New Cytotoxic Terpenoids from Soft Corals Nephthea chabroli and Paralemnalia thyrsoides
Abstract
:1. Introduction
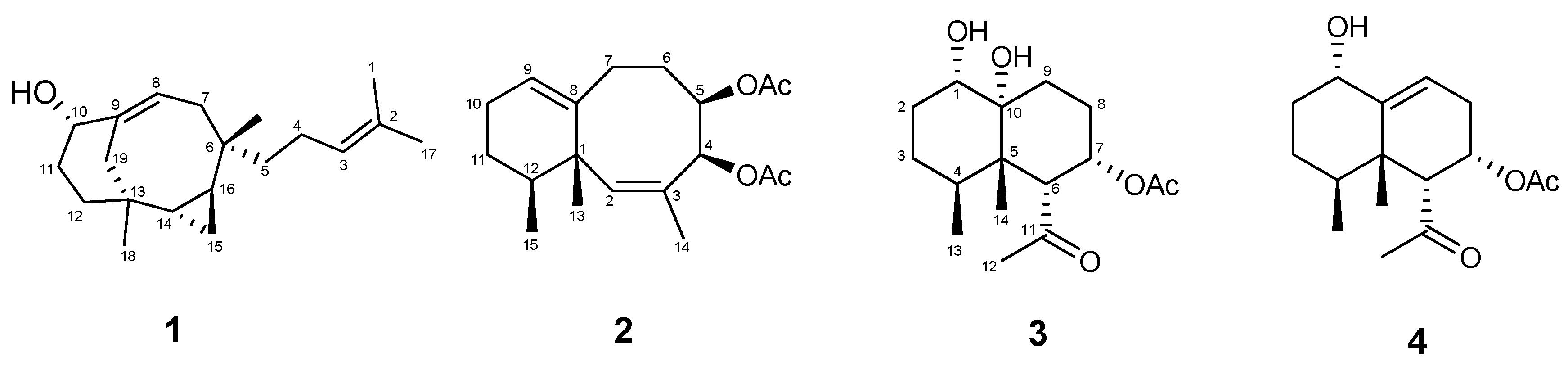
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Separation
3.4. Cytotoxicity Assay
3.5. Anti-HCMV Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Duh, C.-Y.; Wang, S.-K.; Weng, Y.-L.; Chiang, M.Y.; Dai, C.-F. Cytotoxic terpenoids from the Formosan Soft Coral Nephthea brassica. J. Nat. Prod. 1999, 62, 1518–1521. [Google Scholar] [CrossRef] [PubMed]
- Duh, C.-Y.; Wang, S.-K.; Weng, Y.-L. Brassicolene, a novel cytotoxic diterpenoid from the Formosan soft coral Nephthea brassica. Tetrahedron Lett. 2000, 41, 1401–1404. [Google Scholar] [CrossRef]
- Kitagawa, I.; Cui, Z.; Son, B.W.; Kobayashi, M.; Kyogoku, Y. Marine natural products. 17. Nephtheoxydiol, a new cytotoxic hydroperoxy-germacrane sesquiterpene, and related sesquiterpenoids from an Okinawan soft coral of Nephthea sp. (Nephtheidae). Chem. Pharm. Bull. 1987, 35, 124–135. [Google Scholar] [CrossRef] [PubMed]
- El-Gamal, A.A.H.; Wang, S.-K.; Dai, C.-F.; Chen, I.-C.; Duh, C.-Y. Prenylbicyclogermacrane diterpenoids from the Formosan soft coral Nephthea pacifica. J. Nat. Prod. 2005, 68, 74–77. [Google Scholar] [CrossRef] [PubMed]
- El-Gamal, A.A.H.; Wang, S.-K.; Duh, C.-Y. Prenylbicyclogermacrane diterpenoids from the Formosan Soft coral Nephthea elongatae. Chem. Pharm. Bull. 2007, 55, 890–893. [Google Scholar] [CrossRef] [PubMed]
- Su, J.-Y.; Zhong, Y.-L.; Zeng, L.-M. Two new sesquiterpenoids from the soft coral Paralemnalia thyrsoides. J. Nat. Prod. 1993, 56, 288–291. [Google Scholar] [CrossRef]
- Bowden, B.F.; Coll, J.C.; Mitchell, S.J. Studies of Australian soft corals. XIX. Two newsesquiterpenes with the nardosinane skeleton from a Paralemnalia species. Aust. J. Chem. 1980, 33, 885–890. [Google Scholar] [CrossRef]
- Izac, R.R.; Schneider, P.; Swain, M.; Fenical, W. New nor-sesquiterpenoids of apparent nardosinane origin from the pacific soft-coral Paralemnalia thyrsoides. Tetrahedron Lett. 1982, 23, 817–820. [Google Scholar] [CrossRef]
- Huang, H.-C.; Chao, C.-H.; Su, J.-H.; Hsu, C.-H.; Chen, S.-P.; Kuo, Y.-H.; Sheu, J.-H. Neolemnane-type sesquiterpenoids from a Formosan soft coral Paralemnalia thyrsoides. Chem. Pharm. Bull. 2007, 55, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Su, J.-H.; Chen, B.-W.; Wen, Z.-H.; Dai, C.-F.; Shen, J.-H.; Sung, P.-J. Nardosinane-type sesquiterpenoids from the Formosan soft coral Paralemnalia thyrsoides. Mar. Drugs 2011, 9, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-Y.; Lin, E.-H.; Huang, J.-S.; Wen, Z.-H.; Duh, C.-Y. Ylangene-type and nardosinane-type sesquiterpenoids from the soft corals Lemnalia flava and Paralemnalia thyrsoides. Chem. Pharm. Bull. 2010, 58, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Bishara, A.; Yeffet, D.; Sisso, M.; Shmul, G.; Schleyer, M.; Benayahu, Y.; Rudi, A.; Kashman, Y. Nardosinanols A–I and lemnafricanol, sesquiterpenes from several soft corals, Lemnalia sp., Paralemnalia clavata, Lemnalia Africana, and Rhytisma fulvum fulvum. J. Nat. Prod. 2008, 71, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.-M.; Zhong, Y.-L.; Su, J.-Y.; Zhao, D. Sesquiterpenes from the soft coral Paralemnalia thyrsoides and their biogenetic correlation. Chin. Sci. Bull. 1995, 40, 213–216. [Google Scholar]
- Wang, G.-H.; Huang, H.-C.; Su, J.-H.; Wu, Y.-C.; Sheu, J.-H. Paralemnolins J–P, new sesquiterpenoids from the soft coral Paralemnalia thyrsoides. Chem. Pharm. Bull. 2010, 58, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-C.; Chao, C.-H.; Liao, J.-H.; Chiang, M.Y.; Dai, C.-F.; Wu, Y.-C.; Sheu, J.-H. A novel chlorinated norsesquiterpenoid and two related new metabolites from the soft coral Paralemnalia thyrsoides. Tetrahedron Lett. 2005, 46, 7711–7714. [Google Scholar] [CrossRef]
- Huang, H.-C.; Wen, Z.-H.; Chao, C.-H.; Ahmed, A.F.; Chiang, M.Y.; Kuo, Y.-H.; Hsu, C.-H.; Chen, S.-P.; Sheu, J.-H. Novel sesquiterpenoids from the Formosan soft coral Paralemnalia thyrsoides. Tetrahedron Lett. 2006, 47, 8751–8755. [Google Scholar] [CrossRef]
- Daloze, D.; Braekman, J.C.; Georget, P.; Tursch, B. Chemical studies of marine invertebrates. XXII. Two novel sesquiterpenes from soft corals of the genera Lemnalia and Paralemnalia. Bull. Soc. Chim. Belg. 1977, 86, 47–54. [Google Scholar] [CrossRef]
- Wang, S.-K.; Lee, Y.-S.; Duh, C.-Y. Paralemnolide A, an unprecedented bisnorsesquiterpene from the Taiwanese soft coral Paralemnalia thyrsoides. Mar. Drugs 2012, 10, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.-J.; Lee, Y.-S.; Wang, S.-K.; Duh, C.-Y. Parathyrsoidins A–D, Four new sesquiterpenoids from the soft coral Paralemnalia thyrsoides. Mar. Drugs 2013, 11, 2501–2509. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.-S.; Duh, C.-Y.; Chiang, M.Y.; Lin, C.-N. Sinugibberol, a new cytotoxic cembranoid diterpene from the soft coral Sinularia gibberosa. J. Nat. Prod. 1995, 58, 1126–1130. [Google Scholar] [CrossRef] [PubMed]
- Geran, R.I.; Greenberg, N.H.; MacDonald, M.M.; Schumacher, A.M.; Abbott, B.J. Protocols for screening chemical agents and natural products against animal tumors and other biological syatems. Cancer Chemother. Rep. 1972, 3, 1–103. [Google Scholar]
- Stevens, M.; Balzarini, J.; Tabarrini, O.; Andrei, G.; Snoeck, R.; Cecchetti, V.; Fravolini, A.; de Clercq, E.; Pannecouque, C. Cell-dependent interference of a series of new 6-aminoquinolone derivatives with viral (HIV/CMV) transactivation. J. Antimicrob. Chemother. 2005, 56, 847–855. [Google Scholar] [CrossRef] [PubMed]
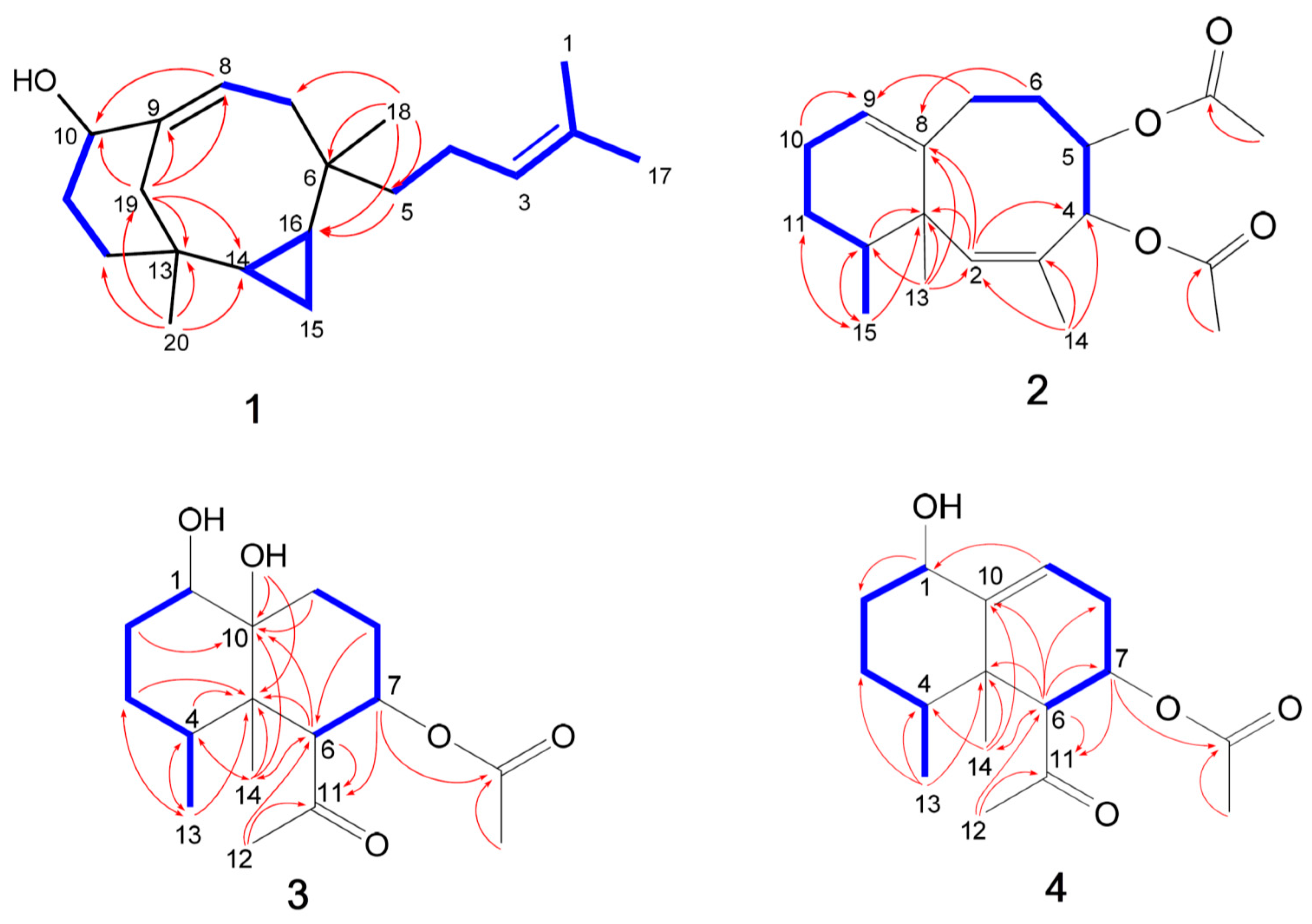


| Position | δH a (J in Hz) | δC b, Type | COSY | HMBC | NOESY |
|---|---|---|---|---|---|
| 1 | 1.62 s | 17.6, CH3 | 3 | 2, 3, 17 | - |
| 2 | - | 130.9, C | - | - | - |
| 3 | 5.13 t (7.2) | 125.3, CH | 1, 4, 17 | 1, 17 | 17 |
| 4 | 1.86 m; 2.04 m | 22.8, CH2 | 3, 5 | - | - |
| 5 | 1.35 m | 45.3, CH2 | 4 | 3, 6, 7, 16, 18 | 16 |
| 6 | - | 39.7, C | - | - | - |
| 7a | 2.24 m | 40.9, CH2 | 8 | 6, 8, 9, 16 | 16 |
| 7b | 1.79 m | 8 | 6, 8, 9, 10 | 8, 18 | |
| 8 | 5.59 t (8.0) | 121.6, CH | 7 | 6, 7, 10 | 10, 18 |
| 9 | - | 144.5, C | - | - | - |
| 10 | 4.29 s | 74.1, CH | 11, 19 | 8 | 8, 11b |
| 11a | 2.05m | 32.4, CH2 | 12 | 10, | - |
| 11b | 1.86 m | 12 | 10, | 14 | |
| 12 | 1.41 m; 1.77 m | 33.6, CH2 | 11 | - | - |
| 13 | - | 37.4, C | - | - | - |
| 14 | 0.78 dd (14.8, 7.2) | 15.6, CH | 15, 16 | 12, 13, 16, 20 | 11b, 15b, 18 |
| 15a | 0.49 m | 8.6, CH2 | 14, 16 | 6, 13, 16 | 20 |
| 15b | 0.36 m | 14, 16 | 6, 13, 16 | 14, 18, | |
| 16 | 0.25 dd (15.2, 7.2) | 23.7, CH | 14, 15 | - | 5, 20 |
| 17 | 1.69 s | 25.7, CH3 | 3 | 1, 2, 3 | 3 |
| 18 | 0.52 s | 15.9, CH3 | - | 5, 6, 7, 16 | 8, 10, 14, 15a |
| 19a | 2.08 m | 38.9, CH2 | 10 | 8, 9, 10, 13, 14 | 16, 20 |
| 19b | 2.16 m | 10 | 8, 9, 10, 13, 14 | 20 | |
| 20 | 0.64 s | 23.2, CH3 | - | 12, 13, 14, 19 | 15a, 16, 19 |
| Position | 2, δH a (Type) | 3, δH b (Type) | 4, δH b (Type) |
|---|---|---|---|
| 1 | 43.2, C | 71.7, CH | 68.9, CH |
| 2 | 137.9, CH | 30.86, CH2 | 35.8, CH2 |
| 3 | 128.0, C | 29.0, CH2 | 28.7, CH2 |
| 4 | 71.0, CH | 30.94, CH | 35.4, CH |
| 5 | 74.7, CH | 46.1, C | 43.9, C |
| 6 | 30.2, CH2 | 59.5, CH | 58.5, CH |
| 7 | 31.2, CH2 | 71.6, CH | 69.4, CH |
| 8 | 141.0, C | 23.1, CH2 | 27.4, CH2 |
| 9 | 125.9, CH | 29.6, CH2 | 114.0, CH |
| 10 | 23.1, CH2 | 72.6, C | 143.6, C |
| 11 | 26.2, CH2 | 214.3, C | 206.9, C |
| 12 | 38.3, CH | 34.3, CH3 | 33.8, CH3 |
| 13 | 23.6, CH3 | 15.5, CH3 | 15.3, CH3 |
| 14 | 21.4, CH3 | 16.4, CH3 | 21.1, CH3 |
| 15 | 15.0, CH3 | - | - |
| 4-OAc | 170.3, C 21.2, CH3 | - | - |
| 5-OAc | 170.1, C 20.8, CH3 | - | - |
| 7-OAc | - | 169.1, C 20.6, CH3 | 169.0, C 20.4, CH3 |
| Position | 2, δH a (J in Hz) | 3, δH b (J in Hz) | 4, δH b (J in Hz) |
|---|---|---|---|
| 1 | - | 3.52 d (5.2) | 3.76 m |
| 2 | 5.29 brs | α: 1.92 m; β: 1.75 m | α: 1.13 m; β: 1.73 m |
| 3 | - | α: 1.16 m; β: 0.98 m | α: 1.13 m; β: 1.04 m |
| 4 | 6.63 d (3.2) | 1.86 m, | 1.63 m |
| 5 | 5.18 ddd (9.2, 4.8, 3.2) | - | - |
| 6 | α: 1.83 m; β: 1.94 m | 2.99 d (6.8) | 3.09 d (5.2) |
| 7 | α: 2.10 ddd (14.8, 12.4, 4.4); β: 2.48 m | 5.16 ddd (12.4, 6.8, 4.8) | 5.38 ddd (10.0, 6.4, 5.2) |
| 8 | - | α: 1.92 m; β: 1.64 ddt (12.4, 4.0, 4.0) | α: 2.42 m; β: 2.34 m |
| 9 | 5.57 dd (3.6, 3.2) | α: 2.22 ddd (14.0, 4.0, 4.0); β: 1.42 td (14.0, 4.0) | 5.67 dd (6.4, 2.8) |
| 10 | 2.03 m | - | - |
| 11 | α: 1.78 m; β: 1.39 m | - | - |
| 12 | 1.71 m | 1.82 s | 1.96 s |
| 13 | 1.05 s | 0.35 d (7.2) | 0.54 d (6.8) |
| 14 | 1.68 d (1.2) | 0.54 s | 0.82 s |
| 15 | 0.87 d (6.8) | - | - |
| 4-OAc | 2.06 s | - | - |
| 5-OAc | 2.03 s | - | - |
| 7-OAc | - | 1.52 s | 1.63 s |
| 12-OH | - | 6.68 d (1.2) | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-S.; Duh, T.-H.; Siao, S.-S.; Chang, R.-C.; Wang, S.-K.; Duh, C.-Y. New Cytotoxic Terpenoids from Soft Corals Nephthea chabroli and Paralemnalia thyrsoides. Mar. Drugs 2017, 15, 392. https://doi.org/10.3390/md15120392
Lee Y-S, Duh T-H, Siao S-S, Chang R-C, Wang S-K, Duh C-Y. New Cytotoxic Terpenoids from Soft Corals Nephthea chabroli and Paralemnalia thyrsoides. Marine Drugs. 2017; 15(12):392. https://doi.org/10.3390/md15120392
Chicago/Turabian StyleLee, Yu-Sheng, Tsai-Hui Duh, Shu-Sheng Siao, Rey-Chang Chang, Shang-Kwei Wang, and Chang-Yih Duh. 2017. "New Cytotoxic Terpenoids from Soft Corals Nephthea chabroli and Paralemnalia thyrsoides" Marine Drugs 15, no. 12: 392. https://doi.org/10.3390/md15120392




