Electrospinning of Nanodiamond-Modified Polysaccharide Nanofibers with Physico-Mechanical Properties Close to Natural Skins
Abstract
:1. Introduction
2. Results and Discussion
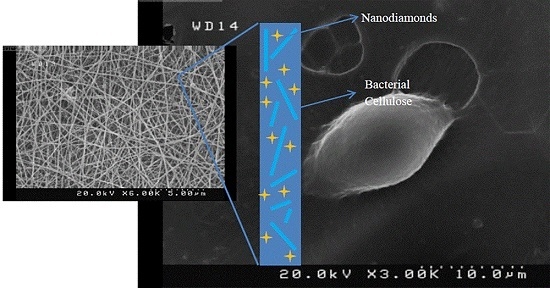
2.1. Size and Morphology of Electrospun Fibers
2.2. Interactions between Nanoparticles and Polymer
2.3. Effect of Nanodiamonds on the Hydrophilicity of Mats
2.4. Mechanical Properties of Electrospun Mats
2.5. Permeability
2.6. Cell Viability Assessment
3. Experimental Procedure
3.1. Materials
3.2. Preparation of Fibrous Mats
3.3. Materials Characterization
3.4. Cell Viability
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.O.; Jafari, S.H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2010, 21, 77–95. [Google Scholar] [CrossRef]
- Kokabi, M.; Sirousazar, M.; Hassan, Z.M. Pva-clay nanocomposite hydrogels for wound dressing. Eur. Polym. J. 2007, 43, 773–781. [Google Scholar] [CrossRef]
- Jiang, T.; Carbone, E.J.; Lo, K.W.-H.; Laurencin, C.T. Electrospinning of polymer nanofibers for tissue regeneration. Prog. Polym. Sci. 2015, 46, 1–24. [Google Scholar] [CrossRef]
- Sun, B.; Jiang, X.-J.; Zhang, S.; Zhang, J.-C.; Li, Y.-F.; You, Q.-Z.; Long, Y.-Z. Electrospun anisotropic architectures and porous structures for tissue engineering. J. Mater. Chem. B 2015, 3, 5389–5410. [Google Scholar] [CrossRef]
- Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Izumi, R.; Osaki, T.; Ifuku, S.; Morimoto, M.; Saimoto, H.; Minami, S.; Okamoto, Y. Chitin, chitosan, and its derivatives for wound healing: Old and new materials. J. Funct. Biomater. 2015, 6, 104–142. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, N.; Ostadhossein, F.; Simchi, A. Physicochemical and antibacterial properties of chitosan-polyvinylpyrrolidone films containing self-organized graphene oxide nanolayers. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Ostadhossein, F.; Mahmoudi, N.; Morales-Cid, G.; Tamjid, E.; Navas-Martos, F.J.; Soriano-Cuadrado, B.; Paniza, J.M.L.; Simchi, A. Development of chitosan/bacterial cellulose composite films containing nanodiamonds as a potential flexible platform for wound dressing. Materials 2015, 8, 6401–6418. [Google Scholar] [CrossRef]
- Zhu, W.; Li, W.; He, Y.; Duan, T. In-situ biopreparation of biocompatible bacterial cellulose/graphene oxide composites pellets. Appl. Surf. Sci. 2015, 338, 22–26. [Google Scholar] [CrossRef]
- Pina, S.; Oliveira, J.M.; Reis, R.L. Natural-based nanocomposites for bone tissue engineering and regenerative medicine: A review. Adv. Mater. 2015, 27, 1143–1169. [Google Scholar] [CrossRef] [PubMed]
- Koosha, M.; Mirzadeh, H. Electrospinning, mechanical properties, and cell behavior study of chitosan/PVA nanofibers. J. Biomed. Mater. Res. A 2015, 103, 3081–3093. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.S.; Nair, S.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.; El-Zairy, E. Chitosan as a Biomaterial—Structure, Properties, and Electrospun Nanofibers. Intech 2015, 4, 7–14. [Google Scholar] [CrossRef]
- Jia, Y.; Huang, G.; Dong, F.; Liu, Q.; Nie, W. Preparation and characterization of electrospun poly (ε-caprolactone)/poly (vinyl pyrrolidone) nanofiber composites containing silver particles. Polym. Compos. 2015. [Google Scholar] [CrossRef]
- Haider, S.; Al-Masry, W.; Al-Zeghayer, Y.; Al-Hoshan, M.; Ali, F.A. Fabrication chitosan nano fibers membrane via electrospinning. Une 2016, 13, 15. [Google Scholar]
- Millon, L.E.; Guhados, G.; Wan, W. Anisotropic polyvinyl alcohol—Bacterial cellulose nanocomposite for biomedical applications. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 86, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Czaja, W.; Krystynowicz, A.; Bielecki, S.; Brown, R.M. Microbial cellulose—The natural power to heal wounds. Biomaterials 2006, 27, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Millon, L.; Wan, W. The polyvinyl alcohol–bacterial cellulose system as a new nanocomposite for biomedical applications. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 79, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Helenius, G.; Bäckdahl, H.; Bodin, A.; Nannmark, U.; Gatenholm, P.; Risberg, B. In vivo biocompatibility of bacterial cellulose. J. Biomed. Mater. Res. A 2006, 76, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhang, J.; Yang, G. Present status and applications of bacterial cellulose-based materials for skin tissue repair. Carbohydr. Polym. 2013, 92, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Jebel, F.S.; Almasi, H. Morphological, physical, antimicrobial and release properties of ZnO nanoparticles-loaded bacterial cellulose films. Carbohydr. Polym. 2016, 149, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Römling, U.; Galperin, M.Y. Bacterial cellulose biosynthesis: Diversity of operons, subunits, products and functions. Trends Microbiol. 2015, 23, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.C.; Oliveira, L.; Freire, C.S.; Silvestre, A.J.; Neto, C.P.; Gandini, A.; Desbriéres, J. Novel transparent nanocomposite films based on chitosan and bacterial cellulose. Green Chem. 2009, 11, 2023–2029. [Google Scholar] [CrossRef] [Green Version]
- Phisalaphong, M.; Jatupaiboon, N. Biosynthesis and characterization of bacteria cellulose–chitosan film. Carbohydr. Polym. 2008, 74, 482–488. [Google Scholar] [CrossRef]
- Lin, W.-C.; Lien, C.-C.; Yeh, H.-J.; Yu, C.-M.; Hsu, S.-H. Bacterial cellulose and bacterial cellulose–chitosan membranes for wound dressing applications. Carbohydr. Polym. 2013, 94, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chen, L.; Zhang, Q.; Hong, F.F. Using in situ dynamic cultures to rapidly biofabricate fabric-reinforced composites of chitosan/bacterial nanocellulose for antibacterial wound dressings. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Azarniya, A.; Eslahi, N.; Mahmoudi, N.; Simchi, A. Effect of graphene oxide nanosheets on the physico-mechanical properties of chitosan/bacterial cellulose nanofibrous composites. Compos. A Appl. Sci. Manuf. 2016, 85, 113–122. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R. A review on electrospinning for membrane fabrication: Challenges and applications. Desalination 2015, 356, 15–30. [Google Scholar] [CrossRef]
- HPS, A.K.; Saurabh, C.K.; Adnan, A.; Fazita, M.N.; Syakir, M.; Davoudpour, Y.; Rafatullah, M.; Abdullah, C.; Haafiz, M.; Dungani, R. A review on chitosan-cellulose blends and nanocellulose reinforced chitosan biocomposites: Properties and their applications. Carbohydr. Polym. 2016, 150, 216–226. [Google Scholar]
- Khalil, H.A.; Davoudpour, Y.; Bhat, A.; Rosamah, E.; Tahir, P.M. Electrospun cellulose composite nanofibers. In Handbook of Polymer Nanocomposites. Processing, Performance and Application; Springer: Berlin/Heidelberg, Germany, 2015; pp. 191–227. [Google Scholar]
- Liu, Y.; Zhou, J.; Tang, J.; Tang, W. Three-dimensional, chemically bonded polypyrrole/bacterial cellulose/graphene composites for high-performance supercapacitors. Chem. Mater. 2015, 27, 7034–7041. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Das, S.; Seal, S. Nanomaterials for wound healing: Scope and advancement. Nanomedicine 2015, 10, 2593–2612. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.-T.; Ma, C.-C.M.; Tien, H.-W.; Liao, W.-H.; Wang, Y.-S.; Li, S.-M.; Chuang, W.-P. Preparation and characterization of silver nanoparticle-reduced graphene oxide decorated electrospun polyurethane fiber composites with an improved electrical property. Compos. Sci. Technol. 2015, 118, 171–177. [Google Scholar] [CrossRef]
- Schrand, A.M.; Dai, L.; Schlager, J.J.; Hussain, S.M.; Osawa, E. Differential biocompatibility of carbon nanotubes and nanodiamonds. Diam. Relat. Mater. 2007, 16, 2118–2123. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, J.; Li, W.; Zhang, Y.; Yang, X.; Chen, N.; Sun, Y.; Zhao, Y.; Fan, C.; Huang, Q. The biocompatibility of nanodiamonds and their application in drug delivery systems. Theranostics 2012, 2, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Badea, I. Nanodiamonds as novel nanomaterials for biomedical applications: Drug delivery and imaging systems. Int. J. Nanomed. 2013, 8, 203. [Google Scholar]
- Hsu, C.M.; Shivkumar, S. N,N-dimethylformamide additions to the solution for the electrospinning of poly (ε-caprolactone) nanofibers. Macromol. Mater. Eng. 2004, 289, 334–340. [Google Scholar] [CrossRef]
- Li, X.; Bian, F.; Lin, J.; Zeng, Y. Effect of electric field on the morphology and mechanical properties of electrospun fibers. RSC Adv. 2016, 6, 50666–50672. [Google Scholar] [CrossRef]
- Naseri, N.; Mathew, A.P.; Girandon, L.; Fröhlich, M.; Oksman, K. Porous electrospun nanocomposite mats based on chitosan–cellulose nanocrystals for wound dressing: Effect of surface characteristics of nanocrystals. Cellulose 2015, 22, 521–534. [Google Scholar] [CrossRef]
- Rakha, S.A.; Raza, R.; Munir, A. Reinforcement effect of nanodiamond on properties of epoxy matrix. Polym. Compos. 2013, 34, 811–818. [Google Scholar] [CrossRef]
- Kartikowati, C.W.; Suhendi, A.; Zulhijah, R.; Ogi, T.; Iwaki, T.; Okuyama, K. Preparation and evaluation of magnetic nanocomposite fibers containing α″-Fe16N2 and α-Fe nanoparticles in polyvinylpyrrolidone via magneto-electrospinning. Nanotechnology 2015, 27. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Shah, N.; Ha, J.H.; Park, J.K. Effect of chitosan penetration on physico-chemical and mechanical properties of bacterial cellulose. Korean J. Chem. Eng. 2011, 28, 1736–1743. [Google Scholar] [CrossRef]
- Mishra, R.; Rao, K.J. On the formation of poly (ethyleneoxide)-poly (vinylalcohol) blends. Eur. Polym. J. 1999, 35, 1883–1894. [Google Scholar] [CrossRef]
- Duan, B.; Dong, C.; Yuan, X.; Yao, K. Electrospinning of chitosan solutions in acetic acid with poly (ethylene oxide). J. Biomater. Sci. Polym. Ed. 2004, 15, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Osswald, S.; Yushin, G.; Mochalin, V.; Kucheyev, S.O.; Gogotsi, Y. Control of sp2/sp3 carbon ratio and surface chemistry of nanodiamond powders by selective oxidation in air. J. Am. Chem. Soc. 2006, 128, 11635–11642. [Google Scholar] [CrossRef] [PubMed]
- Morimune, S.; Kotera, M.; Nishino, T.; Goto, K.; Hata, K. Poly (vinyl alcohol) nanocomposites with nanodiamond. Macromolecules 2011, 44, 4415–4421. [Google Scholar] [CrossRef]
- Zou, Q.; Li, Y.; Zou, L.; Wang, M. Characterization of structures and surface states of the nanodiamond synthesized by detonation. Mater. Charact. 2009, 60, 1257–1262. [Google Scholar] [CrossRef]
- Pan, H.; Xu, D.; Liu, Q.; Ren, H.Q.; Zhou, M. Preparation and Characterization of Corn Starch-Nanodiamond Composite Films. In Applied Mechanics and Materials; Trans Tech. Publ.: Zurich, Switzerland, 2014; pp. 156–161. [Google Scholar]
- Wang, X.; Yu, J.; Sun, G.; Ding, B. Electrospun nanofibrous materials: A versatile medium for effective oil/water separation. Mater. Today 2015. [Google Scholar] [CrossRef]
- Wang, Z.; Macosko, C.W.; Bates, F.S. Fluorine enriched melt blown fibers from polymer blends of poly (butylene terephthalate) and a fluorinated multiblock copolyester. ACS Appl. Mater. Interfaces 2016, 8, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Mao, Y.; Gupta, M.; Gleason, K.K.; Rutledge, G.C. Superhydrophobic fabrics produced by electrospinning and chemical vapor deposition. Macromolecules 2005, 38, 9742–9748. [Google Scholar] [CrossRef]
- Tjong, S. Structural and mechanical properties of polymer nanocomposites. Mater. Sci. Eng. R Rep. 2006, 53, 73–197. [Google Scholar] [CrossRef]
- Cui, W.; Zhou, Y.; Chang, J. Electrospun nanofibrous materials for tissue engineering and drug delivery. Sci. Technol. Adv. Mater. 2016. [Google Scholar] [CrossRef]
- Lee, D.-K.; Kim, S.V.; Limansubroto, A.N.; Yen, A.; Soundia, A.; Wang, C.-Y.; Shi, W.; Hong, C.; Tetradis, S.; Kim, Y. Nanodiamond–gutta percha composite biomaterials for root canal therapy. ACS Nano 2015, 9, 11490–11501. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, W.W.-W.; Hui, Y.Y.; Tsai, P.-C.; Chang, H.-C. Fluorescent nanodiamond: A versatile tool for long-term cell tracking, super-resolution imaging, and nanoscale temperature sensing. Acc. Chem. Res. 2016, 49, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.C.; Vagaska, B.; Edgington, R.; Hébert, C.; Ferretti, P.; Bergonzo, P.; Jackman, R.B. Biocompatibility of nanostructured boron doped diamond for the attachment and proliferation of human neural stem cells. J. Neural Eng. 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- Rieger, K.A.; Birch, N.P.; Schiffman, J.D. Electrospinning chitosan/poly (ethylene oxide) solutions with essential oils: Correlating solution rheology to nanofiber formation. Carbohydr. Polym. 2016, 139, 131–138. [Google Scholar] [CrossRef] [PubMed]







| Concentration | Average Fiber Diameter (nm) | Size Range (nm) |
|---|---|---|
| 0 | 173 ± 44 | 73–308 |
| 1 | 88 ± 18 | 57–160 |
| 2 | 89 ± 14 | 61–128 |
| 3 | 95 ± 22 | 60–157 |
| Concentration | Elastic Modulus (MPa) | Yield Strength (MPa) | Strain to Failure (%) | Permeability (µg·Pa−1·s−1·m−1) |
|---|---|---|---|---|
| 0 | 353 | 21.7 | 15.4 | 423 |
| 1 | 458 | 25.3 | 9.9 | 345 |
| 2 | 393 | 20.2 | 7.9 | 342 |
| 3 | 405 | 15.9 | 4.8 | 359 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahdavi, M.; Mahmoudi, N.; Rezaie Anaran, F.; Simchi, A. Electrospinning of Nanodiamond-Modified Polysaccharide Nanofibers with Physico-Mechanical Properties Close to Natural Skins. Mar. Drugs 2016, 14, 128. https://doi.org/10.3390/md14070128
Mahdavi M, Mahmoudi N, Rezaie Anaran F, Simchi A. Electrospinning of Nanodiamond-Modified Polysaccharide Nanofibers with Physico-Mechanical Properties Close to Natural Skins. Marine Drugs. 2016; 14(7):128. https://doi.org/10.3390/md14070128
Chicago/Turabian StyleMahdavi, Mina, Nafiseh Mahmoudi, Farzad Rezaie Anaran, and Abdolreza Simchi. 2016. "Electrospinning of Nanodiamond-Modified Polysaccharide Nanofibers with Physico-Mechanical Properties Close to Natural Skins" Marine Drugs 14, no. 7: 128. https://doi.org/10.3390/md14070128