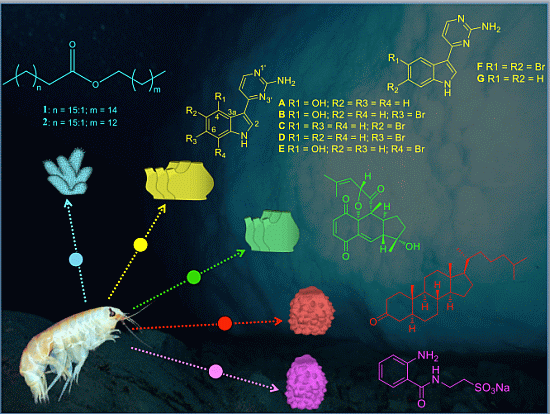
Defensive Metabolites from Antarctic Invertebrates: Does Energetic Content Interfere with Feeding Repellence?
Abstract
:1. Introduction
2. Results and Discussion
| Compounds’ Name and Chemical Structure | Data | |
|---|---|---|
 | Chemical type | Wax ester |
| [N]Comp in DWTOTAL | 25 mg·g−1 | |
| Organic fraction | Ether (EE) | |
| Source organism | Soft coral | |
| Species name | Alcyonium haddoni | |
| Phylum:Class | Cnidaria:Anthozoa | |
| Sample location | Deception Island | |
 | Chemical type | Indole alkaloid |
| [N]Comp DWTOTAL | 24.3 mg·g−1 | |
| Organic fraction | Ether (EE) | |
| Source organism | Colonial ascidian | |
| Species name | Aplidium falklandicum | |
| Phylum:Class | Chordata:Ascidiacea | |
| Sample location | Weddell Sea | |
 | Chemical type | Meroterpene |
| [N]Comp DWTOTAL | 5.1 mg·g−1 * | |
| Organic fraction | Ether (EE) | |
| Source organism | Colonial ascidian | |
| Species name | Aplidium fuegiense | |
| Phylum:Class | Chordata:Ascidiacea | |
| Sample location | Weddell Sea | |
 | Chemical type | Keto-steroid |
| [N]Comp DWTOTAL | 2.8 mg·g−1 | |
| Organic fraction | Ether (EE) | |
| Source organism | Glass sponge | |
| Species name | Rossella nuda | |
| Phylum:Class | Porifera:Hexactinellida | |
| Sample location | Weddell Sea | |
 | Chemical type | Sulfonate acid |
| [N]Comp DWTOTAL | 2.34 mg·g−1 | |
| Organic fraction | Butanol (BE) | |
| Source organism | Glass sponge | |
| Species name | Anoxycalyx (Scolimastra) joubini | |
| Phylum:Class | Porifera:Hexactinellida | |
| Sample location | Weddell Sea | |

2.1. Control (Compound-Free) Diets
| Diets Contrasted | Feeding Preferences | Ingestion Rates | ||
|---|---|---|---|---|
| PhytoPlan® Content | Preference Result | Wilcoxon Test | Ingestion Result | Tukey (HDS) |
| 200 mg vs. 100 mg | 200 mg > 100 mg | p = 0.036 * | 200 mg < 100 mg | p < 0.001 ** |
| 200 mg vs. 50 mg | 200 mg > 50 mg | p = 0.014 * | 200 mg < 50 mg | p < 0.001 ** |
| 100 mg vs. 50 mg | 100 mg > 50 mg | p = 0.089 n.s. | 100 mg > 50 mg | p < 0.001 ** |

2.2. Feeding Repellent Activities of Target Bioactive Metabolites (1–12)
| Compounds | (mg·g−1) | PhytoPlan (mg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 200 | 100 | 50 | ||||||||
| Wax esters (1–2) | 10 | − | − | + | ||||||
| 5 | − | − | − | |||||||
| 2.5 | − | − | − | |||||||
| Meridianins A–G (3–9) | 10 | + | + | + | ||||||
| 5 | − | + | + | |||||||
| 2.5 | − | + | + | |||||||
| Rossinone B (10) | 10 | + | + | + | ||||||
| 5 | + | − | − | |||||||
| 2.5 | − | − | − | |||||||
| 5α(H)-cholestan-3-one (11) | 10 | + | − | − | ||||||
| 5 | − | − | − | |||||||
| 2.5 | − | − | − | |||||||
| Glassponsine (12) | 10 | + | + | + | ||||||
| 5 | − | − | − | |||||||
| 2.5 | − | − | − | |||||||
| 0%–20% | 21%–40% | 41%–60% | 61%–80% | 81%–100% | ||||||
2.3. Interference between Energetic Content and Compounds (1–12) Deterrent Bioactivity

3. Experimental Section
3.1. Sample Collection and Extraction
3.2. Molecular Characterization
3.3. Purification of Target Metabolites
- -
- Wax esters (1–2): The lipophilic (Et2O) fraction from the soft coral Alcyonium haddoni was submitted to silica gel column purification to give a wax ester subfraction (Rf 0.9, light petroleum ether/Et2O, 9:1). LC-MS analysis (250 × 4.60 mm, Phenomenex Kromasil C18, 60 min gradient from 30% to 100% CH3OH in H2O) showed that the wax ester mixture comprised the two main components: wax (1) C34:1 (m/z 529 [M + Na]+), and wax (2) C32:1 (m/z 501 [M + Na]+) (both composed of a C18:1 monounsaturated fatty acid, and C16:0 and C14:0 saturated alcohol esters, respectively). 1–2 were assayed as a mixture, because they are the main components of the wax ester fraction in Alcyonium soft corals. For further details on purification and molecular determination of products 1 and 2 see [27];
- -
- Meridianins A–G (3–9): Column chromatography of the ether fraction of the colonial ascidian Aplidium falklandicum, followed by a TLC purification with preparative (SiO2) plates (Merk Kiesegel 60 F254 0.50 and 1.00 mm), provided an abundant yellowish subfraction (Rf 0.63, chloroform/methanol, 8:2), which corresponded to the alkaloid mixture of meridianins A–G. These products (3–9) were tested as a mixture since in nature they occur together as a rich fraction of bioactively related derivatives. For details on purification and molecular determination of meridianins A–G see [21,25];
- -
- Rossinone B (10): The Et2O inner extract from the ascidian Aplidium fuegiense was fractioned through column and TLC purifications, allowing to recover significant quantities of the meroterpenoid rossinone B (10) (Rf 0.65, light petroleum ether/Et2O, 2:8). Details on purification and molecular determination of product 10 may be found in [21,28];
- -
- 5α(H)-cholestan-3-one (11): The ether extract of the glass sponge Rossella nuda was fractioned by silica gel chromatography, using a gradient of light petroleum ether/Et2O. The fraction eluted with 10% of diethyl ether (Rf 0.51, light petroleum ether/Et2O, 8:2) contained pure 5α(H)-cholestan-3-one (11). More information on the isolation of product 11 is published in [26];
- -
- Glassponsine (12): The fractionation of the BE fraction of the hexactinellid Anoxycalyx (Scolimastra) joubini on Si gel column chromatography (gradient 0%–100% metanol in chloroform) yielded a fraction containing a UV sensitive spot at Rf 0.15 (chloroform/methanol, 8:2). This fraction was further purified by preparative TLC chromatography (SiO2, chloroform/methanol, 65:35) to yield a recently reported pure compound, glassponsine 12, with a structure elucidated by spectroscopic methods elsewhere [29].
3.4. Artificial Food Preparation
| Compounds Tested | Composition of Artificially Prepared Diets (Feeding Pearls) Compound (mg)/PhytoPlan (mg)/Sand (mg)/Alginate Solution (mL) | [Conc] DWTOTAL (mg·g−1) | [Conc] Volume (mg·mL−1) | ||
|---|---|---|---|---|---|
| Wax esters mix (1–2) Meridianins A–G mix (3–9) Rossinone B (10) 5α(H)-cholestan-3-one (11) Glassponsine (12) | 2/200/0/3 | 2/100/100/3 | 2/50/150/3 | 10 | 0.66 |
| 1/200/0/3 | 1/100/100/3 | 1/50/150/3 | 5 | 0.33 | |
| 0.5/200/0/3 | 0.5/100/100/3 | 0.5/50/150/3 | 2.5 | 0.17 | |
| CONTROLS | 0/200/0/3 | 0/100/100/3 | 0/50/150/3 | - | - |
3.5. Feeding-Preference Bioassays with Amphipods
3.6. Statistical Analysis

4. Conclusions
Supplementary Files
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McClintock, J.B.; Baker, B.J. Marine Chemical Ecology; CRC Marine Science Series Press: Boca Raton, FL, USA, 2001; p. 624. [Google Scholar]
- Paul, V.J.; Ritson-Williams, R.; Sharp, K. Marine chemical ecology in benthic environments. Nat. Prod. Rep. 2011, 28, 345–387. [Google Scholar] [CrossRef]
- Pawlik, J.R. Antipredatory defensive roles of natural products from marine invertebrates. In Handbook of Marine Natural Products; Fattorusso, E., Gerwick, W.E., Taglialatela-Scafati, O., Eds.; Springer: New York, NY, USA, 2012; p. 1452. [Google Scholar]
- Begon, M.; Townsend, C.; Harper, J.L. Ecology—From Individuals to Systems; Blackwell Publishing Ltd.: Oxford, UK, 2006; p. 739. [Google Scholar]
- Paul, V.J. Ecological Roles of Marine Natural Products; Comstock Publications Association, Ithaca: New York, NY, USA, 1992. [Google Scholar]
- Paul, V.J.; Arthur, K.E.; Ritson-Williams, R.; Ross, C.; Sharp, K. Chemical defenses: From compounds to communities. Biol. Bull. 2007, 213, 226–251. [Google Scholar] [CrossRef]
- Sotka, E.E.; Forbey, J.; Horn, M.; Poore, A.G.B.; Raubenheimer, D.; Whalen, K.E. The emerging role of pharmacology in understanding consumer-prey interactions in marine and freshwater systems. Integr. Comp. Biol. 2009, 49, 291–313. [Google Scholar] [CrossRef]
- Rhoades, D.F.; Gates, R.G. Towards a general theory of plant antiherbivore chemistry. Recent Adv. Phytochem. 1976, 10, 168–213. [Google Scholar]
- Haslam, E. Secondary metabolism—Evolution and function: Products or processes? Chemoecology 1994, 5–6, 89–95. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2014, 31, 160–258. [Google Scholar] [CrossRef]
- Munro, M.; Blunt, J. MarineLit Database; University Canterbury: Christchurch, New Zealand, 2009. [Google Scholar]
- Hay, M.E.; Kappel, Q.E.; Fenical, W. Synergisms in Plant Defenses against Herbivores—Interactions of Chemistry, Calcification, and Plant-Quality. Ecology 1994, 75, 1714–1726. [Google Scholar] [CrossRef]
- Hay, M.E. Defensive Synergisms? Reply to Pennings. Ecology 1996, 77, 1950–1952. [Google Scholar] [CrossRef]
- Pennings, S.C. Testing for synergisms between chemical and mineral defenses—A comment. Ecology 1996, 77, 1948–1950. [Google Scholar] [CrossRef]
- Chanas, B.; Pawlik, J.R. Does the skeleton of a sponge provide a defense against predatory reef fish? Oecologia 1996, 107, 225–231. [Google Scholar] [CrossRef]
- Duffy, J.E.; Paul, V.J. Prey nutritional quality and the effectiveness of chemical defenses against tropical reef fishes. Oecologia 1992, 90, 333–339. [Google Scholar] [CrossRef]
- Avila, C.; Taboada, S.; Núñez-Pons, L. Marine Antarctic chemical ecology: What is next? Mar. Ecol. 2008, 29, 1–70. [Google Scholar]
- McClintock, J.B.; Amsler, C.D.; Baker, B. Overview of the chemical ecology of benthic marine invertebrates along the western Antarctic Peninsula. Integr. Comp. Biol. 2010, 50, 967–980. [Google Scholar] [CrossRef]
- Núñez-Pons, L.; Rodríguez-Arias, M.; Gómez-Garreta, A.; Ribera-Siguán, A.; Avila, C. Feeding deterrency in Antarctic marine organisms: Bioassays with the omnivore amphipod Cheirimedon femoratus. Mar. Ecol. Progess Ser. 2012, 462, 163–174. [Google Scholar] [CrossRef]
- Kubanek, J.; Whalen, K.E.; Engel, S.; Kelly, S.R.; Henkel, T.P.; Fenical, W.; Pawlik, J.R. Multiple defensive roles for triterpene glycosides from two Caribbean sponges. Oecologia 2002, 131, 125–136. [Google Scholar] [CrossRef]
- Núñez-Pons, L.; Carbone, M.; Vazquez, J.; Rodriguez, J.; Nieto, R.M.; Varela, M.M.; Gavagnin, M.; Avila, C. Natural Products from Antarctic Colonial Ascidians of the Genera Aplidium and Synoicum: Variability and Defensive Role. Mar. Drugs 2012, 10, 1741–1764. [Google Scholar] [CrossRef] [Green Version]
- Herms, D.A.; Mattson, W.J. The Dilemma of Plants: To Grow or Defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar]
- Lebar, M.D.; Heimbegner, J.L.; Baker, B.J. Cold-water marine natural products. Nat. Prod. Rep. 2007, 24, 774–797. [Google Scholar] [CrossRef]
- Dayton, P.K.; Robilliard, G.A.; Paine, R.T.; Dayton, L.B. Biological accomodation in the benthic community at McMurdo Sound, Antarctica. Ecol. Monogr. 1974, 44, 105–128. [Google Scholar]
- Núñez-Pons, L.; Forestieri, R.; Nieto, R.M.; Varela, M.; Nappo, M.; Rodríguez, J.; Jiménez, C.; Castelluccio, F.; Carbone, M.; Ramos-Esplá, A.; et al. Chemical defenses of tunicates of the genus Aplidium from the Weddell Sea (Antarctica). Polar Biol. 2010, 33, 1319–1329. [Google Scholar] [CrossRef]
- Núñez-Pons, L.; Carbone, M.; Paris, D.; Melck, D.; Ríos, P.; Cristobo, J.; Castelluccio, F.; Gavagnin, M.; Avila, C. Chemo-ecological studies on hexactinellid sponges from the Southern Ocean. Naturwissenschaften 2012, 99, 353–368. [Google Scholar] [CrossRef]
- Núñez-Pons, L.; Carbone, M.; Vázquez, J.; Gavagnin, M.; Avila, C. Lipophilic defenses from Alcyonium soft corals of Antarctica. J. Chem. Ecol. 2013, 39, 675–685. [Google Scholar] [CrossRef]
- Carbone, M.; Núñez-Pons, L.; Castelluccio, F.; Avila, C.; Gavagnin, M. Rossinone-related meroterpenes from the Antarctic ascidian Aplidium fuegiense. Tetrahedron 2012, 68, 3541–3544. [Google Scholar] [CrossRef]
- Carbone, M.; Núñez-Pons, L.; Ciavatta, M.L.; Castelluccio, F.; Avila, C.; Gavagnin, M. Occurrence of a Taurine Derivative in an Antarctic Glass Sponge. NPC Nat. Prod. Commun. 2014, 9, 469–470. [Google Scholar]
- Brosnan, J.T.; Brosnan, M.E. The sulfur-containing amino acids: An overview. J. Nutr. 2006, 136, 1636S–1640S. [Google Scholar]
- Cook, A.M.; Denger, K. Metabolism of taurine in microorganisms. Adv. Exp. Med. Biol. 2006, 583, 3–13. [Google Scholar] [CrossRef]
- Leys, S.P.; Mackie, G.O.; Reiswig, H.M. The biology of glass sponges. Adv. Mar. Biol. 2007, 52, 1–145. [Google Scholar] [CrossRef]
- Chanas, B.; Pawlik, J.R. Variability in the chemical defense of the Caribbean reef sponge Xestospongia muta. In Proceedings of the 8th International Coral Reef Symposium, Panama, Latin America, 24–29 June 1996; Lessios, H.A., Macintyre, I.G., Eds.; Smithsonian Tropical Research Institute: Balboa, Panamá, 1997; pp. 1363–1367. [Google Scholar]
- Ge, H.M.; Tan, R.X. Symbionts, an important source of new bioactive natural products. Prog. Chem. 2009, 21, 30–46. [Google Scholar]
- Cutignano, A.; Zhang, W.; Avila, C.; Cimino, G.; Fontana, A. Intrapopulation variability in the terpene metabolism of the Antarctic opisthobranch mollusc Austrodoris kerguelenensis. Eur. J. Org. Chem. 2011, 27, 5383–5389. [Google Scholar]
- Kobayashi, J.; Ishibashi, M. Bioactive metabolites of symbiotic marine microorganisms. Chem. Rev. 1993, 93, 1753–1769. [Google Scholar] [CrossRef]
- Taylor, M.W.; Radax, R.; Steger, D.; Wagner, M. Sponge-associated microorganisms: Evolution, ecology, and biotechnological potential. Microbiol. Mol. Biol. Rev. 2007, 71, 295–347. [Google Scholar] [CrossRef]
- Raubenheimer, D. Tannic acid, protein, and digestible carbohydrate: Dietary imbalance and nutritional compensation in locusts. Ecology 1992, 73, 1012–1027. [Google Scholar] [CrossRef]
- Slansky, F.; Wheeler, S. Caterpillars’ compensatory feeding response to diluted nutrients leads to toxic allelochemical dose. Entomol. Exp. Appl. 1992, 65, 171–186. [Google Scholar] [CrossRef]
- Van Gils, J.A.; de Rooij, S.R.; van Belle, J.; van der Meer, J.; Dekinga, A.; Piersma, T.; Drent, R. Digestive bottleneck affects foraging decisions in red knots Calidris canutus. I. Prey choice. J. Anim. Ecol. 2005, 74, 105–119. [Google Scholar]
- Verlinden, C.; Haven Wiley, R. The constraints of digestive rate: An alternative model of diet selection. Evol. Ecol. 1989, 3, 264–272. [Google Scholar] [CrossRef]
- Taylor, G.D.; Smith, S.O.; Gagosian, R.B. Use of microbial enrichments for the study of the anaerobic degradation of cholesterol. Geochim. Cosmochim. Acta 1981, 45, 2161–2168. [Google Scholar] [CrossRef]
- Sargent, J.R.; Gatten, R.R.; McIntosh, R. Wax esters in the marine environment—Their occurrence, formation, transformation and ultimate fates. Mar. Chem. 1977, 5, 573–584. [Google Scholar] [CrossRef]
- Appleton, D.R.; Chuen, C.S.; Berridge, M.V; Webb, V.L.; Copp, B.R. Rossinones A and B, biologically active meroterpenoids from the Antarctic ascidian, Aplidium species. J. Org. Chem. 2009, 74, 9195–9198. [Google Scholar] [CrossRef]
- Hernández Franco, L.; Bal de Kier Joffé, E.; Puricelli, L.; Tatián, M.; Seldes, A.M.; Palermo, J.A. Indole alkaloids from the Tunicate Aplidium meridianum. J. Nat. Prod. 1998, 61, 1130–1132. [Google Scholar] [CrossRef]
- Gompel, M.; Leost, M.; Bal de Kier Joffé, E.; Puricelli, L.; Hernández Franco, L.; Palermo, J.A.; Meijer, L. Meridianins, a new family of protein kinase inhibitors isolated from the Ascidian Aplidium meridianum. Bioorg. Med. Chem. Lett. 2004, 14, 1703–1707. [Google Scholar] [CrossRef]
- Paul, V.J.; Lindquist, N.; Fenical, W. Chemical defenses of the tropical ascidian Atapozoa sp. and its nudibranch predators Nembrotha spp. Mar. Ecol. Progress Ser. 1990, 59, 109–118. [Google Scholar] [CrossRef]
- Slattery, M.; Hamann, M.T.; McClintock, J.B.; Perry, T.L.; Puglisi, M.P.; Yoshida, W.Y. Ecological roles for water-borne metabolites from Antarctic soft corals. Mar. Ecol. Progress Ser. 1997, 161, 133–144. [Google Scholar] [CrossRef]
- Albert and Ferran Adrià Home Page. Available online: http://www.albertyferranadria.com/eng/texturas.html (accessed on 23 March 2012).
- Atwater, W.O.; Benedict, F.G. Experiments on the Metabolism of Matter and Energy in the Human Body, 1898–1900; Government Printing Office: Washington, DC, USA, 1902; p. 147. [Google Scholar]
- McClintock, J.B. Investigation of the relationship between invertebrate predation and biochemical composition, energy content, spicule armament and toxicity of benthic sponges at McMurdo Sound, Antarctica. Mar. Biol. 1987, 94, 479–487. [Google Scholar] [CrossRef]
- Barthel, D. Tissue composition of sponges from the Weddell Sea, Antarctica—Not much meat on the bones. Mar. Ecol. Prog. Ser. 1995, 123, 149–153. [Google Scholar] [CrossRef]
- Slattery, M.; McClintock, J.B. Population structure and feeding deterrence in three shallow-water Antarctic soft corals. Mar. Biol. 1995, 122, 461–470. [Google Scholar] [CrossRef]
- McClintock, J.B.; Amsler, M.O.; Amsler, C.D.; Southworth, K.J.; Petrie, C.; Baker, B.J. Biochemical composition, energy content and chemical antifeedant and antifoulant defenses of the colonial Antarctic ascidian Distaplia cylindrica. Mar. Biol. 2004, 145, 885–894. [Google Scholar] [CrossRef]
- Bregazzi, P.K. Habitat selection of Cheirimedon femoratus (Pfeffer) and Thryphosella kergueleni (Miers) (Crustacea: Amphipoda). Br. Antarct. Surv. Bull. 1972, 31, 21–31. [Google Scholar]
- De Broyer, C.; Lowry, J.K.; Jazdzewski, K.; Robert, H. Catalogue of the Gammaridean and Corophiidean Amphipoda of the Southern Ocean, with distribution and ecological data. In Census of Antarctic Marine Life: Synopsis of the Amphipoda of the Southern Ocean; de Broyer, C., Ed.; Royal Belgian Institute of Natural Sciences: Brussels, Belgium, 2007; Volume 1, Part 1; pp. 1–325. [Google Scholar]
- Peterson, C.H.; Renaud, P.E. Analysis of feeding preference experiments. Oecologia 1989, 80, 82–86. [Google Scholar] [CrossRef]
- The R Project for Statistical Computing Home Page. Available online: http://www.r-project.org (accessed on 11 April 2013).
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Núñez-Pons, L.; Avila, C. Defensive Metabolites from Antarctic Invertebrates: Does Energetic Content Interfere with Feeding Repellence? Mar. Drugs 2014, 12, 3770-3791. https://doi.org/10.3390/md12063770
Núñez-Pons L, Avila C. Defensive Metabolites from Antarctic Invertebrates: Does Energetic Content Interfere with Feeding Repellence? Marine Drugs. 2014; 12(6):3770-3791. https://doi.org/10.3390/md12063770
Chicago/Turabian StyleNúñez-Pons, Laura, and Conxita Avila. 2014. "Defensive Metabolites from Antarctic Invertebrates: Does Energetic Content Interfere with Feeding Repellence?" Marine Drugs 12, no. 6: 3770-3791. https://doi.org/10.3390/md12063770