Conversion of Squid Pen to Homogentisic Acid via Paenibacillus sp. TKU036 and the Antioxidant and Anti-Inflammatory Activities of Homogentisic Acid
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening and Identification of Strain TKU036
2.2. Comparing the Non-Exopolysaccharide Antioxidants Produced by Paenibacillus Species
2.3. Culture Conditions for Antioxidant Production
2.4. Isolation of Antioxidant Compounds
2.5. Identification of HGA and Tryptophan by NMR
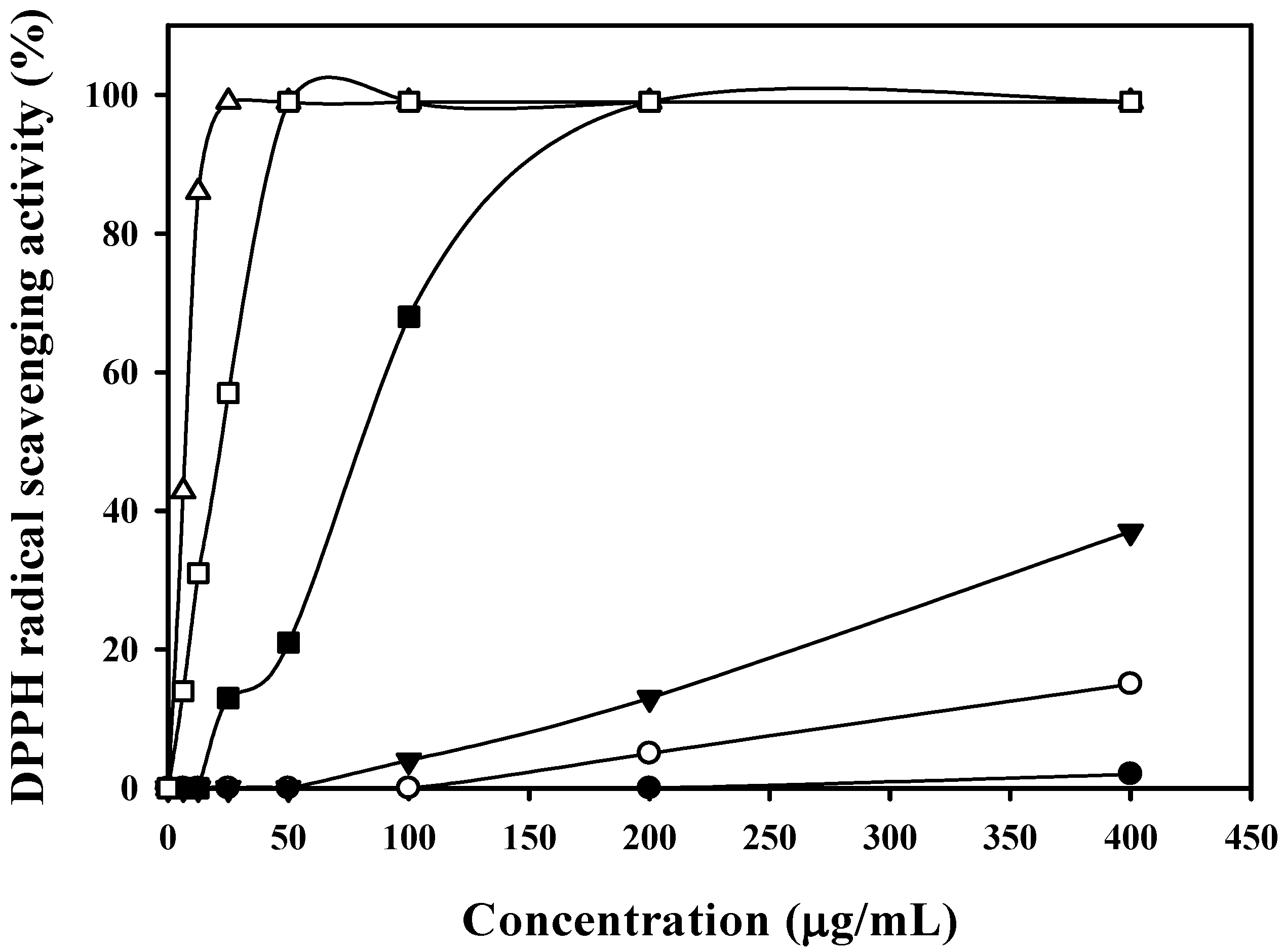
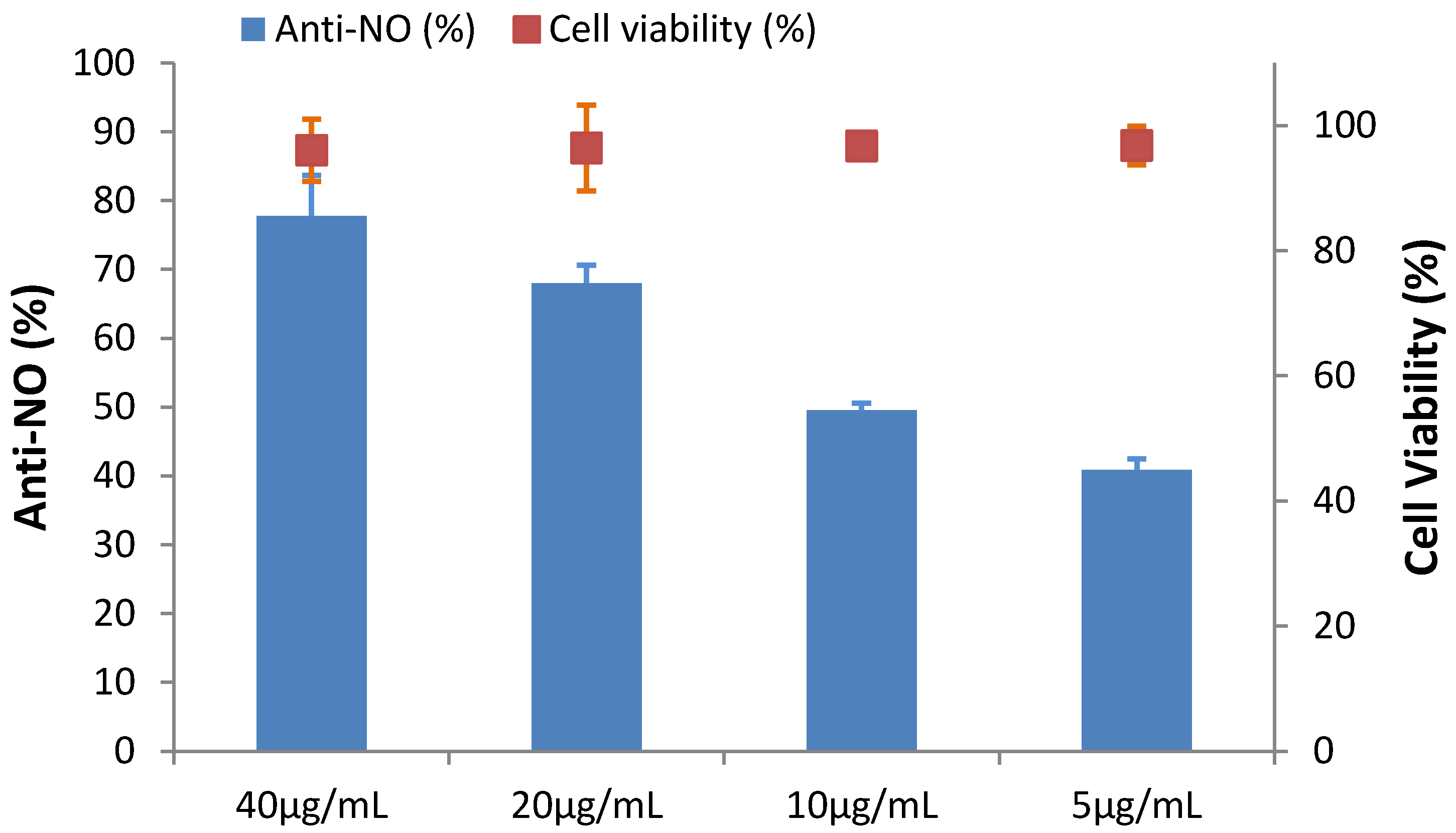
2.6. The Effect of HGA on Cytotoxicity and Anti-Inflammation
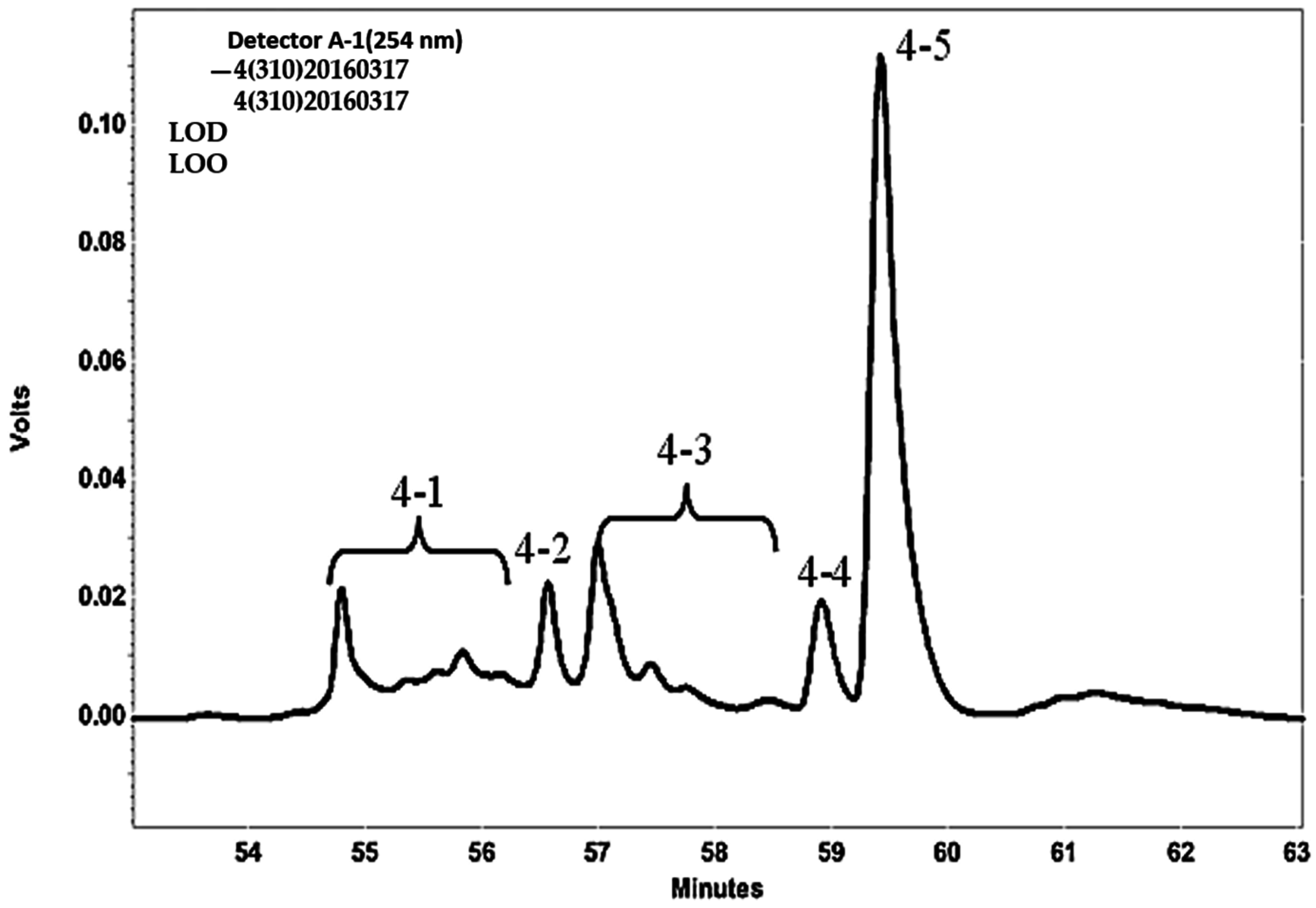
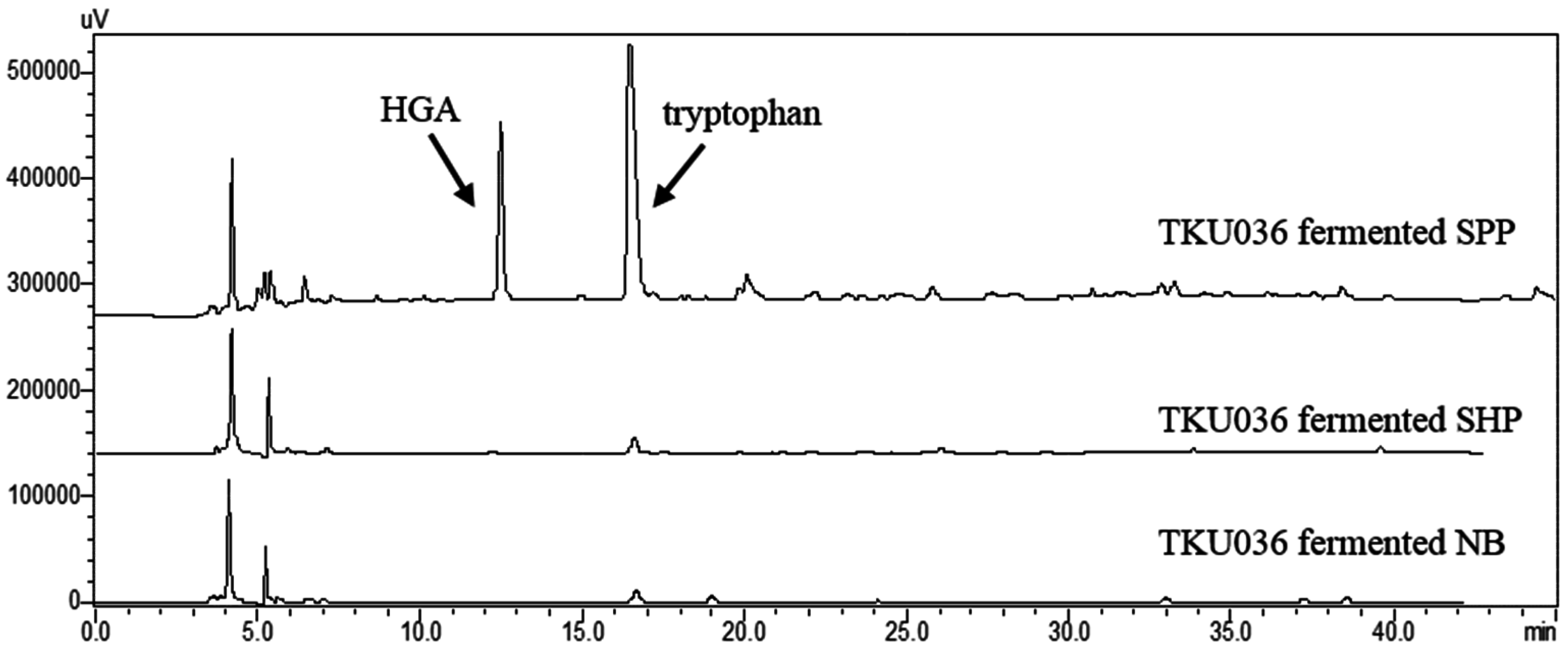
2.7. Confirmation of HGA and Tryptophan Produced from SPP by Fermentation
3. Materials and Methods
3.1. Materials
3.2. Antioxidant Activity Assay
3.3. Screening of Antioxidant-Producing Strain
3.4. Extraction and Isolation of HGA and Tryptophan
3.5. The Analysis of HGA and Tryptophan by HPLC:
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, S.L.; Liang, T.W. Microbial reclamation of squid pen and shrimp shell. Res. Chem. Intermed. 2016. [Google Scholar] [CrossRef]
- Wang, S.L. Microbial reclamation of squid pen. Biocatal. Agric. Biotechnol. 2012, 1, 177–180. [Google Scholar] [CrossRef]
- Liang, T.W.; Chen, W.T.; Lin, Z.H.; Kuo, Y.H.; Nguyen, A.D.; Pan, P.S.; Wang, S.L. An amphiprotic novel chitosanase from Bacillus mycoides and its application in the production of chitooligomers with their antioxidant and anti-inflammatory evaluation. Int. J. Mol. Sci. 2016, 17, 1302. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.D.; Huang, C.C.; Liang, T.W.; Nguyen, V.B.; Pan, P.S.; Wang, S.L. Production and purification of a fungal chitosanase and chitooligomers from Penicillium janthinellum D4 and discovery of the enzyme activators. Carbohydr. Polym. 2014, 108, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Liang, T.W.; Yen, Y.H. Bioconversion of chitin-containing wastes for the production of enzymes and bioactive materials. Carbohydr. Polym. 2011, 84, 732–742. [Google Scholar] [CrossRef]
- Liang, T.W.; Wang, S.L. Recent advances in exopolysaccharides from Paenibacillus spp.: Production, isolation, structure, and bioactivities. Mar. Drugs 2015, 13, 1847–1863. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.W.; Tseng, S.C.; Wang, S.L. Production and characterization of antioxidant properties of exopolysaccharides from Paenibacillus mucilaginosus TKU032. Mar. Drugs 2016, 14, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.H.; Liang, T.W.; Liu, K.C.; Hsu, Y.W.; Hsu, H.C.; Wang, S.L. Isolation and identification of a novel antioxidant with antitumor activity from Serratia ureilytica using squid pen as fermentation substrate. Mar. Biotechnol. 2011, 13, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.H.; Hsu, H.C.; Chen, Y.C.; Liang, T.W.; Wang, S.L. A novel compound with antioxidant activity produced by Serratia ureilytica TKU013. J. Agric. Food Chem. 2012, 60, 9043–9047. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.W.; Chen, C.H.; Wang, S.L. Production of insecticidal materials from Pseudomonas tamsuii. Res. Chem. Intermed. 2015, 41, 7965–7971. [Google Scholar] [CrossRef]
- Wang, S.L.; Chen, S.Y.; Yen, Y.H.; Liang, T.W. Utilization of chitinous materials in pigment adsorption. Food Chem. 2012, 135, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.W.; Lo, B.C.; Wang, S.L. Chitinolytic bacteria-assisted conversion of squid pen and its effect on dyes and pigments adsorption. Mar. Drugs 2015, 13, 4576–4593. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, L.; Aguilera-Carbó, A.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Martínez-Hernández, J.L.; Aguilar, C.N. Optimization of ellagic acid accumulation by Aspergillus niger GH1 in solid state culture using pomegranate shell powder as a support. Process Biochem. 2012, 47, 2199–2203. [Google Scholar] [CrossRef]
- Raghuwanshi, S.; Dutt, K.; Gupta, P.; Misra, S.; Saxena, R.K. Bacillus sphaericus: The highest bacterial tannase producer with potential for gallic acid synthesis. J. Biosci. Bioeng. 2011, 111, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Lambert, F.; Zucca, J.; Ness, F.; Aigle, M. Production of ferulic acid and coniferyl alcohol by conversion of eugenol using a recombinant strain of Saccharomyces cerevisiae. Flavour Fragr. J. 2014, 29, 14–21. [Google Scholar] [CrossRef]
- Kang, K.A.; Chae, S.; Lee, K.H.; Zhang, R.; Jung, M.S.; You, H.J.; Kim, J.S.; Hyun, J.W. Antioxidant effect of homogentisic acid on hydrogen peroxide induced oxidative stress in human lung fibroblast cells. Biotechnol. Bioprocess. Eng. 2005, 10, 556–563. [Google Scholar] [CrossRef]
- Chen, P.; Wang, Y.; Chen, L.; Jiang, W.; Niu, Y.; Shao, Q.; Gao, L.; Zhao, Q.; Yan, L.; Wang, S. Comparison of the anti-inflammatory active constituents and hepatotoxic pyrrolizidine alkaloids in two Senecio plants and their preparations by LC-UV and LC-MS. J. Pharm. Biomed. Anal. 2015, 115, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Huang, T.H.; Liang, T.W.; Wang, S.L. Production and characterization of exopolysaccharides and antioxidant from Paenibacillus sp. TKU023. New Biotechnol. 2011, 28, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.W.; Wu, C.C.; Cheng, W.T.; Chen, Y.C.; Wang, C.L.; Wang, I.L.; Wang, S.L. Exopolysaccharides and antimicrobial biosurfactants produced by Paenibacillus macerans TKU029. Appl. Biochem. Biotechnol. 2014, 172, 933–950. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Chang, C.T.; Chao, W.W.; Lin, C.F.; Chou, S.T. Antioxidative activity and safety of the 50% ethanolic extract from red bean fermented by Bacillus subtilis IMR-NK1. J. Agric. Food Chem. 2002, 50, 2454–2458. [Google Scholar] [CrossRef] [PubMed]
- Dyah, H.W.; Joe, A.V.; Severino, S.P. Mathematical modeling of the development of antioxidant activity in soybeans fermented with Aspergillus oryzae and Aspergillus awamori in the solid state. J. Agric. Food Chem. 2009, 57, 540–544. [Google Scholar]
- Miyake, Y.; Fukumoto, S.; Okada, M.; Sakaida, K.; Nakamura, Y.; Osawa, T. Antioxidative catechol lignans converted from sesamin and sesaminol triglucoside by culturing with Aspergillus. J. Agric. Food Chem. 2005, 53, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.H.; Hung, Y.H.; Chou, C.C. Total phenolic and anthocyanin contents, as well as antioxidant activity, of black been koji fermented by Aspergillus awamori under different culture conditions. Food Chem. 2007, 104, 936–942. [Google Scholar] [CrossRef]
- Kuo, C.F.; Hou, M.H.; Wang, T.S.; Chyau, C.C.; Chen, Y.Y. Enhanced antioxidant activity of Monascus pilosus fermented products by addition of ginger to the medium. Food Chem. 2009, 116, 915–922. [Google Scholar] [CrossRef]
- Wang, S.L.; Lin, C.L.; Liang, T.W.; Liu, K.C.; Kuo, Y.H. Conversion of squid pen by Serratia ureilytica for the production of enzymes and antioxidants. Bioresour. Technol. 2009, 100, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Cabras, P.; Angioni, A.; Tuberoso, C.; Floris, I.; Reniero, F.; Guillou, C.; Ghelli, S. Homogentisic acid: A phenolic acid as a marker of strawberry-tree (Arbutus unedo) honey. J. Agric. Food Chem. 1999, 47, 4064–4067. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Suzuki, M.; Ohnishi-Kameyama, M.; Sada, Y.; Nakanishi, T.; Nagata, T. Extraction and identification of antioxidants in the roots of yacon (Smallanthus sonchifolius). J. Agric. Food Chem. 1999, 47, 4711–4713. [Google Scholar] [CrossRef] [PubMed]
- Carreira, A.; Ferreira, L.M.; Loureiro, V. Brown pigments produced by Yarrowia lipolytica result from extracellular accumulation of homogentisic acid. Appl. Environ. Microbiol. 2001, 67, 3463–3468. [Google Scholar] [CrossRef] [PubMed]
- Kluyver, A.J.; van Zijp, J.C.M. The production of homogentisic acid out of phenylacetic acid by Aspergillus niger. Antonie Van Leeuwenhoek 1951, 17, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Kotob, S.I.; Coon, S.L.; Quintero, E.J.; Weiner, R.M. Homogentisic acid is the primary precursor of melanin synthesis in Vibrio cholerae, a hyphomonas strain, and Shewanella colwelliana. Appl. Environ. Microbiol. 1995, 61, 1620–1622. [Google Scholar] [PubMed]
- Liu, L.; Duan, X.; Wu, J. Modulating the direction of carbon flow in Escherichia coli to improve l-tryptophan production by inactivating the global regulator FruR. J. Biotechnol. 2016, 231, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Katsumata, R. Hyperproduction of tryptophan by Corynebacterium glutamicum with the modified pentose phosphate pathway. Appl. Environ. Microbiol. 1999, 65, 2497–2502. [Google Scholar] [PubMed]
- Chung, M.J.; Park, J.K.; Park, Y.I. Anti-inflammatory effects of low-molecular weight chitosan oligosaccharides in IgE-antigen complex-stimulated RBL-2H3 cells and asthma model mice. Int. Immunopharmcol. 2012, 12, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Drent, M.; de Boer, V.C.; Bast, A.; Haenen, G.R. Quercetin reduces markers of oxidative stress and inflammation in sarcoidosis. Clin. Nutr. 2011, 30, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Huang, E.; Yuan, C.; Zhang, L.; Yousef, A.E. Isolation of a Paenibacillus sp. strain and structural elucidation of its broad-spectrum lipopeptide antibiotic. Appl. Environ. Microbiol. 2012, 78, 3156–3165. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.; Tuberoso, C.I.G.; Atzeri, A.; Melis, M.P.; Bifulco, E.; Dessì, M.A. Antioxidant profile of strawberry tree honey and its marker homogentisic acid in several models of oxidative stress. Food Chem. 2011, 129, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
 —, Fraction 3 (0.1765 g, eluted with 10% methanol); —★—, Fraction 4 (0.1698 g, eluted with 15% methanol); —■—, Fraction 5 (0.1253 g, eluted with 20% methanol); —□—, Fraction 6 (0.0864 g, eluted with 25% methanol); —◆—, Fraction 7 (0.0599 g, eluted with 30% methanol) ;—◇—, Fraction 8 (0.0482 g, eluted with 35% methanol); —▲—, Fraction 9 (0.0363 g, eluted with 40% methanol); —△—, Fraction 10 (0.0275 g, eluted with 45% methanol); —▼—, Fraction 11 (0.0263 g, eluted with 50% methanol); —▽—, Fraction 12 (0.0321 g, eluted with 55% methanol); —✖—, Fraction 13 (0.0324 g, eluted with 60% methanol); and —✕—, Fraction 14 (0.0424 g, eluted with 100% methanol).
—, Fraction 3 (0.1765 g, eluted with 10% methanol); —★—, Fraction 4 (0.1698 g, eluted with 15% methanol); —■—, Fraction 5 (0.1253 g, eluted with 20% methanol); —□—, Fraction 6 (0.0864 g, eluted with 25% methanol); —◆—, Fraction 7 (0.0599 g, eluted with 30% methanol) ;—◇—, Fraction 8 (0.0482 g, eluted with 35% methanol); —▲—, Fraction 9 (0.0363 g, eluted with 40% methanol); —△—, Fraction 10 (0.0275 g, eluted with 45% methanol); —▼—, Fraction 11 (0.0263 g, eluted with 50% methanol); —▽—, Fraction 12 (0.0321 g, eluted with 55% methanol); —✖—, Fraction 13 (0.0324 g, eluted with 60% methanol); and —✕—, Fraction 14 (0.0424 g, eluted with 100% methanol).
 —, Fraction 3 (0.1765 g, eluted with 10% methanol); —★—, Fraction 4 (0.1698 g, eluted with 15% methanol); —■—, Fraction 5 (0.1253 g, eluted with 20% methanol); —□—, Fraction 6 (0.0864 g, eluted with 25% methanol); —◆—, Fraction 7 (0.0599 g, eluted with 30% methanol) ;—◇—, Fraction 8 (0.0482 g, eluted with 35% methanol); —▲—, Fraction 9 (0.0363 g, eluted with 40% methanol); —△—, Fraction 10 (0.0275 g, eluted with 45% methanol); —▼—, Fraction 11 (0.0263 g, eluted with 50% methanol); —▽—, Fraction 12 (0.0321 g, eluted with 55% methanol); —✖—, Fraction 13 (0.0324 g, eluted with 60% methanol); and —✕—, Fraction 14 (0.0424 g, eluted with 100% methanol).
—, Fraction 3 (0.1765 g, eluted with 10% methanol); —★—, Fraction 4 (0.1698 g, eluted with 15% methanol); —■—, Fraction 5 (0.1253 g, eluted with 20% methanol); —□—, Fraction 6 (0.0864 g, eluted with 25% methanol); —◆—, Fraction 7 (0.0599 g, eluted with 30% methanol) ;—◇—, Fraction 8 (0.0482 g, eluted with 35% methanol); —▲—, Fraction 9 (0.0363 g, eluted with 40% methanol); —△—, Fraction 10 (0.0275 g, eluted with 45% methanol); —▼—, Fraction 11 (0.0263 g, eluted with 50% methanol); —▽—, Fraction 12 (0.0321 g, eluted with 55% methanol); —✖—, Fraction 13 (0.0324 g, eluted with 60% methanol); and —✕—, Fraction 14 (0.0424 g, eluted with 100% methanol).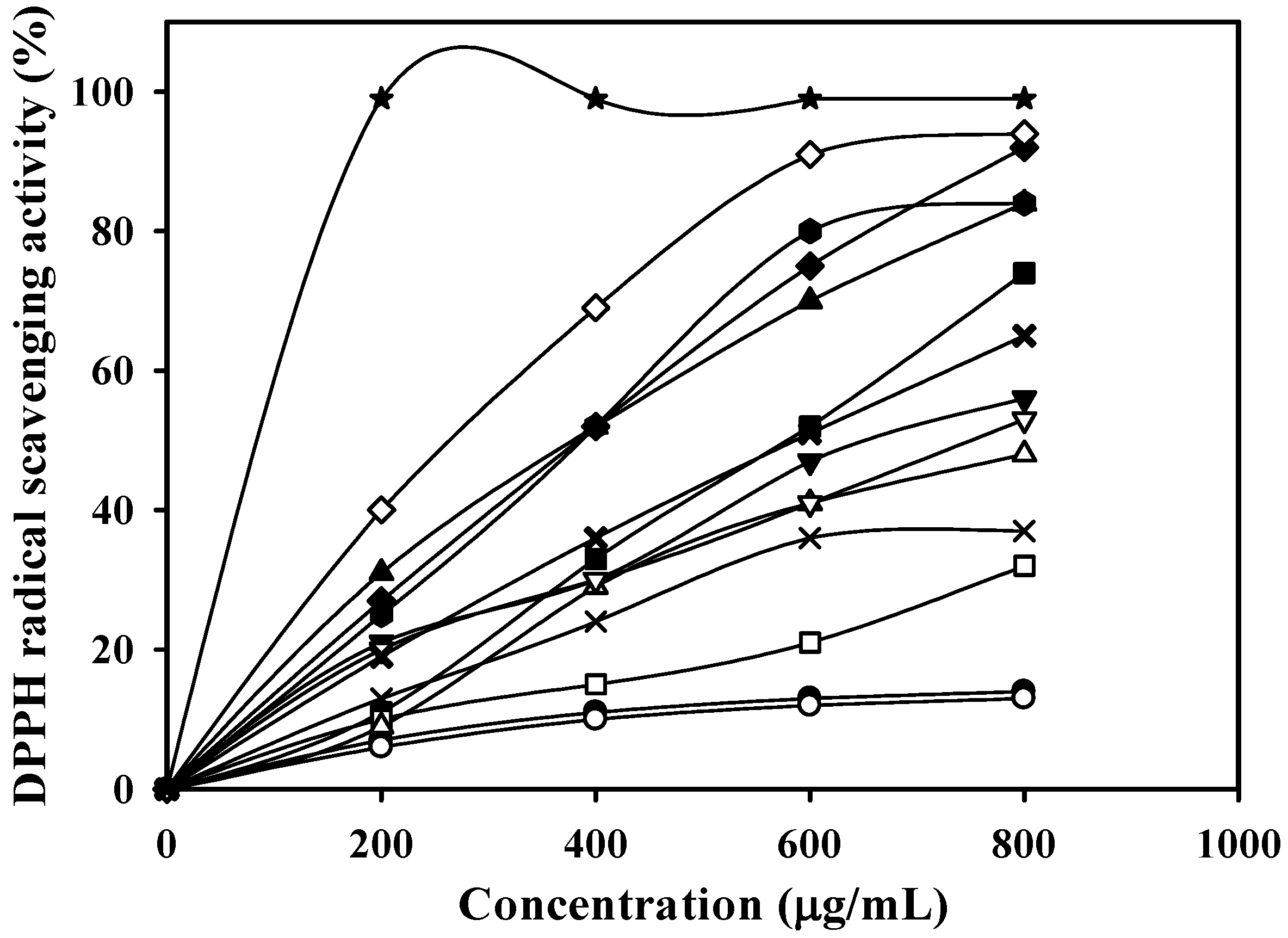

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-L.; Li, H.-T.; Zhang, L.-J.; Lin, Z.-H.; Kuo, Y.-H. Conversion of Squid Pen to Homogentisic Acid via Paenibacillus sp. TKU036 and the Antioxidant and Anti-Inflammatory Activities of Homogentisic Acid. Mar. Drugs 2016, 14, 183. https://doi.org/10.3390/md14100183
Wang S-L, Li H-T, Zhang L-J, Lin Z-H, Kuo Y-H. Conversion of Squid Pen to Homogentisic Acid via Paenibacillus sp. TKU036 and the Antioxidant and Anti-Inflammatory Activities of Homogentisic Acid. Marine Drugs. 2016; 14(10):183. https://doi.org/10.3390/md14100183
Chicago/Turabian StyleWang, San-Lang, Hsin-Ting Li, Li-Jie Zhang, Zhi-Hu Lin, and Yao-Haur Kuo. 2016. "Conversion of Squid Pen to Homogentisic Acid via Paenibacillus sp. TKU036 and the Antioxidant and Anti-Inflammatory Activities of Homogentisic Acid" Marine Drugs 14, no. 10: 183. https://doi.org/10.3390/md14100183