The Skeletal Amino Acid Composition of the Marine Demosponge Aplysina cavernicola
Abstract
:1. Introduction
2. Results and Discussion
2.1. Examination of the Skeletons by SEM

2.2. Amino Acid Extraction, Selection of the Derivatization Agent and the Internal Standard for GC-MS


2.3. GC-MS Analysis of the Skeletal Amino Acid Composition
2.3.1. Skeletal Amino Acid Composition before MeOH Extraction
| Peak | tert-Butyldimethylsilyl (TBDMS)-Derivative | Proteinogenic | Halogenated |
|---|---|---|---|
| 1 | Alanine | X | |
| 2 | Glycine | X | |
| 3 | α-Aminobutyric Acid (AABA) | ||
| 4 | Valine | X | |
| A | Urea | ||
| 5 | Leucine | X | |
| 6a | Serine (2 TBDMS) | X | |
| 7 | Proline | X | |
| 8 | Oxoproline | ||
| 9a | Hydroxyproline (2 TBDMS) | ||
| 6b | Serine (3 TBDMS) | X | |
| 10a | Threonine (3 TBDMS) | X | |
| 10b | Threonine (3 TBDMS) | X | |
| 11 | Phenylalanine | X | |
| 12 | Aspartic Acid | X | |
| 9b | Hydroxyproline (3 TBDMS) | ||
| 13 | Glutamic Acid | X | |
| 14 | Ornithine | ||
| 15 | Lysine | X | |
| B | Aerothionin or its derivatives | ||
| 16 | Arginine | X | |
| 17 | Histidine | X | |
| 18 | Tyrosine | X | |
| 19 | Tryptophan | X | |
| 20 | 3-Monochlorotyrosine | X | |
| 21* | Monobromohistidine | X | |
| 22* | Monobromotyrosine | X | |
| 23* | Dichlorotyrosine | X | |
| 24 | 3-Monoiodotyrosine | X | |
| 25* | Monobromo-monochlorotyrosine | X | |
| 26 | 3,5-Dibromotyrosine | X | |
| 27* | Monochloro-monoiodotyrosine | X | |
| 28* | Monobromo-monoiodotyrosine | X | |
| 29 | 3,5-Diiodotyrosine | X |

| Sample | Arginine/Ornithine Proportion | Arginine/Urea Proportion |
|---|---|---|
| Standard: arginine after Ba(OH)2 | 1:2.6 | 1:1.1 |
| Ba(OH)2 extract of sponge skeletons before MeOH | 1:8.8 | 1:9.1 |

| Amino Acids (AAs) | Present Work | AAs in Hippospongia equina [29] | AAs in Spongia Officinalis obliqua [30] |
|---|---|---|---|
| α-Aminobutyric Acid (AABA) | X | X | |
| γ-Aminobutyric Acid (GABA) | X | ||
| Alanine | X | X | X |
| Arginine | X | X | |
| Aspartic Acid | X | X | X |
| Cystine | X | ||
| Glutamic Acid | X | X | X |
| Glycine | X | X | X |
| Histidine | X | X | |
| Hydroxyproline | X | X | X |
| Leucine | X | X | X |
| Lysine | X | X | X |
| Methionine | X | ||
| Ornithine | X | X | |
| Oxoproline | X | ||
| Phenylalanine | X | X | |
| Proline | X | X | X |
| Serine | X | X | |
| Threonine | X | X | |
| Tryptophan | X | X | X |
| Tyrosine | X | X | X |
| Valine | X | X | X |
| Monobromohistidine | X | ||
| Monobromotyrosine | X | ||
| 3-Monochlorotyrosine | X | ||
| 3-Monoiodotyrosine | X | X | |
| Monochloro-monoiodotyrosine | X | ||
| Monobromo-monochlorotyrosine | X | ||
| Monobromo-monoiodotyrosine | X | ||
| Dichlorotyrosine | X | ||
| 3,5-Dibromotyrosine | X | X | |
| 3,5-Diiodotyrosine | X | X | X |
2.3.2. Skeletal Amino Acid Composition after MeOH Extraction
| TBDMS–Derivative | Decrease 1 |
|---|---|
| Alanine | + |
| Glycine | ++ |
| α-Aminobutyric Acid (AABA) | +++ |
| Valine | +++ |
| Leucine | +++ |
| Serine (2 TBDMS) | − |
| Proline | ++ |
| Oxoproline | + |
| Hydroxyproline (2 TBDMS) | ++ |
| Serine (3 TBDMS) | ++ |
| Threonine (3 TBDMS) | ++++ |
| Threonine (3 TBDMS) | ++++ |
| Phenylalanine | +++ |
| Aspartic Acid | +++ |
| Hydroxyproline (3 TBDMS) | +++ |
| Glutamic Acid | − |
| Ornithine | ++ |
| Lysine | ++ |
| Arginine | ++ |
| Histidine | +++ |
| Tyrosine | + |
| Tryptophan | + |
| 3-Monochlorotyrosine | – |
| Monobromohistidine | ++ |
| Monobromotyrosine | + |
| Dichlorotyrosine | – |
| 3-Monoiodotyrosine | – |
| Monobromo-Monochlorotyrosine | – |
| 3,5-Dibromotyrosine | – |
| Monochloro-Monoiodotyrosine | – |
| Monobromo-Monoiodotyrosine | – |
| 3,5-Diiodotyrosine | – |

2.3.3. Analysis of the MeOH Extract
2.4. LC-MS Analysis of the Skeletal Amino Acid Composition
2.4.1. Skeletal Amino Acid Composition before MeOH Extraction

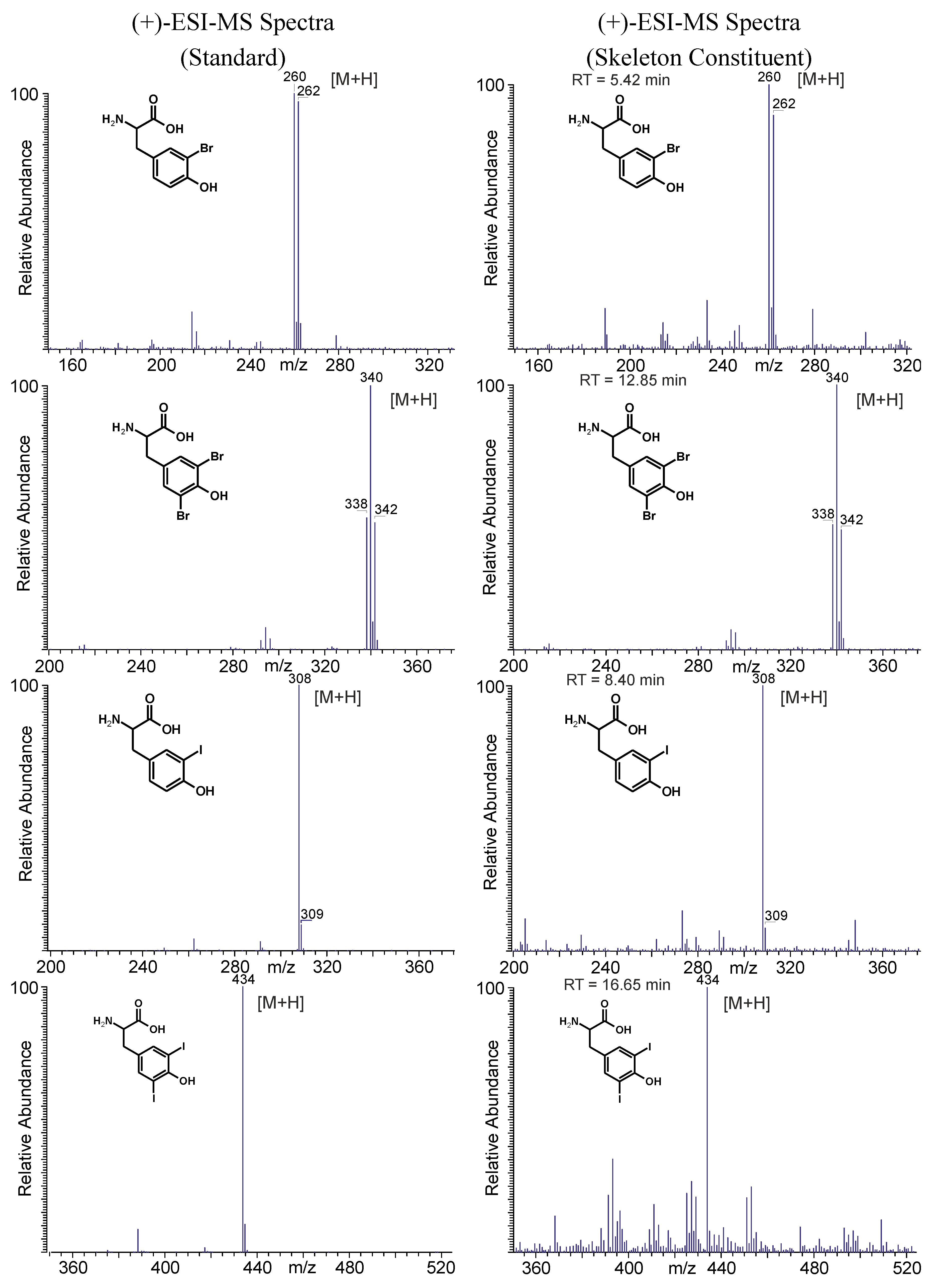
2.4.2. Skeletal Amino Acid Composition after MeOH Extraction
3. Experimental Sections
3.1. Sponge Samples
3.2. Extraction of the Skeletons
3.2.1. Isolation of the Skeleton
3.2.2. MeOH Extraction of the Skeletons
3.2.3. Ba(OH)2 Extraction
3.3. Derivatization
3.3.1. Preparation of Standard Solutions
3.3.2. TBDMS-Derivatization of the Standard Solutions
3.3.3. TBDMS-Derivatization of the Sponge Samples
3.4. GC-MS Measurements
3.5. Liquid Chromatography-Mass Spectrometry
3.5.1. Preparation of Standard Solutions
3.5.2. Preparation of Sponge Extract Samples
3.5.3. Measurement Conditions
| t [min] | Eluent A [%] | Eluent B [%] |
|---|---|---|
| 0 | 90 | 10 |
| 2 | 90 | 10 |
| 35 | 0 | 100 |
| 50 | 0 | 100 |
| 51 | 90 | 10 |
| 60 | 90 | 10 |
3.6. FTIR Spectroscopy
3.7. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDX)
3.8. Light Microscopy
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Müller, W.E.G. Origin of metazoa: Sponges as living fossils. Naturwissenschaften 1998, 85, 11–25. [Google Scholar] [CrossRef]
- Li, C.-W.; Chen, J.-Y.; Hua, T.-E. Precambrian sponges with cellular structures. Science 1998, 279, 879–882. [Google Scholar] [CrossRef]
- Proksch, P.; Putz, A.; Ortlepp, S.; Kjer, J.; Bayer, M. Bioactive natural products from marine sponges and fungal endophytes. Phytochem. Rev. 2010, 9, 475–489. [Google Scholar] [CrossRef]
- Thoms, C.; Wolff, M.; Padmakumar, K.; Ebel, R.; Proksch, P. Chemical defense of Mediterranean sponges Aplysina cavernicola and Aplysina aerophoba. Z. Naturforsch. C 2004, 59, 113–122. [Google Scholar]
- Thoms, C.; Ebel, R.; Proksch, P. Activated chemical defense in Aplysina. sponges revisited. J. Chem. Ecol. 2006, 32, 97–123. [Google Scholar] [CrossRef]
- Paul, V.J.; Ritson-Williams, R.; Sharp, K. Marine chemical ecology in benthic environments. Nat. Prod. Rep. 2011, 28, 345–387. [Google Scholar] [CrossRef]
- Hickman, C.P.; Roberts, L.S.; Larson, A.; l’Anson, H.; Eisenhour, D.J. Zoologie, 13th ed.; Pearson Studium Verlag: München, Germany, 2008; pp. 375–387. [Google Scholar]
- Harris, V.A. Sessile Animals of the Sea Shore; Chapman and Hall: London, UK, 1990; pp. 280–282. [Google Scholar]
- Hill, M.S.; Hill, A.L. Morphological Plasticity in the Tropical Sponge Anthosigmella. varians: Responses to Predators and Wave Energy. Biol. Bull. 2002, 202, 86–95. [Google Scholar] [CrossRef]
- Hill, M.S.; Lopez, N.A.; Young, K.A. Anti-predator defenses in western North Atlantic sponges with evidence of enhanced defense through interactions between spicules and chemicals. Mar. Ecol. Prog. Ser. 2005, 291, 93–102. [Google Scholar] [CrossRef]
- Wehner, R.; Gehring, W.J. Zoologie; Georg Thieme Verlag: Stuttgart, Germany, 2007; pp. 698–699. [Google Scholar]
- Bergquist, P.R.; Cook, S.D.C. Order Verongida Bergquist, 1978. In Systema Porifera: A Guide to the Classification of Sponges; Hooper, J.N.A., van Soest, R.W.M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; Volume I, pp. 1081–1096. [Google Scholar]
- Ehrlich, H.; Maldonado, M.; Spindler, K.-D.; Eckert, C.; Hanke, T.; Born, R.; Goebel, C.; Simon, P.; Heinemann, S.; Worch, H. First evidence of chitin as a component of the skeletal fibers of marine sponges. Part I. Verongidae (Demospongia: Porifera). J. Exp. Zool. B Mol. Dev. Evol. 2007, 308, 347–356. [Google Scholar]
- Brunner, E.; Ehrlich, H.; Schupp, P.; Hedrich, R.; Hunoldt, S.; Kammer, M.; Machill, S.; Paasch, S.; Bazhenov, V.V.; Kurek, D.V.; et al. Chitin-Based scaffolds are an integral part of the skeleton of the marine demosponge Ianthella basta. J. Struct. Biol. 2009, 168, 539–547. [Google Scholar] [CrossRef]
- Uriz, M.-J.; Turon, X.; Becerro, M.A.; Agell, G. Siliceous spicules and skeleton frameworks in sponges: Origin, diversity, ultrastructural patterns, and biological functions. Microsc. Res. Tech. 2003, 62, 279–299. [Google Scholar] [CrossRef]
- Ehrlich, H. Biological Materials of Marine Origin; Biologically-Inspired Systems (Book 1); Springer Verlag: Dordrecht, The Netherlands, 2010; pp. 245–256. [Google Scholar]
- Gross, J.; Sokal, Z.; Rougvie, M. Structural and chemical studies on the connective tissue of marine sponges. J. Histochem. Cytochem. 1956, 4, 227–246. [Google Scholar] [CrossRef]
- Junqua, S.; Robert, L.; Garrone, R.; Pavans de Ceccatty, M.; Vacelet, J. Biochemical and morphological studies on the collagens of Horny Sponges. Ircinia filaments compared to spongines. Connect. Tiss. Res. 1974, 2, 193–203. [Google Scholar]
- Ehrlich, H.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.L.; Shiaparelli, S.; Ereskovsky, A.V.; Schupp, P.; et al. Three-Dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part I. Isolation and identification of chitin. Int. J. Biol. Macromol. 2010, 47, 132–140. [Google Scholar] [CrossRef]
- Ehrlich, H.; Kaluzhnaya, O.V.; Brunner, E.; Tsurkan, M.V.; Ereskovsky, A.; Ilan, M.; Tabachnick, K.R.; Bazhenov, V.V.; Paasch, S.; Kammer, M.; et al. Identification and first insights into the structure and biosynthesis of chitin from the freshwater sponge Spongilla lacustris. J. Struct. Biol. 2013, 183, 474–483. [Google Scholar] [CrossRef]
- Ehrlich, H.; Keith Rigby, J.; Botting, J.P.; Tsurkan, M.V.; Werner, C.; Schwille, P.; Petrášek, Z.; Pisera, A.; Simon, P.; Sivkov, V.N.; et al. Discovery of 505-million-year old chitin in the basal demosponge Vauxia gracilenta. Sci. Rep. 2013, 3, 3497. [Google Scholar]
- Hackman, R.H. Studies on chitin IV. The occurrence of complexes in which chitin and protein are covalently linked. Aust. J. Biol. Sci. 1960, 13, 568–577. [Google Scholar]
- Blackwell, J.; Weih, M.A. Structure of chitin-protein complexes: Ovipositor of the ichneumon fly Megarhyssa. J. Mol. Biol. 1980, 137, 49–60. [Google Scholar] [CrossRef]
- Teeyapant, R.; Woerdenbag, H.; Kreis, P.; Hacker, J.; Wray, V.; Witte, L.; Proksch, P. Antibiotic and cytotoxic activity of brominated compounds from the marine sponge Verongia aerophoba. Z. Naturforsch. C 1993, 48, 939–945. [Google Scholar]
- Faulkner, D.J. Marine pharmacology. Antonie Van Leeuwenhoek 2000, 77, 135–145. [Google Scholar] [CrossRef]
- Thomson, J.E.; Barrow, K.D.; Faulkner, D.J. Localization of two brominated metabolites, aerothionin and homoaerothionin, in spherulous cells of the marine sponge Aplysina. fistularis (=Verongia thiona). Acta Zool. 1981, 64, 199–210. [Google Scholar]
- Turon, X.; Becerro, M.A.; Uriz, M.J. Distribution of brominated compounds within the sponge Aplysina. aerophoba: Coupling X-ray microanalysis with cryofixation techniques. Cell Tissue Res. 2000, 301, 311–322. [Google Scholar] [CrossRef]
- Kunze, K.; Niemann, H.; Ueberlein, S.; Schulze, R.; Ehrlich, H.; Brunner, E.; Proksch, P.; van Pée, K.-H. Brominated Skeletal Components of the Marine Demosponges, Aplysina cavernicola and Ianthella basta: Analytical and Biochemical Investigations. Mar. Drugs 2013, 11, 1271–1287. [Google Scholar] [CrossRef]
- Saper, J.; White, W.E. Amino-acid Composition of Sclero-protein of the Sponge Hippospongia equina. Nature 1958, 181, 285–286. [Google Scholar] [CrossRef]
- Low, E.M. Halogenated amino acids of the bath sponge. J. Mar. Res. 1951, 10, 239–245. [Google Scholar]
- Ebada, S.S.; Edrada, R.A.; Lin, W.; Proksch, P. Methods of isolation, purification and structural elucidation of bioactive secondary metabolites from marine invertebrates. Nat. Protoc. 2008, 3, 1820–1831. [Google Scholar] [CrossRef]
- Sobolevsky, T.G.; Revelsky, A.I.; Miller, B.; Oriedo, V.; Chernetsova1, E.S.; Revelsky, I.A. Comparison of silylation and esterification/acylation procedures in GC-MS analysis of amino acids. J. Sep. Sci. 2003, 26, 1474–1478. [Google Scholar] [CrossRef]
- North, M. Principles and Applications of Stereochemistry; CRC Press: Cheltenham, UK, 1998; pp. 75–77. [Google Scholar]
- Reichenbächer, M.; Popp, J. Strukturanalytik Organischer Und Anorganischer Verbindungen—Ein Übungsbuch; Teubner Verlag: Wiesbaden, Germany, 2007. [Google Scholar]
- Hunt, S.; Breuer, S.W. Isolation of a new naturally occuring halogenated amino acid: Monochloromonobromotyrosine. Biochim. Biophys. Acta 1971, 252, 401–404. [Google Scholar] [CrossRef]
- Čmelik, S. Über einen Farbstoff von Protein-Natur aus dem Schwamme Aplysina aerophoba Nardo. Hoppe-Seyler’s Z. Physiol. Chem. 1952, 289, 218–220. [Google Scholar]
- Institut für angewandte Hydrobiologie; HYDRA AG; HYDRA Institut für Meereswissenschaften AG; HYDRA Büro für Gewässerökologie Mürle & Ortlepp GbR; HYDRA Wiesloch—Dipl-Biol. Andreas Becker. Available online: http://www.hydra-institute.com (accessed on 17 March 2013).
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ueberlein, S.; Machill, S.; Niemann, H.; Proksch, P.; Brunner, E. The Skeletal Amino Acid Composition of the Marine Demosponge Aplysina cavernicola. Mar. Drugs 2014, 12, 4417-4438. https://doi.org/10.3390/md12084417
Ueberlein S, Machill S, Niemann H, Proksch P, Brunner E. The Skeletal Amino Acid Composition of the Marine Demosponge Aplysina cavernicola. Marine Drugs. 2014; 12(8):4417-4438. https://doi.org/10.3390/md12084417
Chicago/Turabian StyleUeberlein, Susanne, Susanne Machill, Hendrik Niemann, Peter Proksch, and Eike Brunner. 2014. "The Skeletal Amino Acid Composition of the Marine Demosponge Aplysina cavernicola" Marine Drugs 12, no. 8: 4417-4438. https://doi.org/10.3390/md12084417





