, P.; Fulco, V.K.; Maranda, L. Diarrhetic shellfish poisoning associated with Prorocentrum lima (Dinophyceae) in Patagonian gulfs (Argentina). J. Shellfish Res. 2002, 21, 461–463. [Google Scholar]
- Foden, J.; Purdie, D.A.; Morris, S.; Nascimento, S. Epiphytic abundance and toxicity of Prorocentrum lima populations in the Fleet Lagoon, UK. Harmful Algae 2005, 4, 1063–1074. [Google Scholar] [CrossRef]
- Reguera, B.; Pizarro, G. Planktonic Dinoflagellates Which Produce Polyether Toxins of the Old “DSP Complex”. In Seafood and Freshwater Toxins: Pharmacology, Physiology and Detection, 2nd ed.; Botana, L.M., Ed.; Taylor & Francis: London, UK, 2008; pp. 257–284. [Google Scholar]
- Lembeye, G.; Yasumoto, Y.; Zhao, J.; Fernández, R. DSP Outbreak in Chilean Fjords. In Toxic Phytoplankton Blooms in the Sea; Smayda, T.J., Shimizu, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 525–529. [Google Scholar]
- Van Egmond, H.P.; Aune, T.; Lassus, P.; Speijers, G.J.A.; Waldock, M. Paralytic and diarrhoeic shellfish poisons: Occurrence in Europe, toxicity, analysis and regulation. J. Nat. Toxins 1993, 2, 41–82. [Google Scholar]
- Suzuki, T.; Mitsuya, T.; Imai, M.; Yamasaki, M. DSP toxin contents in Dinophysis fortii and scallops collected at Mutsu Bay, Japan. J. Appl. Phycol. 1997, 8, 509–515. [Google Scholar]
- Harmful Algal Blooms in the PICES Region of the North Pacific. In PICES Scientific Report No. 23; Taylor, F.J.R.; Trainer, V.L. (Eds.) North Pacific Marine Science Organization: Sydney, Canada, 2002; p. 156.
- Lloyd, J.K.; Duchin, J.S.; Borchert, J.; Flores-Quintana, H.; Robertson, A. Diarrhetic Shellfsh Poisoning, Washington, USA, 2011. Emerg. Infect. Dis. 2013, 19, 1314–1316. [Google Scholar] [CrossRef]
- Esenkulova, S.; Haigh, N. First report of Dinophysis species causing Diarrhetic Shellfish Poisoning in British Columbia, Canada. Harmful Algae News UNESCO 2012, 45, 16–17. [Google Scholar]
- Taylor, M.; McIntyre, L.; Ritson, M.; Stone, J.; Bronson, R.; Bitzikos, O.; Rourke, W.; Galanis, E.; Team, O. Outbreak of diarrhetic shellfish poisoning associated with mussels, British Columbia, Canada. Mar. Drugs 2013, 11, 1669–1676. [Google Scholar] [CrossRef]
- Trainer, V.L.; Moore, L.; Bill, B.; Adams, N.; Harrington, N.; Borchert, J.; da Silva, D.; Eberhart, B. Diarrhetic shellfish toxins and other lipophilic toxins of human health concern in Washington State. Mar. Drugs 2013, 11, 1815–1835. [Google Scholar] [CrossRef]
- Yasumoto, T.; Sugawara, W.; Fukuyo, Y.; Oguri, H.; Igarashi, T.; Fujita, N. Identification of Dinophysis fortii as the causative organism of diarrhetic shellfish poisoning in the Tohoku district. Bull. Jpn. Soc. Sci. Fish. 1980, 46, 1405–1411. [Google Scholar] [CrossRef]
- Lee, J.S.; Igarashi, T.; Fraga, S.; Dahl, E.; Hovgaard, P.; Yasumoto, T. Determination of diarrhetic shellfish toxins in various dinoflagellate species. J. Appl. Phycol. 1989, 1, 147–152. [Google Scholar] [CrossRef]
- Park, M.G.; Kim, S.; Kim, H.S.; Myung, G.; Kang, Y.G.; Yih, W. First successful culture of the marine dinoflagellate Dinophysis acuminata. Aquat. Microb. Ecol. 2006, 45, 101–106. [Google Scholar] [CrossRef]
- Kamiyama, T.; Suzuki, T. Production of dinophysistoxin-1 and pectenotoxin-2 by a culture of Dinophysis acuminata (Dinophyceae). Harmful Algae 2009, 8, 312–317. [Google Scholar] [CrossRef]
- Riisgaard, K.; Hansen, P.J. Role of food uptake for photosynthesis, growth and survival of the mixotrophic dinoflagellate Dinophysis acuminata. Mar. Ecol. Prog. Ser. 2009, 381, 51–62. [Google Scholar] [CrossRef]
- Hackett, J.D.; Tong, M.M.; Kulis, D.M.; Fux, E.; Hess, P.; Bire, R.; Anderson, D.M. DSP toxin production de novo in cultures of Dinophysis acuminata (Dinophyceae) from North America. Harmful Algae 2009, 8, 873–879. [Google Scholar] [CrossRef]
- Smith, J.L.; Tong, M.; Fux, E.; Anderson, D.M. Toxin production, retention, and extracellular release by Dinophysis acuminata during extended stationary phase and culture decline. Harmful Algae 2012, 19, 125–132. [Google Scholar] [CrossRef]
- Rial, P.; Garrido, J.L.; Jaén, D.; Rodríguez, F. Pigment composition in three Dinophysis species (Dinophyceae) and the associated cultures of Mesodinium rubrum and Teleaulax amphioxeia. J. Plankton Res. 2013, 35, 433–437. [Google Scholar] [CrossRef]
- Jaén, D.; Mamán, L.; Domínguez, R.; Martín, E. First report of Dinophysis acuta in culture. Harmful Algal News 2009, 39, 1–2. [Google Scholar]
- Rodríguez, F. Personal Communication, Instituto Español de Oceanografia: Centro Oceanografico de Vigo, Spain, 2013.
- Nielsen, L.T.; Krock, B.; Hansen, P.J. Production and excretion of okadaic acid, pectenotoxin-2 and a novel dinophysistoxin from the DSP-causing marine dinoflagellate Dinophysis acuta—Effects of light, food availability and growth phase. Harmful Algae 2013, 23, 34–45. [Google Scholar] [CrossRef]
- Park, M.G.; Park, J.S.; Kim, M.; Yih, W. Plastid dynamics during survival of Dinophysis caudata without its ciliate prey. J. Phycol. 2008, 44, 1154–1163. [Google Scholar] [CrossRef]
- Nishitani, G.; Nagai, S.; Sakiyama, S.; Kamiyama, T. Successful cultivation of the toxic dinoflagellate Dinophysis caudata (Dinophyceae). Plankton Benthos Res. 2008, 3, 78–85. [Google Scholar] [CrossRef]
- Nagai, S.; Nitshitani, G.; Tomaru, Y.; Sakiyama, S.; Kamiyama, T. Predation by the toxic dinoflagellate Dinophysis fortii on the ciliate Myrionecta rubra and observation of sequestration of ciliate chloroplasts. J. Phycol. 2008, 44, 909–922. [Google Scholar] [CrossRef]
- Nishitani, G.; Nagai, S.; Takano, Y.; Sakiyama, S.; Baba, K.; Kamiyama, T. Growth characteristics and phylogenetic analysis of the marine dinoflagellate Dinophysis infundibulus (Dinophyceae). Aquat. Microb. Ecol. 2008, 52, 209–221. [Google Scholar] [CrossRef]
- Mafra, L.L., Jr. Detecção de Toxinas e Efeitos Tóxicos em Microalgas Cultivadas ou Coletadas na Costa sul do Brasil: Estado da Arte em 2013. In Livro de Resumos da Reunião Latino-Americana sobre Algas Nocivas, Santa Catarina, Brasil, 7–9 de Outubro, 2013; (in Portuguese). Proença, L.A.O., Renan de Souza, K., Eds.; Laboratory of Research and Monitoring of Harmful Algae and Phycotoxins: Santa Catarina, Brazil, 2013; p. 18. [Google Scholar]
- Riobó, P.; Reguera, B.; Franco, J.M.; Rodríguez, F. First report of the toxin profile of Dinophysis sacculus Stein from LC-MS analysis of laboratory cultures. Toxicon 2013, 76, 221–224. [Google Scholar] [CrossRef]
- Rodríguez, F.; Escalera, L.; Reguera, B.; Rial, P.; Riobó, P.; Silva, T.J. Morphological variability, toxinology and genetics of the dinoflagellate Dinophysis tripos (Dinophysiaceae, Dinophysiales). Harmful Algae 2012, 13, 26–33. [Google Scholar] [CrossRef]
- Korringa, P.; Roskam, R.T. An Inusual Case of Mussel Poisoning; C.M./Shellfish Committee, International Council for the Exploration of the Sea: Copenhagen, Denmark, 1961; p. 2. [Google Scholar]
- Guzmán, L.; Campodonico, I. Marea roja en la región de Magallanes. Publ. Inst. Pat. Ser. Mon. 1975, 9, 44. [Google Scholar]
- Lembeye, G.; Campodonico, I.; Guzmán, L.; Kiguel, C. Intoxicaciones por Consumo de Mariscos del Estero de Reloncavi (X Región), Chile (1970–1980). In Resumen Jornadas de Ciencias del Mar, Montemar, 12–14 Agosto 1981; (in Spanish). Montemar: Valparaíso, Chile, 1981; p. 42. [Google Scholar]
- Kat, M. The Occurrence of Prorocentrum Species and Coincidential Gastroenteritis Illness of Mussels Consumers. In Toxic Dinoflagellate Blooms: Proceedings of the Second International Conference on Toxic Dinoflagellate Blooms; Taylor, D.L., Seliger, H.H., Eds.; Elsevier/North-Holland: Key Biscayne, FL, USA, 1979; pp. 215–220. [Google Scholar]
- Moestrup, Ø.; Akselman, R.; Cronberg, G.; Elbraechter, M.; Fraga, S.; Halim, Y.; Hansen, G.; Hoppenrath, M.; Larsen, J.; Lundholm, N.; et al. IOC-UNESCO Taxonomic Reference List of Harmful MicroAlgae. Available online: http://www.marinespecies.org/HAB (accessed on 6 September 2013).
- Yasumoto, Y. Personal Communication, Japan Food Research Laboratory, Tama Laboratory: Nagayama, Tokyo, Japan, 2011.
- Yasumoto, T.; Oshima, Y.; Yamaguchi, M. Occurrence of a New Type of Toxic Shellfish in Japan and Chemical Properties of the Toxin. In Toxic Dinoflagellate Blooms; Taylor, D.L., Seliger, H.W., Eds.; Elsevier: New York, NY, USA, 1979; pp. 395–398. [Google Scholar]
- Tachibana, K.; Scheuer, P.; Tsukitani, Y.; Kikuchi, H.; Enden, V.; Clardy, J.; Gopichand, Y.; Schmitz, F. Okadaic acid, a cytotoxic polyether from two marine sponges of the genus Halichondria. J. Am. Chem. Soc. 1981, 103, 2469–2471. [Google Scholar] [CrossRef]
- Murata, M.; Shimatani, M.; Sugitani, H.; Oshima, Y.; Yasumoto, T. Isolation and structural elucidation of the causative toxin of diarrhetic shellfish poisoning. Bull. Jpn. Soc. Sci. Fish. 1982, 48, 549–552. [Google Scholar] [CrossRef]
- Campos, M.J.; Fraga, S.; Mariño, J.; Sánchez, J. Red Tide Monitoring Programme in NW Spain; Report of 1977–1981; International Council for the Exploration of the Sea: Copenhagen, Denmark, 1982. [Google Scholar]
- Alzieu, C.; Lassus, P.; Maggi, P.; Poggi, R.; Ravoux, G. Contamination des coquillages des cotes bretonnes et normandes par une algue unicelulaire toxique (Dinophysis acuminata). Evolution, nature, consequences. Rapp. Techn. ISTPM 1983, 4, 1–30. [Google Scholar]
- Lassus, P.; Bardouil, M.; Truquet, I.; le Baut, C.; Pierre, M.J. Dinophysis acuminata Distribution and Toxicity along the Southern Brittany Coast (France): Correlation with Hydrological Parameters. In Toxic Dinoflagellates; Anderson, D.M., White, A.W., Baden, D.G., Eds.; Elsevier: New York, NY, USA, 1985; pp. 159–164. [Google Scholar]
- Kat, M. Diarrhetic mussel poisoning in the Netherlands related to the dinoflagellate Dinophysis acuminata. Antonie van Leeuenhoek 1983, 49, 417–427. [Google Scholar]
- Kat, M. Dinophysis acuminata Blooms, the Distinct Cause of Dutch Mussel Poisoning. In Toxic Dinoflagellates; Anderson, D.M., White, A.W., Baden, D.G., Eds.; Elsevier: New York, NY, USA, 1985; pp. 73–78. [Google Scholar]
- Underdahl, B.; Yndestad, M.; Aune, T. DSP. Intoxication in Norway and Sweden, Autumn 1984–Spring 1985. In Toxic Dinoflagellates; Anderson, D.M., White, A.W., Baden, D.G., Eds.; Elsevier: New York, NY, USA, 1985; pp. 489–494. [Google Scholar]
- Krogh, P.; Edler, L.; Graneli, E.; Nyman, U. Outbreak of Diarrheic Shellfish Poisoning on the West Coast of Sweden. In Toxic Dinoflagellates; Anderson, D.M., White, A.W., Baden, D.G., Eds.; Elsevier: New York, NY, USA, 1985; pp. 501–503. [Google Scholar]
- Dahl, E.; Yndestad, M. Diarrhetic Shellfish Poisoning (DSP) in Norway in the Autumn 1984 Related to the Occurence of Dinophysis spp. In Toxic Dinoflagellates; Anderson, D.M., White, A.W., Bden, D.G., Eds.; Elsevier: New York, NY, USA, 1985; pp. 495–500. [Google Scholar]
- Lawley, R. Okadaic Acid Toxins. Food Safety Watch. 30 January 2013. Available online: http://www.foodsafetywatch.org/factsheets/okadaic-acid-toxins/ (accessed on 6 January 2014).
- Lindahl, O. A dividable hose for Phytoplankton Sampling. In Report of the ICES Working Group on Exceptional Algal Blooms; International Council for the Exploration of the Sea: Copenhagen, Denmark, 1986. [Google Scholar]
- Yasumoto, T.; Murata, M.; Lee, J.S.; Torigoe, K. Polyeter Toxins Produced by Dinoflagellates. In Mycotoxins and Phycotoxins; Natori, S., Hashimoto, K., Ueno, Y., Eds.; Elsever: Amsterdam, The Netherlands, 1989; pp. 375–382. [Google Scholar]
- Lee, J.S.; Yanagi, T.; Kenma, R.; Yasumoto, T. Fluorometric determination of diarrhetic shellfish toxins by high-performance liquid chomatography. Agric. Biol. Chem. 1987, 51, 877–881. [Google Scholar] [CrossRef]
- Lee, J.S.; Tangen, K.; Dahl, E.; Hovgaard, P.; Yasumoto, T. Diarrhetic shellfish toxins in Norwegian mussels. Nipppon Suisan Gakkaishi 1989, 54, 1953–1957. [Google Scholar]
- Kumagai, M.; Yanagi, T.; Murata, M.; Yasumoto, T.; Kat, M.; Lassus, P.; Rodríguez-Vázquez, J.A. Okadaic acid as the causative toxin of Diarrhetic Shellfish Poisoning in Europe. Agric. Biol. Chem. 1986, 50, 2853–2857. [Google Scholar] [CrossRef]
- Hu, T.; Doyle, J.; Jackson, D.M.; Marr, J.; Nixon, E.; Pleasance, S.; Quilliam, M.A.; Walter, J.A.; Wright, J.L.C. Isolation of a new diarrhetic shellfish poison from Irish mussels. Chem. Commun. 1992, 1992, 39–44. [Google Scholar]
- Gago, A.; Rodríguez-Vázquez, J.A.; Thibault, P.; Quilliam, M.A. Simultaneus occurrence of diarrhetic and paralytic shellfish poisoning toxins in Spanish mussels in 1993. Nat. Toxins 1996, 4, 72–79. [Google Scholar] [CrossRef]
- Vale, P.; Sampayo, M.A. DTX-2 in Portuguese Bivalves. In Harmful and Toxic Algal Blooms; Yasumoto, T., Oshima, Y., Fukuyo, Y., Eds.; IOC of UNESCO: Sendai, Japan, 1996; pp. 539–542. [Google Scholar]
- Blanco, J.; Fernández, M.L.; Mariño, J.; Reguera, B.; Miguez, A.; Maneiro, J.; Cacho, E.; Martínez, A. From Dinophysis spp. Toxicity to DSP Outbreaks: A preliminary Model of Toxin Accumulation in Mussels. In Harmful Marine Algal Blooms; Lassus, P., Arzul, G., Erard-Le Denn, E., Gentien, P., Marcaillou-Le Baut, C., Eds.; Lavoisier: Paris, France, 1995; pp. 777–782. [Google Scholar]
- Vale, P.; Sampayo, M.A. Dinophysistoxin-2: A rare diarrhoeic toxin associated with Dinophysis acuta. Toxicon 2000, 38, 1599–1606. [Google Scholar] [CrossRef]
- James, K.J.; Bishop, A.G.; Healy, B.M.; Roden, C.; Sherlock, I.R.; Twohig, M.; Draisci, R.; Giannetti, L.; Lucentini, L. Efficient isolation of the rare diarrhoeic shellfish toxin, dinophysistoxin-2, from marine phytoplankton. Toxicon 1999, 37, 343–357. [Google Scholar] [CrossRef]
- Fernández, M.L.; Reguera, B.; Ramilo, I.; Martínez, A. Toxin Content of Dinophysis acuminata, D. acuta and D. caudata from the Galician Rías Baixas. In Harmful Algal Blooms; Hallegraeff, G.M., Blackburn, S.I., Bolch, C.J., Lewis, R.J., Eds.; Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 2001; pp. 360–363. [Google Scholar]
- Marr, J.; Hu, T.; Pleasance, S.; Quilliam, M.A.; Wright, J.L.C. Detection of a new 7-O-acyl derivates of diarrhetic shellfish poisoning toxins by liquid chromatography-mass spectometry. Toxicon 1992, 30, 1621–1630. [Google Scholar] [CrossRef]
- Vale, P.; Sampayo, M.A. Esters of okadaic acid and Dinophysistoxin-2 Portuguese bivalves related to human poisonings. Toxicon 1999, 37, 1109–1121. [Google Scholar] [CrossRef]
- Quilliam, M.A.; Hardstaff, W.R.; Ishida, N.; McLachlan, J.L.; Reeves, A.R.; Ross, N.W.; Windust, A.J. Production of Diarrhetic Shellfish Poisoning (DSP) Toxins by Prorocentrum lima in Culture and Development of Analytical Methods. In Harmful and Toxic Algal Blooms; Yasumoto, T., Oshima, Y., Fukuyo, Y., Eds.; IOC of UNESCO: Sendai, Japan, 1996; pp. 289–292. [Google Scholar]
- McMahon, T.; Silke, J. West coast of Ireland; winter toxicity of unknown aetiology in mussels. Harmful Algae News 1996, 14, 2. [Google Scholar]
- Satake, M.; Ofuji, K.; Naoki, H.; James, K.J.; Furey, A.; McMahon, T.; Silke, J.; Yasumoto, T. Azaspiracid, a new marine toxin having unique spiro ring assemblies, isolated from Irish mussels, Mytilus edulis. J. Am. Chem. Soc. 1998, 120, 9967–9968. [Google Scholar]
- James, K.J.; Furey, A.; Satake, M.; Yasumoto, T. Azaspiracid Poisoning (AZP): A New Shellfish Toxic Syndrome in Europe. In Harmful Algal Blooms; Hallegraeff, G., Blackburn, S., Lewis, R., Bolch, C., Eds.; Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 2000; pp. 250–253. [Google Scholar]
- James, K.J.; Furey, A.; Lehane, M.; Ramstad, H.; Aune, T.; Hovgaard, P.; Morris, S.; Higman, W.; Satake, M.; Yasumoto, T. First evidence of an extensive northern European distribution of azaspiracid poisoning (AZP) toxins in shellfish. Toxicon 2002, 40, 909–915. [Google Scholar] [CrossRef]
- Magdalena, A.B.; Lehane, M.; Krys, S.; Fernández, M.L.; Furey, A.; James, K.J. The first identification of azaspiracids in shellfish from France and Spain. Toxicon 2003, 42, 105–108. [Google Scholar] [CrossRef]
- James, K.J.; Moroney, C.; Roden, C.; Satake, M.; Yasumoto, T.; Lehane, M.; Furey, A. Ubiquitous benign alga emerges as the cause of shellfish contamination responsible for the human toxic syndrome, azaspiracid poisoning. Toxicon 2003, 41, 145–151. [Google Scholar] [CrossRef]
- Moran, S.; Silke, J.; Cusack, C.; Hess, P. Correlations between known toxic phytoplankton species and toxin levels in shellfish in Irish waters 2002–2006. In Proceedings of the 6th International Conference on Molluscan Shellfish Safety, Blenheim, New Zealand, 5–7 October 2005.
- Krock, B.; Tillmann, U.; John, U.; Cembella, A.D. Characterization of azaspiracids in plankton size-fractions and isolation of an azaspiracid-producing dinoflagellate from the North Sea. Harmful Algae 2009, 8, 254–263. [Google Scholar] [CrossRef]
- Tillmann, U.; Elbrächter, M.; Krock, B.; John, U.; Cembella, A. Azadinium spinosum gen. et sp. nov. (Dinophyceae) identified as a primary producer of azaspiracid toxins. Eur. J. Phycol. 2009, 44, 63–79. [Google Scholar] [CrossRef]
- Satake, M.; MacKenzie, A.L.; Yasumoto, Y. Identification of Protoceratium reticulatum as the biogenetic origin of yessotoxin. Nat. Toxins 1997, 5, 164–167. [Google Scholar]
- MacKenzie, L.; Truman, P.; Satake, M.; Yasumoto, T.; Adamson, J.; Mounfort, D.; White, D. Dinoflagellate Blooms and Associated DSP—Toxicity in Shellfish in New Zeland. In Harmful Algae; Reguera, B., Blanco, J., Fernández, M.L., Wyatt, T., Eds.; Xunta de Galicia and IOC of UNESCO: Santiago de Compostela, Spain, 1998; pp. 74–77. [Google Scholar]
- Paz, B.; Riobó, P.; Fernández, M.L.; Fraga, S.; Franco, J.M. Production and release of yessotoxins by the dinoflagellates Protoceratium reticulatum and Lingulodinium polyedrum in culture. Toxicon 2004, 44, 251–258. [Google Scholar] [CrossRef]
- Rhodes, L.; McNabb, P.; de Salas, M.; Briggs, L.; Beuzenberg, V.; Gladstone, M. Yessotoxin production by Gonyaulax spinifera. Harmful Algae 2006, 5, 148–155. [Google Scholar] [CrossRef]
- Draisci, R.; Lucentini, L.; Giannetti, L.; Boria, P.; Poletti, R. First report of pectenotoxin-2 (PTX-2) in algae (Dinophysis fortii) related to seafood poisoning in Europe. Toxicon 1996, 34, 923–935. [Google Scholar] [CrossRef]
- Daiguji, M.; Satake, M.; James, K.J.; Bishop, A.; MacKenzie, L.; Naoki, H.; Yasumoto, T. Structures of new pectenotoxin analogs, pectenotoxin-2 seco acid and 7-epi-pectenotoxin-2 seco acid, isolated from a dinoflagellate and greenshell mussels. Chem. Lett. 1998, 7, 653–654. [Google Scholar]
- Cembella, A.D.; Krock, B. Cyclic Imine Toxins: Chemistry, Biogeography, Biosynthesis, and Pharmacology. In Seafood and Freshwater Toxins: Pharmacology, Physiology and Detection, 2nd ed.; Botana, L., Ed.; Taylor & Francis: London, UK, 2008; pp. 561–580. [Google Scholar]
- Hastrup-Jensen, M.; Daugbjerg, N. Molecular phylogeny of selected species of the order Dinophysiales (Dinophyceae)—testing the hypothesis of a dinophysioid radiation. J. Phycol. 2009, 45, 1136–1152. [Google Scholar] [CrossRef]
- Gómez, F.; López-García, P.; Moreira, D. Molecular phylogeny of dinophysoid dinoflagellates: The systematic position of Oxyphysis oxytoxoides and the Dinophysis hastata group (Dinophysales, Dinophyceae). J. Phycol. 2011, 47, 393–406. [Google Scholar] [CrossRef]
- Reguera, B.; Velo-Suárez, L.; Raine, R.; Park, M. Harmful Dinophysis species: A review. Harmful Algae 2012, 14, 87–106. [Google Scholar] [CrossRef]
- González-Gil, S.; Pizarro, G.; Paz, B.; Velo-Suárez, L.; Reguera, B. Considerations on the toxigenic nature and prey sources of Phalacroma rotundatum. Aquat. Microb. Ecol. 2011, 64, 197–203. [Google Scholar] [CrossRef]
- France, J.; Mozetic, P. Ecological characterization of toxic phytoplankton species (Dinophysis spp., Dinophyceae) in Slovenian mariculture areas (Gulf of Trieste, Adriatic Sea) and the implications for monitoring. Mar. Pollut. Bull. 2006, 52, 1504–1516. [Google Scholar] [CrossRef]
- Madigan, T.L.; Lee, K.G.; Padula, D.J.; McNabb, P.; Pointon, A.M. Diarrhetic shellfish poisoning (DSP) toxins in South Australian shellfish. Harmful Algae 2006, 5, 119–123. [Google Scholar] [CrossRef]
- MacKenzie, L. Does Dinophysis (Dinophyceae) have a sexual life cycle? J. Phycol. 1992, 28, 399–406. [Google Scholar]
- Pitcher, G.C.; Calder, D. Harmful algal blooms of the southern Benguela Current: A review and appraisal of monitoring from 1989 to 1997. S. Afr. J. Mar. Sci. 2000, 22, 255–271. [Google Scholar]
- Weber, R.; Silver, M. Detection of DSP toxins on the US west coast. In Harmful Algal Blooms 2000, Proceedings of the IX International Conference on Harmful Algal Blooms, Tasmania, Australia, 7–11 February 2000; Hallegraeff, G.M., Blackburn, S.I., Bolch, C.J., Lewis, R.J., Eds.; Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 2001; p. 245. [Google Scholar]
- Wallace, G.M. Diarrhetic shellfish toxins in Tasmanian coastal waters: Causative dinoflagellate organisms, dissolved toxins and shellfish depuration. Ph.D. Thesis, University of Tasmania, Hobart, Tasmania, June 2011. [Google Scholar]
- Hattenrath-Lehmann, T.K.; Marcoval, M.A.; Berry, D.L.; Fire, S.; Wang, Z.; Morton, S.L.; Gobler, C.J. The emergence of Dinophysis acuminata blooms and DSP toxins in shellfish in New York waters. Harmful Algae 2013, 26, 33–44. [Google Scholar] [CrossRef]
- Deeds, J.R.; Wiles, K.; Heideman VI, G.B.; White, K.D.; Abraham, A.; First, U. First U.S. report of shellfish harvesting closures due to confirmed okadaic acid in Texas Gulf coast oysters. Toxicon 2010, 55, 1138–1146. [Google Scholar] [CrossRef]
- Clément, A.; Lembeye, G.; Lassus, P.; le Baut, C. Bloom superficial no tóxico de Dinophysis cf. acuminata en el estero de Reloncaví. (in Spanish). In Proceedings of the XIV Marine Science Session and I Chilean Salmon Cultivation Session, Puerto Montt, Chile, 23–25 May 1994; 1994; p. 83. [Google Scholar]
- Alves-de-Souza, C.; Varela, D.; Contreras, C.; de La Iglesia, P.; Fernández, P.; Hipp, B.; Hernández, C.; Riobó, P.; Reguera, B.; Franco, J.M.; et al. Seasonal variability of Dinophysis spp. and Protoceratium reticulatum associated to lipophilic shellfish toxins in a strongly stratified Chilean fjord. Deep Sea Res. II Top. Stud. Oceanogr. 2013. [Google Scholar] [CrossRef]
- Proença, L.A.O.; Tamanaha, M.S.; Schramm, M.A.; Alves, T.P.; Fonseca, R. Recurrent DSP outbreaks in Southern Brazil. In Harmful Algae 2008, Proceedings of the 13th International Conference on Harmful Algae, Hong Kong, 3–7 November 2008; Ho, K.C., Zhou, M., Qi, Y., Eds.; International Society For the Study of Harmful Algae: Copenhagen, Denmark, 2010; p. 110. [Google Scholar]
- Méndez, S.; Ferrari, G. Floraciones algales nocivas en Uruguay: Antecedentes, proyectos en curso y revisión de resultados. In Floraciones Algales Nocivas en el Cono Sur Americano; (in Spanish). Sar, E.A., Ferrario, M.E., Reguera, B., Eds.; Instituto Español de Oceanografía: Madrid, Spain, 2002; pp. 271–288. [Google Scholar]
- Sar, E.A.; Sunesen, I.; Lavigne, A.; Goya, A. Dinophysis spp. asociadas a detección de toxinas diarreicas (DSTs) en moluscos y a intoxicación diarreica en humanos (Provincia de Buenos Aires, Argentina). Rev. Biol. Mar. Oceanogr. 2010, 45, 451–460. (in Galician). [Google Scholar] [CrossRef]
- Lassus, P.; Bardouil, M. Le complexe Dinophysis acuminata: Identification des espèces le long des côtes Françaises. Cryptogam. Algol. 1991, 12, 1–9. (in French). [Google Scholar]
- Zingone, A.; Montresor, M.; Marino, D. Morphological variability of the potentially toxic dinoflagellate Dinophysis sacculus (Dinophyceae) and its taxonomic relationship with D. pavillardii and D. acuminata. Eur. J. Phycol. 1998, 33, 259–273. [Google Scholar] [CrossRef]
- Raho, N.; Pizarro, G.; Escalera, L.; Reguera, B.; Marin, I. Morphology, toxin composition and molecular analysis of Dinophysis ovum Schutt, a dinoflagellate of the “Dinophysis acuminata complex”. Harmful Algae 2008, 7, 839–848. [Google Scholar] [CrossRef]
- Nielsen, L.T.; Krock, B.; Hansen, P.J. Effects of light and food availability on toxin production, growth and photosynthesis in Dinophysis acuminata. Mar. Ecol. Prog. Ser. 2012, 471, 37–50. [Google Scholar] [CrossRef]
- Jørgensen, K.; Andersen, P. Relation between the concentration of Dinophysis acuminata and diarrheic shellfish poisoning toxins in blue mussels (Mytilus edulis) during a toxic episode in the Limfjord (Denmark), 2006. J. Shellfish Res. 2007, 26, 1081–1087. [Google Scholar] [CrossRef]
- Miles, C.O.; Wilkins, A.L.; Samdal, I.A.; Sandvik, M.; Petersen, D.; Quilliam, M.A.; Naustvoll, L.J.; Jensen, D.J.; Cooney, J.M. A novel pectenotoxin, PTX-12, in Dinophysis sp. and shellfish from Norway. Chem. Res. Toxicol. 2004, 17, 1423–1433. [Google Scholar] [CrossRef]
- MacKenzie, L.; Beuzenberg, V.; Holland, P.; McNabb, P.; Suzuki, T.; Selwood, A. Pectenotoxin and okadaic acid-based toxin profiles in Dinophysis acuta and Dinophysis acuminata from New Zealand. Harmful Algae 2005, 4, 75–85. [Google Scholar] [CrossRef]
- Palma, A.S.; Vilarinho, M.G.; Moita, M.T. Interannual Trends in the Longshore Distribution of Dinophysis off the Portuguese Coast. In Harmful Algae; Reguera, B., Blanco, J., Fernández, M.L., Wyatt, T., Eds.; Xunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO: Santiago de Compostela, Spain, 1998; pp. 124–127. [Google Scholar]
- Reguera, B.; Bravo, I.; Fraga, S. Autoecology and some life history stages of Dinophysis acuta Ehrenberg. J. Plankton Res. 1995, 17, 999–1015. [Google Scholar] [CrossRef]
- Aune, T.; Torgesen, T.; Arff, J.; Tangen, K. Detection of Pectenotoxin in Norwegian Blue Mussels (Mytilus edulis). In Harmful Algae 2002; Steidinger, K.A., Landsberg, J.H., Tomas, C.R., Vargo, G.A., Eds.; Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography and Intergovernmental Oceanographic Commission of UNESCO: St. Petersburg, FL, USA, 2004; pp. 306–308. [Google Scholar]
- Lindahl, O.; Lundve, B.; Johansen, M. Toxicity of Dinophysis spp. in relation to population abundance and environmental condition on the Swedish west coast. Harmful Algae 2007, 6, 218–231. [Google Scholar]
- Dahl, E.; Johannessen, T. Relationship between occurrence of Dinophysis species (Dinophyceae) and shellfish toxicity. Phycologia 2001, 40, 223–227. [Google Scholar] [CrossRef]
- Bayne, B.L.; Iglesias, J.I.P.; Hawkins, A.J.S.; Navarro, E.; Heral, M.; Deslous-Paoli, J.M. Feeding behaviour of the mussel, Mytilus edulis: Responses to variations in quantity and organic content of the seston. J. Mar. Biol. Assoc. UK 1993, 73, 813–829. [Google Scholar] [CrossRef]
- Fernández-Puente, P.; Fidalgo Sáez, M.J.; Hamilton, B.; Furey, A.; James, K.J. Studies of polyether toxins in the marine phytoplankton, Dinophysis acuta, in Ireland using multiple tandem mass spectrometry. Toxicon 2004, 44, 919–926. [Google Scholar] [CrossRef]
- Pizarro, G.; Paz, B.; Gonzalez-Gil, S.; Franco, J.M.; Reguera, B. Seasonal variability of lipophilic toxins during a Dinophysis acuta bloom in Western Iberia: Differences between picked cells and plankton concentrates. Harmful Algae 2009, 8, 926–937. [Google Scholar] [CrossRef]
- Fernández, M.L.; Reguera, B.; González-Gil, S.; Míguez, A. Pectenotoxin-2 in single-cell isolates of Dinophysis caudata and Dinophysis acuta from the Galician Rías (NW Spain). Toxicon 2006, 48, 477–490. [Google Scholar] [CrossRef]
- Aune, T.; Strand, O.; Aase, B.; Weidemann, J.; Dahl, E.; Hovgard, P. The Sognefjord in Norway, a Possible Location for Mussel Farming? In Harmful and Toxic Algal Blooms; Yasumoto, T., Oshima, Y., Fukuyo, Y., Eds.; Intergovernmental Oceanographic Commission of UNESCO: Sendai, Japan, 1996; pp. 73–75. [Google Scholar]
- Taylor, F.J.R. Dinoflagellates from the International Indian Ocean Expedition; A Report of the Material Collocted by the R.V. “AntonBruun” 1963-1964; Bilbliotheca Botanica: Stuttgart, Germany, 1976; p. 234. [Google Scholar]
- Santhanam, R.; Srinivasan, A. Impact of Dinoflagellate Dinophysis caudata Bloom on the Hydrography and Fishery Potentials of Tuticorin Bay, South India. In Harmful and Toxic Algal Blooms; Yasumoto, Y., Oshima, Y., Fukuyo, Y., Eds.; IOC of UNESCO: Sendai, Japan, 1996; pp. 41–44. [Google Scholar]
- Poletti, R.; Cettul, K.; Bovo, F.; Milandri, A.; Pompei, M.; Frate, R. Distribution of Toxic Dinoflagellates and Their Impact on Shellfish along the Northwest Adriatic Coast. In Harmful Algae; Reguera, B., Blanco, J., Fernandez, M.L., Wyatt, T., Eds.; Xunta de Galicia and Intergovernamental Oceanographic Commission of UNESCO: Santiago de Compostela, Spain, 1998; pp. 88–90. [Google Scholar]
- Morton, S.T.; Vershinin, A.; Smith, L.L.; Leighfield, T.A.; Pankov, S.; Quilliam, M.A. Seasonality of Dinophysis spp. and Prorocentrum lima in Black Sea phytoplankton and associated shellfish toxicity. Harmful Algae 2009, 8, 629–639. [Google Scholar] [CrossRef]
- Pizarro, G.; Moroño, A.; Paz, P.; Franco, J.M.; Pazos, Y.; Reguera, B. Evaluation of passive samplers as a monitoring tool for early warning of Dinophysis toxins in shellfish. Mar. Drugs 2013, 11, 3823–3845. [Google Scholar] [CrossRef]
- Tahri-Joutei, L. Gymnodinium catenatum Graham Blooms on Moroccan Waters. In Harmful Algae; Reguera, B., Blanco, J., Fernandez, M.L., Wyatt, T., Eds.; Xunta de Galicia and Intergovernmental Ocenographic Commission of UNESCO: Santiago de Compostela, Spain, 1998; pp. 66–67. [Google Scholar]
- Flores, M.; Franco, J.M.; Lluch Cota, S.E.; Lluch Cota, D.B.; Cortés Altamirano, R.; Sierra-Beltrán, A.P. Recent species shifts on the HAB occurrences in Acapulco Bay, Mexico. In Harmful Algae 2002, Proceedings of the Xth International Conference on Harmful Algae, St. Pete Beach, FL, USA, 21–25 October 2002; Steidinger, K.A., Landsberg, J.H., Tomas, C.R., Vargo, G.A., Eds.; Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography and Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 2002; p. 96. [Google Scholar]
- Dickey, R.; Fryxell, G.; Granade, R.; Roelke, D. Detection of the marine toxins okadaic acid and domoic acid in shellfish and phytoplankton in the gulf of Mexico. Toxicon 1992, 30, 355–359. [Google Scholar] [CrossRef]
- Fukuyo, Y.; Toyoda, Y.; Miyazaki, S. Dinoflagellates found in Sanriku Coast—I. Genus Dinophysis. Otsuchi Mar. Res. Center Rep. 1981, 7, 13–23. [Google Scholar]
- Tseng, C.K.; Zhou, M.J.; Zou, J.Z. Toxic Phytoplankton Studies in China. In Toxic Phytoplankton Blooms in the Sea; Smayda, T.J., Shimizu, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 347–352. [Google Scholar]
- Holmes, M.J.; Teo, S.L.M. Toxic marine dinoflagellates in Singapore waters that cause seafood poisonings. Clin. Exp. Pharmacol. Physiol. 2002, 29, 829–836. [Google Scholar] [CrossRef]
- Karunasagar, I.; Segar, K.; Karunasagar, I. Potentially Toxic Dinoflagellates in Shellfish Harvesting Areas along the Coast of Karnataka State (India). In Red Tides. Biology, Enviromental Science and Toxicology; Okaichi, T., Anderson, D.M., Nemoto, T., Eds.; Elsevier: New York, NY, USA, 1989; pp. 65–68. [Google Scholar]
- Marasigan, A.N.; Sato, S.; Fukuyo, Y.; Kodama, M. Accumulation of a high level of diarrhetic shellfish toxins in the green mussel Perna viridis during a bloom of Dinophysis caudata and Dinophysis miles in Saipan Bay, Panay Island, the Philippines. Fish. Sci. 2001, 67, 994–996. [Google Scholar] [CrossRef]
- Burgess, V.; Shaw, G. Investigations into the Toxicology of Pectenotoxin-2-seco Acid and 7-epi Pectenotoxin 2-seco Acid to Aid in a Health Risk Assessment for the Consumption of Shellfish Contaminated with These Shellfish Toxins in Australia. Report on Project No. 2001/258. National Research Centre for Environmental Toxicology: Archerfield, Australia, 2003. [Google Scholar]
- Chou, R.; Lee, H.B. Commercial marine fish farming in Singapore. Aquac. Res. 1997, 28, 767–776. [Google Scholar] [CrossRef]
- Vilicic, D.; Djakovac, T.; Buric, Z.; Bosak, S. Composition and annual cycle of phytoplankton assemblages in the northeastern Adriatic Sea. Bot. Mar. 2009, 52, 291–305. [Google Scholar]
- Trainer, V.L.; Pitcher, G.C.; Reguera, B.; Smayda, T.J. The distribution and impacts of harmful algal bloom species in eastern boundary upwelling systems. Prog. Oceanogr. 2010, 85, 33–52. [Google Scholar] [CrossRef]
- García-Mendoza, E.; Sánchez-Bravo, Y.A.; Blanco, J.; Turner, A.; Mancera-Flores, J.; Rivas, D.; Pérez-Brunius, P.; Almazán-Becerril, A. Lipophilic toxins in Mediterranean Mussels from the northwest coast of Baja California, México, Marine and Freshwater Toxins Analysis. In Proceedings of the Fourth Joint Symposium and AOAC Task Force Meeting, Baiona, Pontevedra, Spain, 5–9 May 2013; University of Vigo: Vigo, Spain, 2013; p. 32. [Google Scholar]
- Southerland, C.M. Diarrhetic Shellfish Toxins Linked to Local Dinophysis Populations in the California Coastal Waters of Monterey Bay. Master Thesis, University of California, Santa Cruz, CA, USA, 2008. [Google Scholar]
- Sato, S.; Koike, K.; Kodama, M. Seasonal Variation of Okadaic Acid and Dinophysistoxin-1 in Dinophysis spp. in Association with the Toxicity of Scallop. In Harmful and Toxic Algal Blooms; Yasumoto, T., Oshima, Y., Fukuyo, Y., Eds.; Intergovernmental Oceanographic Commission of UNESCO: Sendai, Japan, 1996; pp. 285–288. [Google Scholar]
- Suzuki, T.; Miyazono, A.; Baba, K.; Sugawara, R.; Kamiyama, T. LC-MS/MS analysis of okadaic acid analogues and other lipophilic toxins in single-cell isolates of several Dinophysis species collected in Hokkaido, Japan. Harmful Algae 2009, 8, 233–238. [Google Scholar] [CrossRef]
- Nagai, S.; Suzuki, T.; Nishikawa, T.; Kamiyama, T. Differences in the production and excretion kinetics of okadaic acid, dinophysistoxin-1, and pectenotoxin-2 between cultures of Dinophysis acuminata and Dinophysis fortii isolated from western Japan. J. Phycol. 2011, 47, 1326–1337. [Google Scholar] [CrossRef]
- Shahi, N.; Nayak, B.B.; Mallik, S.K. Dinophysis norvegica: First report of the toxic temperate water Dinophysis in Manori creek of Mumbai water. Harmful Algae News 2010, 42, 14–15. [Google Scholar]
- Hernández-Becerril, D.U. La Diversidad del Fitoplancton Marino de México. Un Acercamiento Actual. In Planctología Mexicana; Barreiro, M.T., Meave, M.E., Signoret, M., Figueroa, M.G., Eds.; Sociedad Mexicana de Planctología (SOMPAC): Universidad Autónoma Metropolitana, México, 2003; pp. 1–17. [Google Scholar]
- Carpenter, E.J.; Janson, S.; Boje, R.; Pollehne, F.; Chang, J. The dinoflagellate Dinophysis norvegica: biological and ecological observations in the Baltic Sea. Eur. J. Phycol. 1995, 30, 1–9. [Google Scholar]
- Subba Rao, D.V.; Pan, Y.; Zitko, V.; Bugden, G.; Mackelgan, K. Diarrhetic shellfish poisoning (DSP) associated with a subsurface bloom of Dinophysis norvergica in Bedford Basin, eastern Canada. Mar. Ecol. Prog. Ser. 1993, 97, 117–126. [Google Scholar] [CrossRef]
- Cembella, A.D. Occurence of okadaic acid, a major diarrhetic shellfish toxin, in natural populations of Dinophysis spp. from the eastern of North America. J. Appl. Phycol. 1989, 1, 307–310. [Google Scholar]
- Papaefthimiou, D.; Aligizaki, K.; Nikolaidis, G. Exploring the identity of the Greek Dinophysis cf acuminata. Harmful Algae 2010, 10, 1–8. [Google Scholar] [CrossRef]
- Swanson, K.M.; Flewelling, L.J.; Byrd, M.; Nunez, A.; Villareal, T.A. The 2008 Texas Dinophysis ovum bloom: Distribution and toxicity. Harmful Algae 2010, 9, 190–199. [Google Scholar] [CrossRef]
- Campbell, L.; Olson, R.; Sosik, H.M.; Abraham, A.; Henrichs, D.W.; Hyatt, C.; Buskey, E.B. First harmful Dinophysis (Dinophyceae, Dinophysiales) bloom in the U.S. is revealed by automated imaging flow cytometry. J. Phycol. 2010, 46, 66–75. [Google Scholar] [CrossRef]
- Fux, E.; Smith, J.L.; Tong, M.; Guzmán, L.; Anderson, D.M. Toxin profiles of five geographical isolates of Dinophysis spp. from North and South America. Toxicon 2011, 57, 275–287. [Google Scholar] [CrossRef]
- Louppis, A.P.; Badeka, A.V.; Katikou, P.; Paleologos, E.P.; Kontominas, M.G. Determination of okadaic acid, dinophysistoxin-1 and related esters in Greek mussels using HPLC with fluorometric detection, LC-MS/MS and mouse bioassay. Toxicon 2010, 55, 724–733. [Google Scholar] [CrossRef]
- Reguera, B.; Mariño, J.; Campos, M.J.; Bravo, I.; Fraga, S.; Carbonell, A. Trends in the Occurrence of Dinophysis spp. in Galician Coastal Waters. In Toxic Phytoplankton Blooms in the Sea; Smayda, T.J., Shimizu, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 559–564. [Google Scholar]
- Cañete, E.; Caillaud, A.; Fernández, M.; Mallat, E.; Blanco, J.; Diogène, J. Dinophysis sacculus from Alfacs Bay, NW Mediterranean. Toxin profiles and cytotoxic potential. In Proceedings of the 12th International Conference on Harmful Algae, Copenhagen, Denmark, 4–8 September 2006; Moestrup, Ø., Ed.; International Society for the Study of Harmful Algae and Intergovernmental Oceanographic Commission of UNESCO: Copenhagen, Denmark, 2008; pp. 279–281. [Google Scholar]
- Alvito, P.; Sousa, I.; Franca, S.; Sampayo, M.A. Diarrhetic Shellfish Toxins in Bivalve Molluscs along the Coast of Portugal. In Toxic Marine Phytoplankton; Graneli, E., Sundström, B., Edler, L., Anderson, D.M., Eds.; Elsevier: New York, NY, USA, 1990; pp. 443–448. [Google Scholar]
- Giacobbe, M.G.; Penna, A.; Ceredi, A.; Milandri, A.; Poletti, R.; Yang, X. Toxicity and ribosomal DNA of the dinoflagellate Dinophysis sacculus (Dinophyta). Phycologia 2000, 39, 177–182. [Google Scholar] [CrossRef]
- Marasović, I.; Nincevic, Z.; Orhanovic, S.; Pavela-Vrančič, M. A survey of shellfish toxicity in the Central Adriatic Sea. J. Mar. Biol. Assoc. UK 1998, 78, 745–754. [Google Scholar] [CrossRef]
- Tahri-Joutei, L.; Maghraoui, M.; Boutaïb, R. Toxic Phytoplankton and Phycotoxins in the Mediterranean coast of Morocco from 1994 to 2000. In Molluscan Shellfish Safety; Villalba, A., Reguera, B., Romalde, J.L., Beiras, R., Eds.; Consellería de Pesca e Asuntos Marítimos da Xunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO: Santiago de Compostela, Spain, 2003; pp. 187–195. [Google Scholar]
- Armi, Z.; Turki, S.; Trabelsi, E.; Ceredi, A.; Riccardi, E.; Milandri, A. Occurrence of diarrhetic shellfish poisoning (DSP) toxins in clams (Ruditapes decussatus) from Tunis north lagoon. Environ. Monit. Assess. 2012, 184, 5085–5095. [Google Scholar] [CrossRef]
- Masselin, P.; Lassus, P.; Bardouil, M. High performance liquid chromatography analysis of diarrhetic toxins in Dinophysis spp. from the French coast. J. Appl. Phycol. 1992, 4, 385–389. [Google Scholar] [CrossRef]
- Delgado, M.; Garcés, E.; Camp, J. Growth and behaviour of Dinophysis sacculus from NW Mediterranean Sea. In Harmful and Toxic Algal Blooms, Proceedings of the Seventh International Conference on Toxic Phytoplankton, Sendai, Japan, 12–16 July 1995; Yasumoto, T., Oshima, Y., Fukuyo, Y., Eds.; Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 1996; pp. 261–264. [Google Scholar]
- Nincevic-Gladan, Z.; Skejic, S.; Buzancic, M.; Marasovic, I.; Arapov, J.; Ujevic, I.; Bojanic, N.; Grbec, B.; Kuspilic, G.; Vidjak, O. Seasonal variability in Dinophysis spp. abundances and diarrhetic shellfish poisoning outbreaks along the eastern Adriatic coast. Bot. Mar. 2008, 51, 449–463. [Google Scholar]
- Larsen, J.; Moestrup, Ø. Potentially Toxic Phytoplankton 2. Genus Dinophysis (Dinophyceae). In ICES Identification Leaflets for Plankton 180; Lindley, J.A., Ed.; International Council for the Exploration of the Sea: Denmark, Copenhagen, 1992; pp. 1–12. [Google Scholar]
- Johnsen, T.M.; Lømsland, E.R. Observations of Dinophysis tripos in Norwegian coastal waters. In Proceedings of the 14th International Conference on Harmful Algae, Creta, Greece, 1–2 November 2010; Pagou, P., Hallegraeff, G.M., Eds.; International Society for the Study of Harmful Algae and Intergovernmental Oceanographic Commission of UNESCO: Copenhagen, Denmark, 2012; pp. 54–56. [Google Scholar]
- Anonymous. Report of the ICES-IOC Working Group on Harmful Algal Bloom Dynamics (WGHABD); International Council for the Exploration of the Sea: Copenhagen, Denmark, 2010. [Google Scholar]
- Nagai, S.; Suzuki, T.; Kamiyama, T. Successful cultivation of the toxic dinoflagellate Dinophysis tripos (Dinophyceae). Plankton Benthos Res. 2013, 8, 171–177. [Google Scholar]
- Hansen, P.J. Dinophysis: A planktonic dinoflagellate genus which can act both as a prey and as a predator of a ciliate. Mar. Ecol. Prog. Ser. 1991, 69, 201–204. [Google Scholar] [CrossRef]
- Caroppo, C.; Congestri, R.; Bruno, M. On the presence of Phalacroma rotundatum in the southern Adriatic Sea (Italy). Aquat. Microb. Ecol. 1999, 17, 301–310. [Google Scholar] [CrossRef]
- HAEDAT. ICES-IOC Harmful Algae Event Data Base. 2013. Available online: http://www.iode.org/haedat (accessed on 20 October 2013).
- Vale, P.; Botelho, M.J.; Rodrigues, S.M.; Gomes, S.S.; Sampayo, M.A.M. Two decades of marine biotoxin monitoring in bivalves from Portugal (1986-2006): A review of exposure assessment. Harmful Algae 2008, 7, 11–25. [Google Scholar] [CrossRef]
- Blanco, J.; Correa, J.; Muñíz, S.; Mariño, C.; Martín, H.; Arévalo, F. Evaluación del impacto de los métodos y niveles utilizados para el control de toxinas en el mejillón. Revista Galega dos Recursos Mariños 2013, 3, 1–55. (in Spanish). [Google Scholar]
- Instituto Tecnolóxico Para o Control do Medio Mariño de Galicia. (in Galician). Available online: http://www.intecmar.org (accessed on 30 October 2013).
- CEFAS. Biotoxin Monitoring Programme for Scotland; Center for Environment, Fisheries and Aquacuture Science: London, UK, 2012. Available online: http://cefas.defra.gov.uk/ (accessed on 8 January 2014).
- Batifoulier, F.; Lazure, P.; Velo-Suarez, L.; Maurer, D.; Bonneton, P.; Charria, G.; Dupuy, C.; Gentien, P. Distribution of Dinophysis species in the Bay of Biscay and possible transport pathways to Arcachon Bay. J. Mar. Syst. 2012, 109–110, S273–S283. [Google Scholar]
- Haamer, J.; Andersson, P.-O.; Lindahl, O.; Lange, S.; Li, X.P.; Edebo, L. Geographic and seasonal variation of okadic acid content in farmed mussels, Mytilus edulis Linnaeus, 1758, along the Swedish west coast. J. Shellfish Res. 1990, 9, 103–108. [Google Scholar]
- Ramstad, H.; Hovgaard, P.; Yasumoto, T.; Larsen, S.; Aune, T. Monthly variations in diarrhetic toxins and yessotoxin in shellfish from coast to the inner part of the Sognefjord, Norway. Toxicon 2001, 39, 1035–1043. [Google Scholar] [CrossRef]
- Raine, R. A review of the biophysical interactions relevant to the promotion of HABs in stratified systems: The case study of Ireland. Deep Sea Res. II Top. Stud. Oceanogr. 2013, in press. [Google Scholar]
- Pazos, Y. Personal Communication, INTECMAR: Vilagarcia de Arousa, Pontevedra, Spain, 2006.
- Vale, P.; Maia, A.J.; Correia, A.; Rodrigues, S.M.; Botelho, M.J.; Casanova, G.; Silva, A.; Vilarinho, M.G.; Silva, A.D. An outbreak of diarrhetic shellfish poisoning after ingestion of wild mussels at the northern coast in summer 2002. Elect. J. Environ. Agri. Food Chem. 2003, 2, 449–452. [Google Scholar]
- Torgersen, T.; Aasen, J.; Aune, T. Diarrhetic shellfish poisoning by okadaic acid esters from Brown crabs (Cancer pagurus) in Norway. Toxicon 2005, 46, 572–578. [Google Scholar] [CrossRef]
- Vale, P.; Sampayo, M.A. First confirmation of human diarrhoeic poisonings by okadaic acid esters after ingestion of razor clams (Solen marginatus) and geen crabs (Carcinus maenas) in Aveiro lagoon, Portugal and detection of okadaic acid esters in phytoplankton. Toxicon 2002, 40, 989–996. [Google Scholar] [CrossRef]
- Vershinin, A.; Moruchkov, A.; Morton, S.T.; Leighfield, T.A.; Quilliamc, M.A.; Ramsdell, J.S. Phytoplankton composition of the Kandalaksha Gulf, Russian White Sea: Dinophysis and lipophilic toxins in the blue mussel (Mytilus edulis). Harmful Algae 2006, 5, 558–564. [Google Scholar] [CrossRef]
- Hällfors, H.; Hajdu, S.; Kuosa, H.; Larsson, U. Vertical and temporal distribution of the dinoflagellates Dinophysis acuminata and D. norvegica in the Baltic Sea. Boreal Environ. Res. 2011, 16, 121–135. [Google Scholar]
- Kuuppo, P.; Uronen, P.; Petermann, A.; Tamminen, T.; Granéli, E. Pectenotoxin-2 and dinophysistoxin-1 in suspended and sedimenting organic matter in the Baltic Sea. Limnol. Oceanogr. 2006, 51, 2300–2307. [Google Scholar] [CrossRef]
- Prassopoulou, E.; Katikou, P.; Georgantelis, D.; Kyritsakis, A. Detection of okadaic acid and related esters in mussels during diarrhetic shellfish poisoning (DSP) episodes in Greece using the mouse bioassay, the PP2A inhibition assay and HPLC with fluorimetric detection. Toxicon 2009, 53, 214–227. [Google Scholar] [CrossRef]
- Ciminiello, P.; Dell’Aversano, C.; Fattorusso, E.; Forino, M.; Magno, S.; Santelia, F.; Tsoukatou, M. Investigation of the toxin profile of Greek mussels Mytilus galloprovincialis by liquid chromatography-Mass spectrometry. Toxicon 2006, 47, 174–181. [Google Scholar] [CrossRef]
- Koukaras, K.; Nikolaidis, G. Dinophysis blooms in Greek coastal waters (Thermaikos Gulf, NW Aegean Sea). J. Plankton Res. 2004, 26, 445–457. [Google Scholar] [CrossRef]
- Boni, L.; Milandri, A.; Poletti, R.; Pompei, M. DSP Cases along the Coasts of Emilia-Romagna (Northwestern Adriatic Sea). In Toxic Phytoplankton Blooms in the Sea; Smayda, T.J., Shimizu, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 475–481. [Google Scholar]
- Della Loggia, R.; Cabrini, M.; Delnegro, P.; Honsell, G.; Tubaro, A. Relationship between Dinophysis spp in Seawater and DSP Toxins in Mussels in the Northern Adriatic Sea. In Toxic Phytoplankton Blooms in the Sea; Smayda, T.J., Shimizu, Y., Eds.; Elsevier: Amsterdam, The Netherlankds, 1993; pp. 483–488. [Google Scholar]
- Pavela-Vrancic, M.; Mestrovic, V.; Marasovic, I.; Gillman, M.; Furey, A.; James, K.J. DSP toxin profile in the coastal waters of the central Adriatic Sea. Toxicon 2002, 40, 1601–1607. [Google Scholar] [CrossRef]
- Amzil, Z.; Sibat, M.; Royer, F.; Masson, N.; Abadie, E. Report on the first detection of pectenotoxin-2, spirolide-A and their derivatives in French shellfish. Mar. Drugs 2007, 5, 168–179. [Google Scholar] [CrossRef]
- Elgarch, A.; Vale, P.; Rifai, S.; Fassouane, A. Detection of diarrheic shellfish poisoning and azaspiracid toxins in Moroccan mussels: Comparison of the LC-MS method with the commercial immunoassay kit. Mar. Drugs 2008, 6, 587–594. [Google Scholar] [CrossRef]
- Tahri-Joutei, L. Gymnodinium catenatum GRAHAM: Aspects Dynamique et Toxicologique. Ph.D. Thesis, University of Rabat, Morocco, June 2007. [Google Scholar]
- Ennaffah, B.; Chafik, A. Distribution of Toxic Dinophysis Species and Contamination of Shellfish along the Doukkala Coast (Moroccan Atlantic Water). In Proceedings of the 12th International Conference on Harmful Algae, Copenhagen, Denmark, 4–8 September 2006; International Society for the Study of Harmful Algae: Copenhagen, Denmark, 2008; p. 168. [Google Scholar]
- Fawcett, A.; Pitcher, G.; Bernard, S.; Cembella, A.; Kudela, R. Contrasting wind patterns and toxigenic phytoplankton in the southern Benguela upwelling system. Mar. Ecol. Prog. Ser. 2007, 348, 19–31. [Google Scholar] [CrossRef]
- Pitcher, G.C.; Krock, B.; Cembella, A.D. Accumulation of diarrhetic shellfish poisoning toxins in the oyster Crassostrea gigas and the mussel Choromytilus meridionalis in the southern Benguela ecosystem. Afr. J. Marine Sci. 2011, 33, 273–281. [Google Scholar] [CrossRef]
- Hubbart, B.; Pitcher, G.C.; Krock, B.; Cembella, A.D. Toxigenic phytoplankton and concomitant toxicity in the mussel Choromytilus meridionalis off the west coast of South Africa. Harmful Algae 2012, 20, 30–41. [Google Scholar] [CrossRef]
- Velikova, V.; Louw, D.; Pienaar, R.; Sym, S. Dinoflagellates in the Benguela Current Waters off Namibia. In Harmful Algae 2004, Proceedings of the 11th International Conference on Harmful Algal Blooms, Cape Town, South Africa, 14–19 November 2004; Pitcher, G.C., Probyn, T.A., Verheye, H.M., Eds.; International Society for the Study of Harmful Algae: Cape Town, South Africa, 2006; p. 252. [Google Scholar]
- Rangel, I.; Coelho, P.; Rodrigues, S.; Vale, P.; Wilar, A. Okadaic acid in the clam Semele proficua in Luanda Bay, Angola. Harmful Algae News 2008, 38, 12. [Google Scholar]
- Turki, S. Occurrence of harmful microalgae in two different ecosystems in Tunisian marine waters: The Lagoon of Bizerte and the Gulf of Gabès. In Harmful Algae 2004, Proceedings of the XI International Conference on Harmful Algal Blooms, Cape Town, South Africa, 14–19 November 2004; Pitcher, G.C., Probyn, T.A., Verheye, H.M., Eds.; International Society for the Study of Harmful Algae: Cape Town, South Africa, 2006; p. 247. [Google Scholar]
- Kacem, I.; Hajjem, B.; Bouaïcha, N. First evidence of okadaic acid in Mytilus galloprovincialis Mussels, Collected in a Mediterranean Lagoon, Tunisia. Bull. Environ. Contam. Toxicol. 2009, 82, 660–664. [Google Scholar] [CrossRef]
- Fukuyo, Y.; Imai, I.; Kodama, M.; Tamai, K. Red Tides and Harmful Algal Blooms in Japan. In PICES Scientific Report No. 23; Taylor, F.J.R., Trainer, V.L., Eds.; North Pacific Marine Science Organization: Sydney, Canada, 2002; pp. 7–20. [Google Scholar]
- Adachi, M.; Okamoto, N.; Matsubara, M.; Nishijima, T.; Suzuki, T. Occurrence of toxic Dinophysis acuminata (Dinophyceae) in Uranouchi Inlet, Japan. Fish. Sci. 2008, 74, 1315–1321. [Google Scholar] [CrossRef]
- Suzuki, T.; Miyazono, A.; Okumura, Y.; Kamiyama, T. LC-MS/MS Analysis of Lipophilic Toxins in Japanese Dinophysis Species. Available online: http://www.pices.int/publications/presentations/PICES_15/Ann15_W4/W4_Suzuki.pdf (accessed on 20 October 2013).
- Suzuki, T.; Mitsuya, T. Comparison of dinophysistoxin-1 and esterified dinophysistoxin-1 (dinophysistoxin-3) contents in the scallop Patinopecten yessoensis and the mussel Mytilus galloprovincialis. Toxicon 2001, 39, 905–908. [Google Scholar] [CrossRef]
- Zhou, M.; Li, J.; Luckas, B.; Yu, R.C.; Yan, T.; Hummert, C. A recent shellfish toxin investigation in China. Mar. Pollut. Bull. 1999, 39, 331–334. [Google Scholar] [CrossRef]
- Li, A.; Ma, J.; Cao, J.; McCarron, P. Toxins in mussels (Mytilis galloprovincialis) associated with diarrhetic shellfish poisoning episodes in China. Toxicon 2012, 60, 420–425. [Google Scholar] [CrossRef]
- Jiang, T.; Xu, Y.; Li, Y.; Qi, Y.; Jiang, T.; Wu, F.; Zhang, F. Lipophilic shellfish toxins (LST) in bivalves associated with the occurrence of Dinophysis caudata in Nanji Islands, East China Sea. Chin. J. Oceanol. Limnol. 2013, in press. [Google Scholar]
- Luo, X. Population Dynamics and Toxin Production of Dinophysis Species in the Coastal Waters of Qingdao. Ph.D. Thesis, Chinese Academy of Sciences, Beijing, China, 2011. [Google Scholar]
- Wang, J.; Qin, Y.; Liu, C.; Chen, X.; Xu, R. Dinophysis spp: The Abundance, Distribution and the Toxicity of DSP in East China Sea. Available online: http://www.pices.int/publications/presentations/PICES_15/Ann15_W4/W4_Wang.pdf (accessed on 13 January 2014).
- FAO. The State of World Fisheries and Aquaculture; FAO Fisheries and Aquaculture Department: Rome, Italy, 2012; p. 230. [Google Scholar]
- Kim, J.H.; Suzuki, T.; Lee, K.J.; Kim, P.H.; Kamiyama, T.; Lee, T.S. The first detection of okadaic acid and its derivatives in Korean bivalves by liquid chromatography-mass spectrometry. Fish. Res. 2008, 74, 433–438. [Google Scholar]
- Kim, J.H.; Lee, K.J.; Suzuki, T.; Kang, Y.S.; Ho Kim, P.; Song, K.C.; Lee, T.S. Seasonal variability of lipophilic shellfish toxins in bivalves and waters, and abundance of Dinophysis spp. in Jinhae Bay, Korea. J. Shellfish Res. 2010, 29, 1061–1067. [Google Scholar] [CrossRef]
- Holmes, M.; Teo, S.; Lee, F.; Khoo, H. Persistent low concentratrions of diarrhetic shellfish toxins in green mussels Perna viridis from the Johor Strait, Singapure: First record of diarrhetic shellfish toxins from South-East Asia. Mar. Ecol. Prog. Ser. 1999, 181, 257–268. [Google Scholar]
- Karunasagar, I.; Segar, K.; Karunasagar, I. Incidence of PSP and DSP in shellfish along the coast of Karnataka State (India). In Red Tides. Biology, Enviromental Science and Toxicology; Okaichi, T., Anderson, D.M., Nemoto, T., Eds.; Elsevier: New York, NY, USA, 1989; pp. 61–64. [Google Scholar]
- Almuftah, A.R. Some Potentially Toxic Species of Dinophysis in Qatari Waters. In Harmful Algae 2004, Proceedings of the XI International Conference on Harmful Algal Blooms, Cape Town, South Africa, 14–19 November 2004; Pitcher, G.C., Probyn, T.A., Verheye, H.M., Eds.; International Society For the Study of Harmful Algae: Cape Town, South Africa, 2006; p. 56. [Google Scholar]
- Maranda, L.; Shimizu, Y. Diarrhetic shellfish poisoning on Narragansett Bay. Estuaries 1987, 10, 298–302. [Google Scholar] [CrossRef]
- Freudenthal, A.R.; Jijina, J. Shellfish Poisoning Involving or Coincidental with Dinoflagellates. In Toxic Dinoflagellates; Anderson, D.M., White, A.W., Baden, D.G., Eds.; Elsevier: New York, NY, USA, 1985; pp. 461–466. [Google Scholar]
- Morton, S.L.; Leighfield, T.A.; Haynes, B.L.; Petitpain, D.L.; Busman, M.A.; Moeller, P.D.R.; Bean, L.; McGowan, J.; John, W.; Hurst, J.; et al. Evidence of diarrhetic shellfish poisoning along the coast of Maine. J. Shellfish Res. 1999, 18, 681–686. [Google Scholar]
- Tango, P.; Butler, W.; Lacouture, R.; Goshorn, D.; Magnien, R.; Michael, B.; Hall, S.; Browhawn, K.; Wittman, R.; Beatty, W. An unprecedented bloom of Dinophysis acuminata in Chesapeake Bay. In Harmful Algae 2002, Proceedings of the Xth International Conference on Harmful Algae, St. Pete Beach, FL, USA, 21–25 October 2002; Steidinger, K.A., Landsberg, J.H., Tomas, C.R., Vargo, G.A., Eds.; Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography and Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 2002; pp. 358–361. [Google Scholar]
- Marshall, H.; Egerton, T.; Stem, T.; Hicks, J.; Kokocinski, M. Extended Bloom Concentration of the Toxic Dinoflagellate Dinophysis acuminata in Virginia Estuaries Late Winter through Early Spring, 2002. In Harmful Algae 2002, Proceedings of the 10th International Conference on Harmful Algae, St. Pete Beach, FL, USA, 21–25 October 2002; Steidinger, K.A., Landsberg, J.H., Tomas, C.R., Vargo, G.A., Eds.; Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography and Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 2002; pp. 364–366. [Google Scholar]
- Trainer, V.L.; Trick, C.G. Dinophysis and Cochlodinium spp. in North American Waters, Review of Selected Harmful Algae in the PICES Region: II. In Proceedings of the Dinophysis and Cochlodinium and HAB-S Meeting, Yokohama, Japan, 13–22 October 2006.
- Ochoa, J.L.; Sierra-Beltrán, A.P.; Alonso-Colmenara, G.; Barradas-Sánchez, H.; Cruz-Villacorta, A.; Nuñez-Vazquez, E.; Sánchez-Paz, A. Biotoxins in the Pacific Coast of Mexico. In Mycotoxins and Phycotoxins—Developments in Chemistry, Toxicology and Food Safety; Miraglia, M., van Egmond, H., Brera, C., Gilbert, J., Eds.; Alaken, Inc.: Fort Collins, CO, USA, 1998; pp. 441–448. [Google Scholar]
- García-Mendoza, E.; Mancera-Flores, J.; Peña-Manjarrez, J.L. Monitoring of marine biotoxins in Baja California, Mexico: Status, problems and perspectives. In Proceedings of the Second Joint Symposium and AOAC Task Force Meeting on Marine and Freshwater Toxins AnalysisBaiona, Pontevedra, Spain, 1–5 May 2011; University of Vigo: Vigo, Spain, 2011; p. 63. [Google Scholar]
- COFEPRIS, Comunicados de la Comisión Federal para la Protección Contra Riesgos Sanitarios, en materia de Marea Roja en 2008. Available online: http://201.147.97.103/wb/cfp/emergencias_sanitarias_estatales_por_marea_roja/_rid/319?page=2 (accessed on 13 January 2014).
- Sernapesca. Anuario Estadistico de Pesca; Servicio Nacional de Pesca: Valparaiso, Chile, 2011. [Google Scholar]
- Millanao, M.O.; Díaz, P.A.; Díaz, M.; Molinet, C. La mitilicultura en Chile: Evolución, estado actual y perspectivas futuras. In XV Foro dos Recursos Mariños e da Acuicultura das Rías Galegas; (in Spanish). Rey, M., Fernández, J., Lodeiros, C., Guerra, A., Eds.; Libromar Ediciones y Gestión S.L: A Coruña, Spain, 2013; pp. 285–292. [Google Scholar]
- IOC. Second IOC Regional Science Planning Workshop on Harmful Algal Blooms in South America. Mar del Plata, Argentina, 30 October–1 November, 1995; p. 75. Available online: http://hab.ioc-unesco.org/index.php?option=com_oe&task=viewDocumentRecord&docID=2847 (accessed on 28 October 2013).
- Zhao, J.; Lembeye, G.; Cenci, G.; Wall, B.; Yasumoto, T. Determination of okadaic acid and dinophysistoxin-1 in mussels from Chile, Italy and Ireland. In Toxic Phytoplankton Blooms in the Sea; Smayda, T.J., Shimizu, Y, Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 587–592. [Google Scholar]
- Villarroel, O. Detección de toxina paralizante, diarreica y amnésica en mariscos de la XI región por Cromatografía de Alta Resolución (HPLC) y bioensayo de ratón. Cienc. Tecnol. Mar. 2004, 27, 33–42. (in Spanish). [Google Scholar]
- García, C.; Rodriguez-Unda, N.; Contreras, C.; Barriga, A.; Lagos, N. Lipophilic toxin profiles detected in farmed and benthic mussels populations from the most relevant production zones in Southern Chile. Food Addit. Contam. 2012, 29, 1011–1020. [Google Scholar] [CrossRef]
- Goto, H.; Igarashi, T.; Watai, M.; Yasumoto, T.; Villarroel, O.; Lembeye, G.; Noren, F.; Gisselson, G.; Graneli, E. Worldwide Occurrence of Pectenotoxins and Yessotoxins in Shellfish and Phytoplankton. In Harmful Algal Blooms 2000, Proceedings of the IX International Conference on Harmful Alga Blooms, Hobart, Tasmania, Australia, 7–11 February 2000; Hallegraeff, G.M., Blackburn, S.I., Bolch, C.J., Lewis, R.J., Eds.; Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 2001; p. 49. [Google Scholar]
- Blanco, J.; Álvarez, G.; Uribe, E. Identification of pectenotoxins in plankton, filter feeders, and isolated cells of a Dinophysis acuminata with an atypical toxin profile from Chile. Toxicon 2007, 49, 710–716. [Google Scholar] [CrossRef]
- Uribe, J.C.; García, C.; Rivas, M.; Lagos, N. First report of diarrhetic shellfish toxins in Magellanic fjord, Southern Chile. J. Shellfish Res. 2001, 20, 69–74. [Google Scholar]
- Trefault, N.; Krock, B.; Delherbe, N.; Cembella, A.; Vásquez, M. Latitudinal transects in the southeastern Pacific Ocean reveal a diverse but patchy distribution of phycotoxins. Toxicon 2011, 58, 389–397. [Google Scholar] [CrossRef]
- Salgado, P.; Pizarro, G.; Franco, J.M.; Riobó, P.; Bravo, I. Perfil de toxinas de Prorocentrum lima (Dinophyceae) aislado desde la costa de Magallanes, sur de Chile. In Proceedings of the XXXII Congreso de Ciencias del Mar, Punta Arenas, Chile, 22–26 October 2012; p. 228.
- Krock, B.; Seguel, C.G.; Valderrama, K.; Tillmann, U. Pectenotoxins and Yessotoxin from Arica Bay, North Chile as determined by Tandem Mass Spectrometry. Toxicon 2009, 54, 364–367. [Google Scholar] [CrossRef]
- Pizarro, G.; Alarcón, C.; Franco, J.M.; Palma, M.; Escalera, L.; Reguera, B.; Vidal, G.; Guzmán, L. Distribución espacial de Dinophysis spp. y detección de toxinas DSP en el agua mediante resinas DIAION (verano 2006, Región de Los Lagos, Chile). Cienc. Tecnol. Mar. 2011, 34, 31–48. [Google Scholar]
- IOC. Sixth IOC Regional Science Planning Workshop on Harmful Algal Blooms in South America, Guayaquil, Ecuador, 22–24 October, 2003. p. 111. Available online: http://hab.ioc-unesco.org/index.php?option=com_oe&task=viewDocumentRecord&docID=11753 (accessed on 25 October 2013).
- Anonymous. Mesa Redonda: Demanda Regional para Capacitação e Pesquisas. In Proceedings of the Livro de Reumos da Reunião Latino-Americana sobre Algas Nocivas, Santa Catarina, Brasil, 7–9 October 2013; Proença, L.A.O., Renan de Souza, K., Eds.; Instituto Federal de Santa Catarina: Santa Catarina, Brazil, 2013. [Google Scholar]
- Proença, L.A.O.; Schramm, M.A.; Tamanaha, M.S.; Alves, T.P. Diarrhoetic shellfish poisoning (DSP) outbreak in Subtropical Southwest Atlantic. Harmful Algae News 2007, 33, 19–20. [Google Scholar]
- Mafra, L.L., Jr.; Prokopiak, L.K.; Fernandes, L.F.; Proença, L.A.O. Harmful Algae and Toxins in a Brazilian Port Area. In Proceedings of the XI International Conference on Harmful Algal Blooms, Cape Town, South Africa, 14–19 November 2004; International Society For the Study of Harmful Algae: Cape Town, South Africa, 2004; p. 176. [Google Scholar]
- Sar, E.A.; Sunesen, I.; Goya, A.B.; Lavigne, A.S.; Tapia, E.; Garcia, C.; Lagos, N. First report of diarrheic shellfish toxins in mollusks from Buenos Aires province (Argentina) associated with Dinophysis spp.: Evidence of okadaic acid, dinophysistoxin-1 and their acyl-derivatives. Bol. Soc. Argent. Bot. 2012, 47, 5–14. [Google Scholar]
- Quaine, J.; Kraa, E.; Holloway, J.; White, K.; McCarthy, R.; Delpech, V.; Trent, M.; McAnulty, J. Outbreak of gastroenteritis linked to eating pipis. Infect. Dis. NSW 1997, 8, 103–107. [Google Scholar]
- MacKenzie, L. An Evaluation of the Risk to Consumers of Pectenotoxin 2 Seco Acid (PTX2-SA) Contamination of Greenshell™ Mussels; Cawthron Report 750; Cawthron Institute: Nelson, New Zealand, 2002. [Google Scholar]
- MacKenzie, L.; Holland, P.; McNabb, P.; Beuzenberg, V.; Selwood, A.; Suzuki, T. Complex toxin profiles in phytoplankton and Greenshell mussels (Perna canaliculus), revealed by LC-MS/MS analysis. Toxicon 2002, 40, 1321–1330. [Google Scholar] [CrossRef]
- Flynn, K.J.; Flynn, K. Dinoflagellate Physiology: Nutrient Stress and Toxicity. In Harmful Marine Algal Blooms; Lassus, P., Arzul, G., Erard-le Denn, E., Gentien, P., Marcaillou-le Baut, C., Eds.; Lavoisier Publishing: Paris, France, 1995; pp. 541–550. [Google Scholar]
- MacKenzie, L.; Beuzenberg, V.; Holland, P.; McNabb, P.; Selwood, A. Solid phase adsorption toxin tracking (SPATT): A new monitoring tool that simulates the biotoxin contamination of filter feeding bivalves. Toxicon 2004, 44, 901–918. [Google Scholar] [CrossRef]
- Pizarro, G.; Escalera, L.; González-Gil, S.; Franco, J.; Reguera, B. Growth, behaviour and cell toxin quota of Dinophisis acuta during a daily cycle. Mar. Ecol. Prog. Ser. 2008, 353, 89–105. [Google Scholar] [CrossRef]
- Johansen, M. On Dinophysis—Occurrence and Toxin Content. Ph.D. Thesis, University of Gothenburg, Gothenburg, Sweden, February 2008. [Google Scholar]
- Reguera, B.; Garcés, E.; Pazos, I.; Bravo, I.; Ramillo, I.; Gonzáles-Gil, S. Cell cycle patterns and estimates of in situ division rates of dinoflagellates of the genus Dinophysis by a postmitotic index. Mar. Ecol. Prog. Ser. 2003, 249, 117–131. [Google Scholar] [CrossRef]
- Reguera, B.; Rodríguez, F.; Blanco, J. Harmful Algae Blooms and Food Safety: Physiological and Environmental Factors Affecting Toxin Production and Their Accumulation in Shellfish. In New Trends in Marine Freshwater Toxins: Food Safety Concerns; Cabado, A.G., Vieites, J.M., Eds.; Nova Science Publishers, Inc: New York, NY, USA, 2012. [Google Scholar]
- Hawkins, A.J.S.; Navarro, E.; Iglesias, J.I.P. Comparative allometries of gut-passage time, gut content and metabolic faecal loss in Mytilus edulis and Cerastoderma edule. Mar. Biol. 1990, 105, 197–204. [Google Scholar] [CrossRef]
- Navarro, E.; Iglesias, J.I.P.; Ortega, M.M.; Larretxea, X. The basis for a functional response to variable food quantity and quality in cockles Cerastoderma edule (Bivalvia, Cardiidae). Physiol. Zool. 1994, 67, 468–496. [Google Scholar]
- Sampayo, M.A.; Alvito, P.; Franca, S.; Sousa, I. Dinophysis spp. Toxicity and Relation to Accompanying Species. In Toxic Marine Phytoplankton; Granéli, E., Sundström, B., Edler, L., Anderson, D.M., Eds.; Elsevier: New York, NY, USA, 1990; pp. 215–220. [Google Scholar]
- Rossignoli, A.E. Acumulación de Toxinas DSP en el Mejillón Mytilus galloprovincialis. Ph.D. Thesis, University of Santiago de Compostela, Santiago de Compostela, Spain, 18 July 2011. [Google Scholar]
- Daranas, A.H.; Cruz, P.G.; Creus, A.H.; Norte, M.; Fernández, J.J. Self-assembly of okadaic acid as a pathway to the cell. Org. Lett. 2007, 9, 4191–4194. [Google Scholar] [CrossRef]
- Jauffrais, T.; Marcaillou, C.; Herrenknecht, C.; Truquet, P.; Séchet, V.; Nicolau, E.; Tillmann, U.; Hess, P. Azaspiracid accumulation, detoxification and biotransformation in blue mussels (Mytilus edulis) experimentally fed Azadinium spinosum. Toxicon 2012, 60, 582–595. [Google Scholar] [CrossRef]
- Fux, E.; Bire, R.; Hess, P. Comparative accumulation and composition of lipophilic marine biotoxins in passive samplers and in mussels (M. edulis) on the West Coast of Ireland. Harmful Algae 2009, 8, 523–537. [Google Scholar]
- Blanco, J. Modelling as a Mitigation Strategy for Harmful Algal Blooms. In Shellfish Safety and Quality; Shumway, S.E., Rodrick, G.E., Eds.; Woodhead Publishing: Cambridge, UK, 2009; pp. 200–227. [Google Scholar]
- Moroño, A.; Arevalo, F.; Fernandez, M.; Maneiro, J.; Pazos, Y.; Salgado, C.; Blanco, J. Accumulation and transformation of DSP toxins in mussels Mytilus galloprovincialis during a toxic episode caused by Dinophysis acuminata. Aquat. Toxicol. 2003, 62, 269–280. [Google Scholar] [CrossRef]
- Vale, P. Differential Dynamics of Dinophysistoxins and Pectenotoxins, Part II: Offshore Bivalve Species. Toxicon 2006, 47, 163–173. [Google Scholar] [CrossRef]
- Mafra, L.L.; Bricelj, V.M.; Ouellette, C.; Bates, S.S. Feeding mechanics as the basis for differential uptake of the neurotoxin domoic acid by oysters, Crassostrea virginica, and mussels, Mytilus edulis. Aquat. Toxicol. 2010, 97, 160–171. [Google Scholar] [CrossRef]
- Ward, J.E.; Shumway, S.E. Separating the grain from the chaff: Particle selection in suspension- and deposit-feeding bivalves. J. Exp. Mar. Biol. Ecol. 2004, 300, 83–130. [Google Scholar] [CrossRef]
- Sidari, L.; Nichetto, P.; Cok, S.; Sosa, S.; Tubaro, A.; Honsell, G.; DellaLoggia, R. Phytoplankton selection by mussels, and diarrhetic shellfish poisoning. Mar. Biol. 1998, 131, 103–111. [Google Scholar] [CrossRef]
- Lindegarth, S.; Torgersen, T.; Lundve, B.; Sandvik, M. Differential retention of Okadaic Acid (OA) group toxins and Pectenotoxins (PTX) in the blue mussel, Mytilus edulis (l.), and European flat oyster, Ostrea edulis (l.). J. Shellfish Res. 2009, 28, 313–323. [Google Scholar] [CrossRef]
- Kacem, I.; Bouaïcha, N.; Hajjem, B. Comparison of okadaic acid profiles in mussels and oysters collected in Mediterranean lagoon, Tunisia. Int. J. Biol. 2010, 2, 238–245. [Google Scholar]
- Morton, B. Feeding and Digestion in Bivalvia. In The Mollusca. Physiology Part 2; Saleuddin, A.S.M., Wilbur, K.M., Eds.; Academic Press: New York, NY, USA, 1983; pp. 65–147. [Google Scholar]
- Palmer, R.E. A histological and histochemical study of digestion in the Bivalve Arctica Islandica L. Biol. Bull. 1979, 156, 115–129. [Google Scholar] [CrossRef]
- Ibarrola, I.; Etxeberria, M.; Iglesias, J.I.P.; Urrutia, M.B.; Angulo, E. Acute and acclimated digestive responses of the cockle Cerastoderma edule (L.) to changes in the food quality and quantity: II. Enzymatic, cellular and tissular responses of the digestive gland. J. Exp. Mar. Biol. Ecol. 2000, 252, 199–219. [Google Scholar] [CrossRef]
- Luedeking, A.; van Noorden, C.J.F.; Koehler, A. Identification and characterisation of a multidrug resistance-related protein mRNA in the blue mussel Mytilus edulis. Mar. Ecol. Prog. Ser. 2005, 286, 167–175. [Google Scholar] [CrossRef]
- Faria, M.; Navarro, A.; Luckenbach, T.; Piña, B.; Barata, C. Characterization of the multixenobiotic resistance (MXR) mechanism in embryos and larvae of the zebra mussel (Dreissena polymorpha) and studies on its role in tolerance to single and mixture combinations of toxicants. Aquat. Toxicol. 2011, 101, 78–87. [Google Scholar] [CrossRef]
- Luckenbach, T.; Epel, D. ABCB-and ABCC-type transporters confer multixenobiotic resistance and form an environment-tissue barrier in bivalve gills. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1919–R1929. [Google Scholar] [CrossRef]
- Luedeking, A.; Koehler, A. Regulation of expression of multixenobiotic resistance (MXR) genes by environmental factors in the blue mussel Mytilus edulis. Aquat. Toxicol. 2004, 69, 1–10. [Google Scholar]
- Lozano, V. Identificación de Genes Implicados en la Eliminación de Biotoxinas en el Mejillón Mytilus galloprovincialis Lmk.: Clonación y Expresion de los cDNA que Codifican para tres Proteinas Transportadoras ABC Pertenecientes a las Subfamilias C (proteínas MRP) y G. Ph.D. Thesis, Universidade de Santiago de Compostela, Santiago de Compostela, Spain, 18 July 2013. [Google Scholar]
- Martínez-Escauriaza, R. Identificación de Genes Implicados en la Eliminación de Biotoxinas en el Mejillón Mytilus galloprovincialis Lmk.: Clonación y Expresion de los cDNA que Codifican para dos Proteinas Transportadoras ABC de la Subfamilia B (Proteínas MDR). Ph.D. Thesis, Universidade de Santiago de Compostela, Santiago de Compostela, Spain, 18 July 2013. [Google Scholar]
- Windust, A.J.; Hu, T.M.; Wright, J.L.C.; Quilliam, M.A.; McLachlan, J.L. Oxidative metabolism by Thalassiosira weissflogii (Bacillariophyceae) of a diol-ester of okadaic acid, the diarrhetic shellfish poisoning. J. Phycol. 2000, 36, 342–350. [Google Scholar]
- MacKenzie, L.A.; Selwood, A.I.; Marshall, C. Isolation and characterization of an enzyme from the Greenshell (TM) mussel Perna canaliculus that hydrolyses pectenotoxins and esters of okadaic acid. Toxicon 2012, 60, 406–419. [Google Scholar] [CrossRef]
- Fernández, M.L.; Miguez, A.; Moroño, A.; Cacho, E.; Martínez, A.; Blanco, J. Detoxification of Low Polarity Toxins (DTX3) from Mussels Mytilis galloprovincialis in Spain. In Harmful Algae; Reguera, B., Blanco, J., Fernández, M.L., Wyatt, T., Eds.; Xunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO: Santiago de Compostela, Spain, 1998; pp. 449–452. [Google Scholar]
- Svensson, S. Depuration of Okadaic acid (Diarrhetic Shellfish Toxin) in mussels, Mytilus edulis (Linnaeus), feeding on different quantities of nontoxic algae. Aquaculture 2003, 218, 277–291. [Google Scholar] [CrossRef]
- Vale, P.; Sampayo, M.A. Pectenotoxin-2 seco acid, 7-epi-pectenotoxin-2 seco acid and pectenotoxin-2 in shellfish and plankton from Portugal. Toxicon 2002, 40, 979–987. [Google Scholar] [CrossRef]
- Suzuki, T.; Walter, J.A.; LeBlanc, P.; MacKinnon, S.; Miles, C.O.; Wilkins, A.L.; Munday, R.; Beuzenberg, V.; MacKenzie, L.; Jensen, D.J.; et al. Identification of pectenotoxin-11 as 34S-Hydroxypectenotoxin-2, a new pectenotoxin analogue in the toxic dinoflagellate Dinophysis acuta from New Zealand. Chem. Res. Toxicol. 2006, 19, 310–318. [Google Scholar] [CrossRef]
- Suzuki, T.; Mitsuya, T.; Matsubara, H.; Yamasaki, M. Determination of pectenotoxin-2 after solid-phase extraction from seawater and from the dinoflagellate Dinophysis fortii by liquid chromatography with electrospray mass spectrometry and ultraviolet detection. Evidence of oxidation of pectenotoxin-2 to pectenotoxin-6 in scallop. J. Chromatogr. A 1998, 815, 155–160. [Google Scholar] [CrossRef]
- Blanco, J.; Mariño, C.; Martín, H.; Acosta, C.P. Anatomical distribution of Diarrhetic Shellfish Poisoning (DSP) toxins in the mussel Mytilus galloprovincialis. Toxicon 2007, 50, 1011–1018. [Google Scholar] [CrossRef]
- Suzuki, T.; Ota, H.; Yamasaki, M. Direct evidence of transformation of dinophysistoxin-1 to 7-O-acyl-dinophysistoxin-1 (Dinophysis-3) in the scallop Patinopecten yessoensis. Toxicon 1999, 37, 187–198. [Google Scholar] [CrossRef]
- Torgersen, T.; Sandvik, M.; Lundve, B.; Lindegarth, S. Profiles and levels of fatty acid esters of okadaic acid group toxins and pectenotoxins during toxin depuration. Part II: Blue mussels (Mytilus edulis) and flat oyster (Ostrea edulis). Toxicon 2008, 52, 418–427. [Google Scholar] [CrossRef]
- Wilkins, A.L.; Rehmann, N.; Torgersen, T.; Rundberget, T.; Keogh, M.; Petersen, D.; Hess, P.; Rise, F.; Miles, C.O. Identification of fatty acid esters of pectenotoxin-2 seco acid in blue mussels (Mytilus edulis) from Ireland. J. Agric. Food Chem. 2006, 54, 5672–5678. [Google Scholar] [CrossRef]
- Rossignoli, A.E.; Fernández, D.; Regueiro, J.; Mariño, C.; Blanco, J. Esterification of okadaic acid in the mussel Mytilus galloprovincialis. Toxicon 2011, 57, 712–720. [Google Scholar] [CrossRef]
- Konoki, K.; Onoda, T.; Watanabe, R.; Cho, Y.; Kaga, S.; Suzuki, T.; Yotsu-Yamashita, M. In Vitro acylation of okadaic acid in the presence of various bivalves’ extracts. Mar. Drugs 2013, 11, 300–315. [Google Scholar] [CrossRef]
- Vale, P.; Sampayo, M.A. Esterification of DSP toxins by Portuguese bivalves from the northwest coast determined by LC-MS-a widespread phenomenon. Toxicon 2002, 40, 33–42. [Google Scholar] [CrossRef]
- Torgersen, T.; Miles, C.O.; Rundberget, T.; Wilkins, A.L. New esters of okadaic acid in seawater and blue mussels (Mytilus edulis). J. Agric. Food Chem. 2008, 56, 9628–9635. [Google Scholar] [CrossRef]
- Miles, C.O. Pectenotoxins. In Phycotoxins: Chemistry and Biochemistry; Botana, L.M., Ed.; Wiley: Oxford, UK, 2008; pp. 159–186. [Google Scholar]
- Blanco, J.; Acosta, C.P.; de la Puente, M.B.; Salgado, C. Depuration and anatomical distribution of the amnesic shellfish poisoning (ASP) toxin domoic acid in the king scallop Pecten maximus. Aquat. Toxicol. 2002, 60, 111–121. [Google Scholar] [CrossRef]
- Bricelj, V.M.; Cembella, A.D. Fate of Gonyautoxins in Surfclams, Spisula solidissima, Grazing upon Toxigenic Alexandrium. In Harmful Marine Algal Blooms; Lassus, P., Arzul, G., Erard, E., Gentien, P., Marcaillou, C., Eds.; Lavoisier, Intercept Ltd.: Paris, France, 1995; pp. 413–418. [Google Scholar]
- Rossignoli, A.E.; Blanco, J. Subcellular distribution of okadaic acid in the digestive gland of Mytilus galloprovincialis: First evidences of lipoprotein binding to okadaic acid. Toxicon 2010, 55, 221–226. [Google Scholar] [CrossRef]
- Johansen, M.; Rundberget, T. The sampling technique greatly affects the toxin content in Dinophysis spp. cells. In Proceedings of the 12th International Conference on Harmful Algae, Copenhagen, Denmark, 4–8 September 2006; International Society for the Study of Harmful Algae: Copenhagen, Denmark, 2008; p. 200. [Google Scholar]
- Fux, E.; Marcaillou, C.; Mondeguer, F.; Bire, R.; Hess, P. Field and mesocosm trials on passive sampling for the study of adsorption and desorption behaviour of lipophilic toxins with a focus on OA and DTX1. Harmful Algae 2008, 7, 574–583. [Google Scholar] [CrossRef]
- Maneiro, I.; Guisande, C.; Frangópulos, M.; Riveiro, I. Importance of copepod faecal pellets to the fate of the DSP toxins produced by Dinophysis spp. Harmful Algae 2002, 1, 333–342. [Google Scholar] [CrossRef]
- Setälä, O.; Riitta, A.; Harri, K.; Janne, R.; Pasi, Y. Survival and photosynthetic activity of different Dinophysis acuminata populations in the northern Baltic Sea. Harmful Algae 2005, 4, 337–350. [Google Scholar] [CrossRef]
- McCarron, P.; Kilcoyne, J.; Hess, P. Effects of cooking and heat treatment on concentration and tissue distribution of okadaic acid and dinophysistoxin-2 in mussels (Mytilus edulis). Toxicon 2008, 51, 1081–1089. [Google Scholar] [CrossRef]
- Yasumoto, T.; Murata, M.; Oshima, T.; Matsumoto, C.; Clardy, J. Diarrhetic Shellfish Poisoning. In Seafood Toxins; Ragelis, E.P., Ed.; American Chemical Society: Washington, DC, USA, 1984; pp. 207–214. [Google Scholar]
- Marcaillou-Le, C.; Masselin, P. Recent data on Diarrhetic Shellfish Poisoning in France. In Toxin Marine Phytoplankton; Graneli, E., Sundström, B., Edler, L., Andersen, D., Eds.; Elsevier-North Holland: New York, NY, USA, 1990; pp. 485–488. [Google Scholar]
- Anonymous. Standard Operating Procedure for Detection of Okadaic acid, Dinophysistoxins and Pectenotoxins by Mouse Bioassay. Working group CRLMB/NRL.Version 4.0 (April 2007). Available online: https://www.aesa.msc.es/crlmb/web/CRLMB.jsp (accessed on 15 October 2013).
- Anonymous. Opinion of the Scientific Panel on Contaminants in the Food chain on a request from the European Commission on marine biotoxins in shellfish okadaic acid and analogues. EFSA J. 2008, 589, 1–62. [Google Scholar]
- Simon, J.F.; Vemoux, J.P. Highly sensitive assay of okadaic acid using protein phosphatase and paranitrophenyl phosphate. Nat. Toxins 1994, 2, 293–301. [Google Scholar] [CrossRef]
- Honkanen, R.; Stapleton, J.D.; Bryan, D.E.; Abercrombie, J. Development of a protein phosphatase-based assay for the detection of phosphatase inhibitors in crude whole cell and animal extracts. Toxicon 1996, 34, 1385–1392. [Google Scholar] [CrossRef]
- Vieytes, M.R.; Fontal, O.I.; Leira, F.; Baptista de Sousa, J.M.V.; Botana, L.M. A fluorescent microplate assay for diarrheic shellfish toxins. Anal. Biochem. 1997, 248, 258–264. [Google Scholar] [CrossRef]
- Mountfort, D.O.; Kennedy, G.; Garthwaite, I.; Quilliam, M.A.; Truman, P.; Hannah, D.J. Evaluation of the fluorometric protein phosphatase inhibition assay in the determination of okadaic acid in mussels. Toxicon 1999, 37, 909–922. [Google Scholar] [CrossRef]
- Ramstad, H.; Shen, J.L.; Larsen, S.; Aune, T. The validity of two HPLC methods and a colorimetric PP2A assay related to the mouse bioassay in quantification of diarrhetic toxins in blue mussels (Mytilus edulis). Toxicon 2001, 39, 1387–1391. [Google Scholar] [CrossRef]
- Tubaro, A.; Florio, C.; Luxich, E.; Sosa, S.; Loggia, R.D.; Yasumoto, T. A protein phosphatase 2A inhibition assay for a fast and sensitive assessment of okadaic acid contamination in mussels. Toxicon 1996, 34, 743–752. [Google Scholar] [CrossRef]
- Smienk, H.; Dominguez, E.; Rodríguez-Velasco, M.L.; Clarke, D.; Kapp, K.; Katikou, P.; Cabado, A.G.; Otero, A.; Vieites, J.M.; Razquin, P.; et al. Quantitative determination of the okadaic acid toxins group by a colorimetric phosphatase inhibition assay: Interlaboratory study. J. AOAC Int. 2013, 96, 77–85. [Google Scholar] [CrossRef]
- Aune, T.; Yasumoto, T.; Engeland, E. Light and scanning electron microscopic studies on effects of marine algal toxins toward freshly prepared hepatocytes. J. Toxicol. Environ. Health 1991, 34, 1–9. [Google Scholar] [CrossRef]
- Amzil, Z.; Pouchus, Y.F.; le Boterff, J.; Roussakis, C.; Verbist, J.-F.; Marcaillou-Lebaut, C.; Masselin, P. Short-time cytotoxicity of mussel extracts: A new bioassay for okadaic acid detection. Toxicon 1992, 30, 1419–1425. [Google Scholar] [CrossRef]
- Diogène, J.; Fessard, V.; Ammar, M.; Dubreuil, A.; Puiseux-Dao, S. Evaluation of Cytotoxic Responses to a Maitotoxin Extract and Okadaic Acid. In Harmful Marine Algal Blooms; Lassus, P., Arzul, G., Erard, E., Gentien, P., Marcaillou-Le Baut, C., Eds.; Lavoisier: Paris, France, 1995; pp. 285–290. [Google Scholar]
- Cañete, E.; Campàs, M.; de la Iglesia, P.; Diogène, J. NG108–15 cell-based and protein phosphatase inhibition assays as alternative semiquantitative tools for the screening of lipophilic toxins in mussels. Okadaic acid detection. Toxicol. in Vitro 2010, 24, 611–619. [Google Scholar] [CrossRef]
- Tubaro, A.; Florio, C.; Luxich, E.; Vertua, R.; Della loggia, R.; Yasumoto, T. Suitability of the MTT-based cytotoxicity assay to detect okadaic acid contamination of mussels. Toxicon 1996, 34, 965–974. [Google Scholar] [CrossRef]
- Levine, L.; Fujiki, H.; Yamada, K.; Ojika, M.; Gjika, H.B.; van Vunakis, H. Production of antibodies and development of a radioimmunoassay for okadaic acid. Toxicon 1988, 26, 1123–1128. [Google Scholar] [CrossRef]
- Usagawa, T.; Nishimura, M.; Itoh, Y.; Uda, T.; Yasumoto, T. Preparation of monoclonal antibodies against okadaic acid prepared from the sponge Halichondria okadai. Toxicon 1989, 27, 1323–1330. [Google Scholar] [CrossRef]
- Shestowsky, W.S.; Quilliam, M.A.; Sikorska, H.M. An idiotypic-anti-idiotypic competitive immunoassay for quantitation of okadaic acid. Toxicon 1992, 30, 1441–1448. [Google Scholar] [CrossRef]
- Shestowsky, W.S.; Holmes, C.F.B.; Hu, T.M.; Marr, J.; Wright, J.L.C.; Chin, J.; Sikorska, H.M. An anti-okadaic acid-anti-idiotypic antibody bearing an internal image of okadaic acid inhibits protein phosphatase PP1 and PP2A catalytic activity. Biochem. Biophys. Res. 1993, 192, 302–310. [Google Scholar] [CrossRef]
- Chin, J.D.; Quilliam, M.A.; Fremy, J.M.; Mohatrapa, S.K.; Sikorska, H.M. Screening for okadaic acid by inmunoassay. J. AOAC Int. 1995, 78, 508–513. [Google Scholar]
- Carmody, E.P.; James, K.J.; Kelly, S.S. Diarrhetic shellfish poisoning: Evaluation of enzyme-linked immunosorbent assay methods for dinophysistoxin-2 determination. J. AOAC Int. 1995, 78, 1408–1408. [Google Scholar]
- Anonymous. Food stuffs Determination of Okadaic Acid and Dinophysis toxin in Mussels HPLC Method with Solid Phase Extraction Clean-up after Derivatization and Fluorimetric Detection; European Standardization Committee (CEN): Brussels, Belgium, 2004; p. 14524. [Google Scholar]
- Anonymous. Commision Regulation (EU) No 15/201. Amending Regulation (EC) No 2074/2005 as regards recognised testing methods for detecting marine biotoxins in live bivalve molluscs. Off. J. Eur. Union 2011, L6, 3–6. [Google Scholar]
- Nogueiras, M.J.; Gago-Martinez, A.; Paniello, A.I.; Twohig, M.; James, K.; Lawrence, J.F. Comparison of different fluorometric HPLC method for analysis of acidic polyether toxins in marine phytoplankton. Anal. Bioanal. Chem. 2003, 377, 1202–1206. [Google Scholar] [CrossRef]
- Yasumoto, T.; Fukui, M.; Sasaki, K.; Sugiyama, K. Determinations of marine toxins in foods. J. AOAC Int. 1995, 78, 574–582. [Google Scholar]
- Draisci, R.; Giannetti, L.; Lucentini, L.; Ferretti, E.; Palleschi, L.; Marchiafava, C. Direct identification of yessotoxin in shellfish by liquid chromatography coupled with mass spectrometry and tandem mass spectrometry. Rapid Commun. Mass Spectrom. 1998, 12, 1291–1296. [Google Scholar] [CrossRef]
- Pleasance, S.; Quilliam, M.A.; de Freitas, A.S.W.; Marr, J.C.; Cembella, A.D. Ion-spray mass spectrometry of marine toxins. II. Analysis of diarrhetic shellfish toxins in plankton by liquid chromatrography/mass spectrometry. Rapid Commun. Mass Spectrom. 1990, 4, 206–213. [Google Scholar] [CrossRef]
- Quilliam, M.A. Analysis of Diarrhetic Shellfish Poisoning Toxins in shellfih tissue by LC with fluorometric and Mass Spectrometric detection. J. AOAC Int. 1995, 78, 555–569. [Google Scholar]
- Suzuki, T.; Yasumoto, T. Liquid chromatography electrospray ionization mass spectrometry of the diarrhetic shellfish poisoning toxins okadaic acid, dinophysistoxin-1 and pectenotoxin-6 in bivalves. J. Chomatogr. A 2000, 874, 199–206. [Google Scholar] [CrossRef]
- Hu, T.; Marr, J.; Defreitas, A.S.W.; Quilliam, M.A.; Walter, J.A.; Wright, J.L.C. New diolester (of okadaic acid) isolated from cultures of Prorocentrum lima and Prorocentrum concavum. J. Nat. Prod. 1992, 55, 1631–1637. [Google Scholar] [CrossRef]
- Suzuki, H.; Beuzenberg, V.; MacKenzie, A.L.; Quilliam, M.A. Discovery of okadaic acid esters in the toxic dinoflagellate Dinophysis acuta from New Zealand using liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 1131–1138. [Google Scholar] [CrossRef]
- Paz, B.; Daranas, A.H.; Cruz, P.G.; Franco, J.M.; Pizarro, G.; Souto, M.L.; Norte, M.; Fernández, J.J. Characterisation of okadaic acid related toxins by liquid chromatography coupled with mass spectrometry. Toxicon 2007, 50, 225–235. [Google Scholar] [CrossRef]
- Gerssen, A.; McElhinney, M.A.; Mulder, P.; Bire, R.; Hess, P.; Boer, J. Solid phase extraction for removal of matrix effects in lipophilic marine toxin analysis by liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2009, 394, 1213–1226. [Google Scholar] [CrossRef]
- Paz, B.; Riobó, P.; Franco, J.M. Preliminary study for rapid determination of phycotoxins in microalgae whole cells using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 3627–3639. [Google Scholar] [CrossRef] [Green Version]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Reguera, B.; Riobó, P.; Rodríguez, F.; Díaz, P.A.; Pizarro, G.; Paz, B.; Franco, J.M.; Blanco, J. Dinophysis Toxins: Causative Organisms, Distribution and Fate in Shellfish. Mar. Drugs 2014, 12, 394-461. https://doi.org/10.3390/md12010394
Reguera B, Riobó P, Rodríguez F, Díaz PA, Pizarro G, Paz B, Franco JM, Blanco J. Dinophysis Toxins: Causative Organisms, Distribution and Fate in Shellfish. Marine Drugs. 2014; 12(1):394-461. https://doi.org/10.3390/md12010394
Chicago/Turabian StyleReguera, Beatriz, Pilar Riobó, Francisco Rodríguez, Patricio A. Díaz, Gemita Pizarro, Beatriz Paz, José M. Franco, and Juan Blanco. 2014. "Dinophysis Toxins: Causative Organisms, Distribution and Fate in Shellfish" Marine Drugs 12, no. 1: 394-461. https://doi.org/10.3390/md12010394




