Variation Quality and Kinetic Parameter of Commercial n-3 PUFA-Rich Oil during Oxidation via Rancimat
Abstract
:1. Introduction
2. Results and Discussion
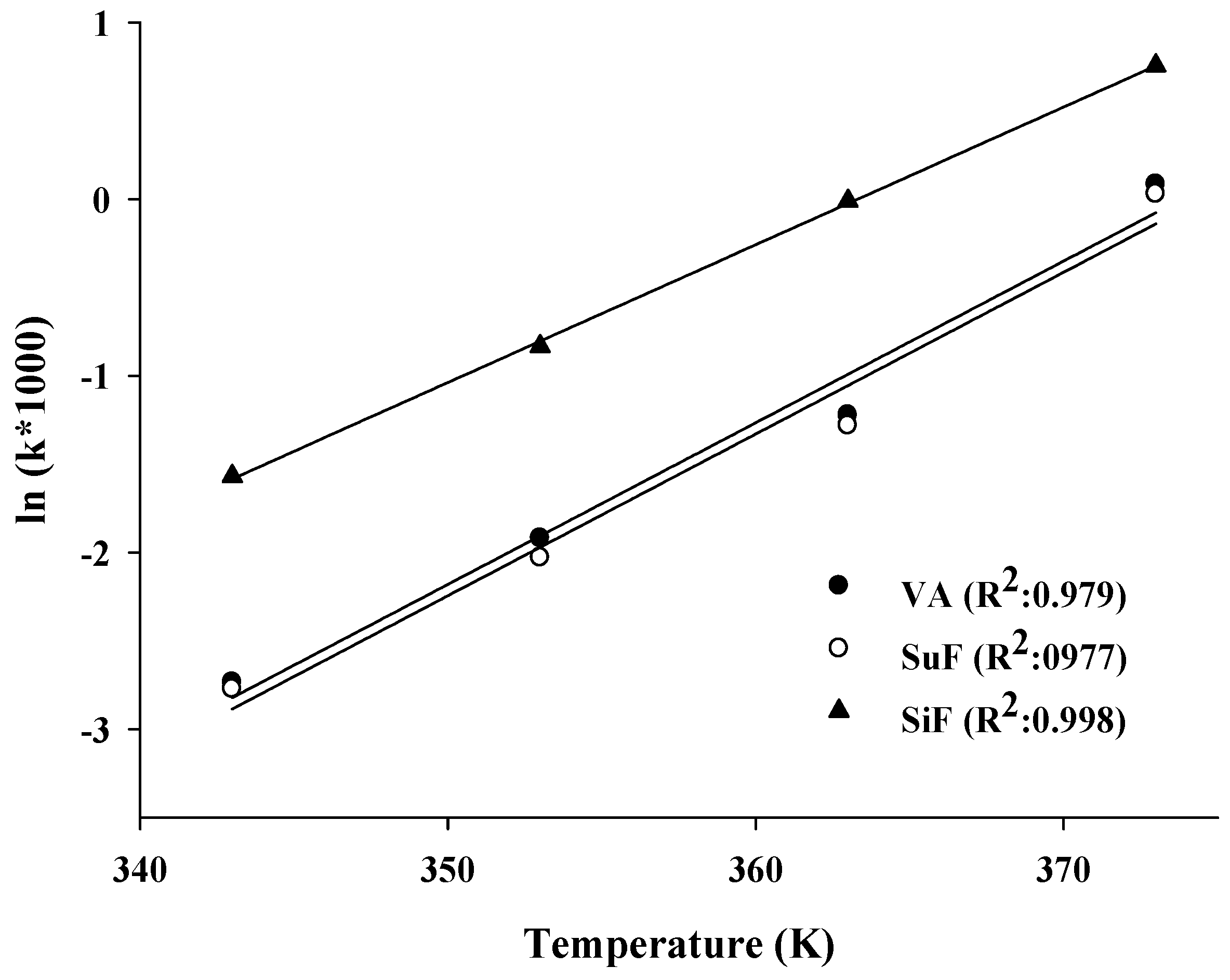
2.1. Kinetic Analysis
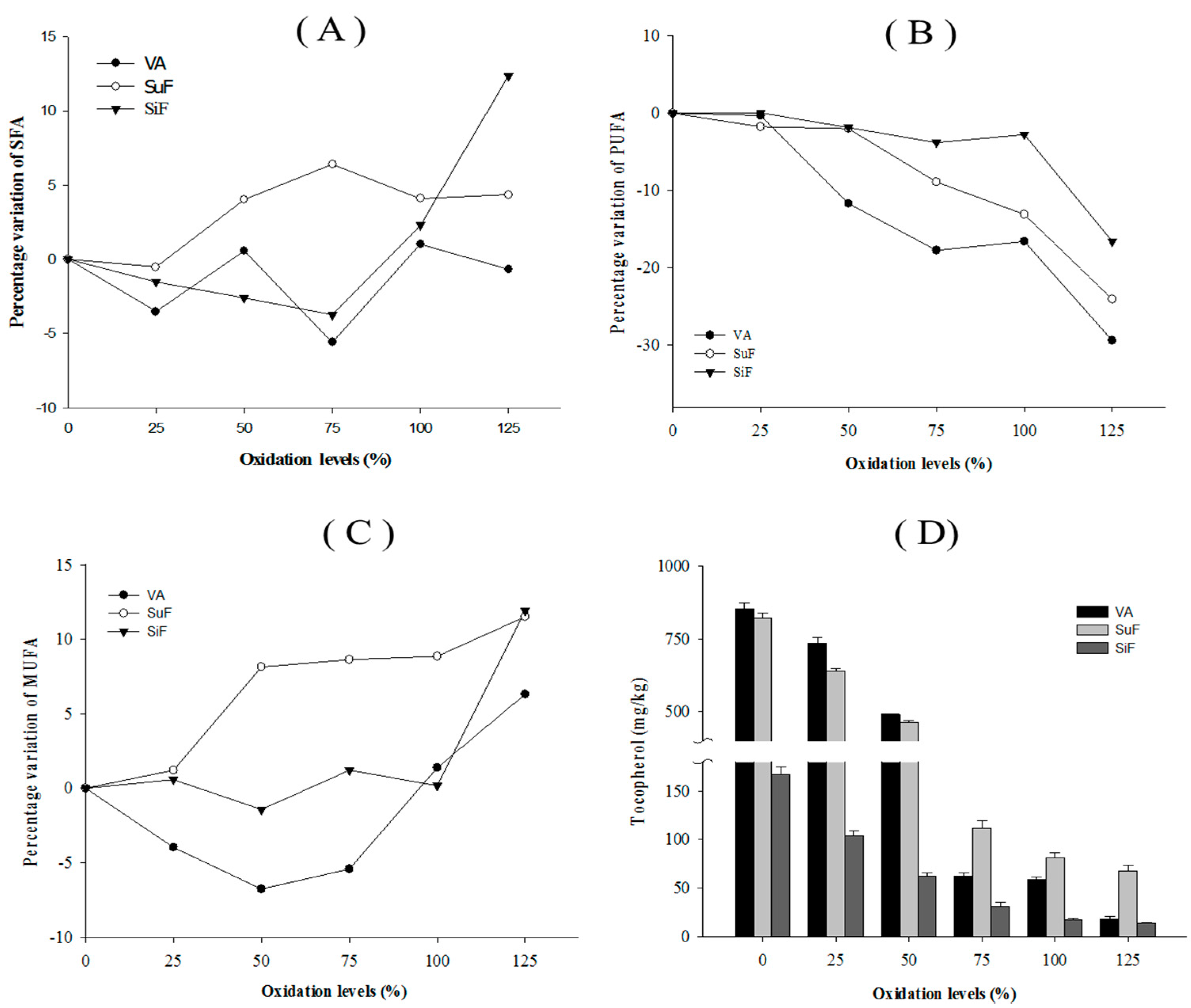
2.2. Monitoring Substrate Variants
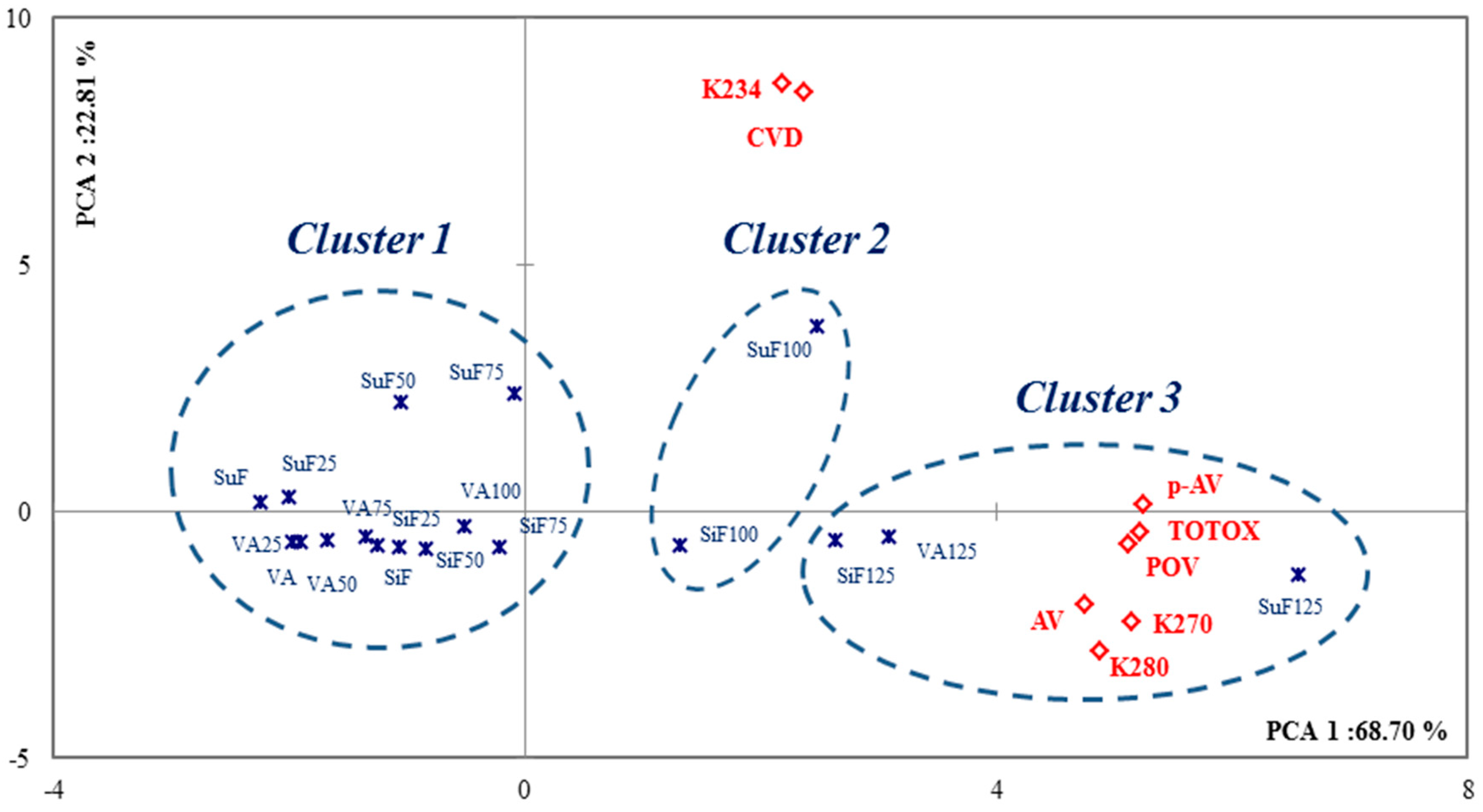
2.3. Monitoring Oxidation Products
3. Materials and Methods
3.1. Materials
3.2. Rancimat Test
3.3. Kinetic Data Analysis
3.4. Analysis of Tocopherol
3.5. Fatty Acid Analysis
3.6. Quality Analytical Determination
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SFA | Saturated fatty acids |
| MUFA | Monounsaturated fatty acid |
| PUFA | Polyunsaturated fatty acid |
| AA | Arachidonic acid |
| EPA | Eicosapentaenoic acid |
| DPA | Docosapentaenoic acid |
| DHA | Docosahexaenoic acid |
| GOED | Global Organization for EPA and DHA |
| IP | Induction period |
| Ea | Activation energies |
| POV | Peroxide value |
| p-AV | Anisidine value |
| AV | Acid value |
| CD | Conjugated dienes |
| TOTOX | Total oxidation value |
| PCA | Principal component analysis |
| AHC | Agglomerative hierarchical cluster |
References
- Pike, I.H. Fish oil: Supply and demand as a source of long-chain n-3 polyunsaturated fatty acids in the human diet. Eur. J. Lipid Sci. Technol. 2015, 117, 747–750. [Google Scholar] [CrossRef]
- Nichols, P.D.; McManus, A.; Krail, K.; Sinclair, A.J.; Miller, M. Recent advances in omega-3: Health benefits, Sources, Products and bioavailability. Nutrients 2014, 9, 3727–3733. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J.; Committee, N. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Onuma, K.; Fujimoto, K.; Miyake, S.; Nakamura, T. Long-term effect of an enteral diet with a different n-6/n-3 ratio on fatty acid composition and blood parameters in rats. J. Oleo Sci. 2011, 60, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.K.; Garg, S.; Timmins, M.; Zhang, E.S.; Thomas-Hall, S.R.; Schuhmann, H.; Li, Y.; Schenk, P.M. Isolation and evaluation of oil-producing microalgae from subtropical coastal and brackish waters. PLoS ONE 2012, 7, e40751. [Google Scholar] [CrossRef] [PubMed]
- Opperman, M.; Marais, D.W.; Benadé, A.S. Analysis of omega-3 fatty acid content of South African fish oil supplements. Cardiovasc. J. Afr. 2011, 22, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Facts, P. Global Market for EPA/DHA Omega-3 Products; Packaged Facts: Rockville, MD, USA, 2012. [Google Scholar]
- Adarme-Vega, T.C.; Thomas-Hall, S.R.; Schenk, P.M. Towards sustainable sources for omega-3 fatty acids production. Curr. Opin. Biotechnol. 2014, 26, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Adarme-Vega, T.C.; Lim, D.K.; Timmins, M.; Vernen, F.; Li, Y.; Schenk, P.M. Microalgal biofactories: A promising approach towards sustainable omega-3 fatty acid production. Microb. Cell Fact. 2012, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Kuratko, C.N.; Norman, S. Docosahexaenoic acid from algal oil. Eur. J. Lipid Sci. Technol. 2013, 115, 965–976. [Google Scholar] [CrossRef]
- Martins, D.A.; Custódio, L.; Barreira, L.; Pereira, H.; Ben-Hamadou, R.; Varela, J.; Abu-Salah, K.M. Alternative sources of n-3 long-chain polyunsaturated fatty acids in marine microalgae. Mar. Drugs 2013, 11, 2259–2281. [Google Scholar] [CrossRef] [PubMed]
- Drusch, S.; Groß, N.; Schwarz, K. Efficient stabilization of bulk fish oil rich in long-chain polyunsaturated fatty acids. Eur. J. Lipid Sci. Technol. 2008, 110, 351–359. [Google Scholar] [CrossRef]
- Guillén, M.D.; Goicoechea, E. Toxic oxygenated α, β-unsaturated aldehydes and their study in foods: A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Guillén, M.D.; Cabo, N.; Ibargoitia, M.L.; Ruiz, A. Study of both sunflower oil and its headspace throughout the oxidation process. Occurrence in the headspace of toxic oxygenated aldehydes. J. Agric. Food Chem. 2005, 53, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- GOED. Available online: http://www.goedomega3.com/healthcare (accessed on 10 January 2016).
- Tan, C.; Man, Y.C.; Selamat, J.; Yusoff, M. Application of Arrhenius kinetics to evaluate oxidative stability in vegetable oils by isothermal differential scanning calorimetry. J. Am. Oil Chem. Soc. 2001, 78, 1133–1138. [Google Scholar] [CrossRef]
- Farhoosh, R.; Niazmand, R.; Rezaei, M.; Sarabi, M. Kinetic parameter determination of vegetable oil oxidation under Rancimat test conditions. Eur. J. Lipid Sci. Technol. 2008, 110, 587–592. [Google Scholar] [CrossRef]
- Chen, M.H.; Huang, T.C. Volatile and Nonvolatile Constituents and Antioxidant Capacity of Oleoresins in Three Taiwan Citrus Varieties as Determined by Supercritical Fluid Extraction. Molecules 2016, 21, 1735. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.L. Shelf life of packaged foods, its measurements and prediction. In Developing New Food Products for a Changing Marketplace; CRC Press: Boca Raton, FL, USA, 2000; pp. 329–353. [Google Scholar]
- Rupasinghe, H.V.; Erkan, N.; Yasmin, A. Antioxidant protection of eicosapentaenoic acid and fish oil oxidation by polyphenolic-enriched apple skin extract. J. Agric. Food Chem. 2009, 58, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Adhvaryu, A.; Erhan, S.; Liu, Z.; Perez, J. Oxidation kinetic studies of oils derived from unmodified and genetically modified vegetables using pressurized differential scanning calorimetry and nuclear magnetic resonance spectroscopy. Thermochim. Acta 2000, 364, 87–97. [Google Scholar] [CrossRef]
- Yoshii, H.; Furuta, T.; Siga, H.; Moriyama, S.; Baba, T.; Maruyama, K.; Misawa, Y.; Hata, N.; Linko, P. Autoxidation kinetic analysis of docosahexaenoic acid ethyl ester and docosahexaenoic triglyceride with oxygen sensor. Biosci. Biotechnol. Biochem. 2002, 66, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, T.; Ning, Z.; Wang, Y.; Yang, B.; Ma, Y.; Yang, X. A process for the synthesis of PUFA-enriched triglycerides from high-acid crude fish oil. J. Food Eng. 2012, 109, 366–371. [Google Scholar] [CrossRef]
- De Leonardis, A.; Macciola, V. Heat-oxidation stability of palm oil blended with extra virgin olive oil. Food Chem. 2012, 135, 1769–1776. [Google Scholar] [CrossRef] [PubMed]
- Kulås, E.; Ackman, R.G. Properties of α-, γ-, and δ-tocopherol in purified fish oil triacylglycerols. J. Am. Oil Chem. Soc. 2001, 78, 361–367. [Google Scholar] [CrossRef]
- Fujisawa, S.; Kadoma, Y. Kinetic study of the radical-scavenging activity of vitamin E and ubiquinone. In Vivo 2005, 19, 1005–1011. [Google Scholar] [PubMed]
- Verleyen, T.; Kamal-Eldin, A.; Dobarganes, C.; Verhé, R.; Dewettinck, K.; Huyghebaert, A. Modeling of α-tocopherol loss and oxidation products formed during thermoxidation in triolein and tripalmitin mixtures. Lipids 2001, 36, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Navas, J.A.; Tres, A.; Codony, R.; Guardiola, F. Optimization of analytical methods for the assessment of the quality of fats and oils used in continuous deep fat frying. Grasas Aceites 2007, 58, 154–162. [Google Scholar]
- Shahid Chatha, S.A.; Anwar, F.; Manzoor, M.; Rehman Bajwa, J.U. Evaluation of the antioxidant activity of rice bran extracts using different antioxidant assays. Grasas Aceites 2006, 57, 328–335. [Google Scholar]
- Navarro, T.; de Lorenzo, C.; Pérez, R. SPME analysis of volatile compounds from unfermented olives subjected to thermal treatment. Anal. Bioanal. Chem. 2004, 379, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Tu, D.; Li, H.; Wu, Z.; Zhao, B.; Li, Y. Application of headspace solid-phase microextraction and multivariate analysis for the differentiation between edible oils and waste cooking oil. Anal. Bioanal. Chem. 2014, 7, 1263–1270. [Google Scholar] [CrossRef]
- Yang, K.M.; Cheng, M.C.; Chen, C.W.; Tseng, C.Y.; Lin, L.Y.; Chiang, P.Y. Characterization of Volatile Compounds with HS-SPME from Oxidized n-3 PUFA Rich Oils via Rancimat tests. J. Oleo Sci. 2017, 66, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Grau, A.; Guardiola, F.; Boatella, J.; Baucells, M.D.; Codony, R. Evaluation of lipid ultraviolet absorption as a parameter to measure lipid oxidation in dark chicken meat. J. Agric. Food Chem. 2000, 48, 4128–4135. [Google Scholar] [CrossRef] [PubMed]
- Shehata, A.B.; Rizk, M.S.; Farag, A.M.; Tahoun, I.F. Development of two reference materials for all trans-retinol, retinyl palmitate, α- and γ-tocopherol in milk powder and infant formula. J. Food Drug Anal. 2015, 23, 82–92. [Google Scholar] [CrossRef]
- American Oil Chemists’ Society (AOCS). Official Methods and Recommended Practices of the American Oil Chemists’ Society; Firestone, D., Ed.; AOCS Press: Champaign, IL, USA, 1998. [Google Scholar]
- Regulation, H. Commission Regulation (EEC) No. 2568/91 of 11 July 1991 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis Official Journal L 248, 5 September 1991. Off. J. L 1991, 248, 1–83. [Google Scholar]
| Groups | VA | SuF | SiF |
|---|---|---|---|
| ln(k) = a(1/T) + b | |||
| a | −11.66 | −11.66 | −9.96 |
| b | 31.16 | 31.1 | 27.44 |
| R2 | 0.973 | 0.971 | 0.998 |
| Ea (kJ/mol) | 96.98 | 96.97 | 82.84 |
| Groups | VA | SuF | SiF |
|---|---|---|---|
| Fatty Acid (g/100 g) | |||
| SFA a | 34.4 | 44.5 | 43.9 |
| C14:0 | 2.6 | 8.6 | 15.6 |
| C16:0 | 30.2 | 29.8 | 25.6 |
| C18:0 | 1.6 | 6.1 | 2.7 |
| MUFA b | 34.5 | 20.2 | 24.0 |
| C14:1 | 1.3 | 1.9 | 1.1 |
| C16:1 | N.D d | 4.8 | 18.0 |
| C18:1 | 33.2 | 11.7 | 7.1 |
| C20:1 | N.D | 1.8 | 1.8 |
| PUFA c | 30.3 | 17.6 | 24.0 |
| C18:2 | 3.5 | 1.2 | 3.5 |
| C20:2 | N.D | N.D | 3.3 |
| C20:3 | N.D | 1.9 | 0.6 |
| AA | N.D | N.D | 0.9 |
| EPA | N.D | 4.6 | 9.9 |
| DPA | 3.5 | 1.0 | 1.1 |
| DHA | 23.3 | 8.9 | 4.7 |
| Tocopherol (mg/kg) | |||
| δ- | 219.3 | 252.9 | 167.3 |
| γ- | 529.4 | 445.3 | N.D |
| α- | 106.6 | 124.4 | N.D |
| Groups | VA | SuF | SiF |
|---|---|---|---|
| Quality Indicators | |||
| AV (mg KOH/g) | 0.48 ± 0.02 | 0.49 ± 0.01 | 0.65 ± 0.01 |
| CVD (%) | 0.22 ± 0.05 | 1.18 ± 0.17 | 0.58 ± 0.03 |
| POV (meq/kg) | 1.98 ± 0.27 | 4.12 ± 0.34 | 13.62 ± 0.42 |
| p-AV (meq/kg) | 6.33 ± 0.71 | 15.12 ± 0.64 | 29.23 ± 1.84 |
| TOTOX (meq/kg) | 10.30 ± 0.92 | 23.26 ± 1.24 | 56.46 ± 2.11 |
| Visible Spectra (10 mg/mL) | |||
| K234 a | 3.33 ± 0.07 | 14.79 ± 0.14 | 7.58 ± 0.17 |
| K270 | 1.12 ± 0.01 | 0.66 ± 0.02 | 1.78 ± 0.04 |
| K280 | 1.08 ± 0.02 | 0.51 ± 0.01 | 1.48 ± 0.07 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.-M.; Chiang, P.-Y. Variation Quality and Kinetic Parameter of Commercial n-3 PUFA-Rich Oil during Oxidation via Rancimat. Mar. Drugs 2017, 15, 97. https://doi.org/10.3390/md15040097
Yang K-M, Chiang P-Y. Variation Quality and Kinetic Parameter of Commercial n-3 PUFA-Rich Oil during Oxidation via Rancimat. Marine Drugs. 2017; 15(4):97. https://doi.org/10.3390/md15040097
Chicago/Turabian StyleYang, Kai-Min, and Po-Yuan Chiang. 2017. "Variation Quality and Kinetic Parameter of Commercial n-3 PUFA-Rich Oil during Oxidation via Rancimat" Marine Drugs 15, no. 4: 97. https://doi.org/10.3390/md15040097