Construction of a Fluorescent H2O2 Biosensor with Chitosan 6-OH Immobilized β-Cyclodextrin Derivatives
Abstract
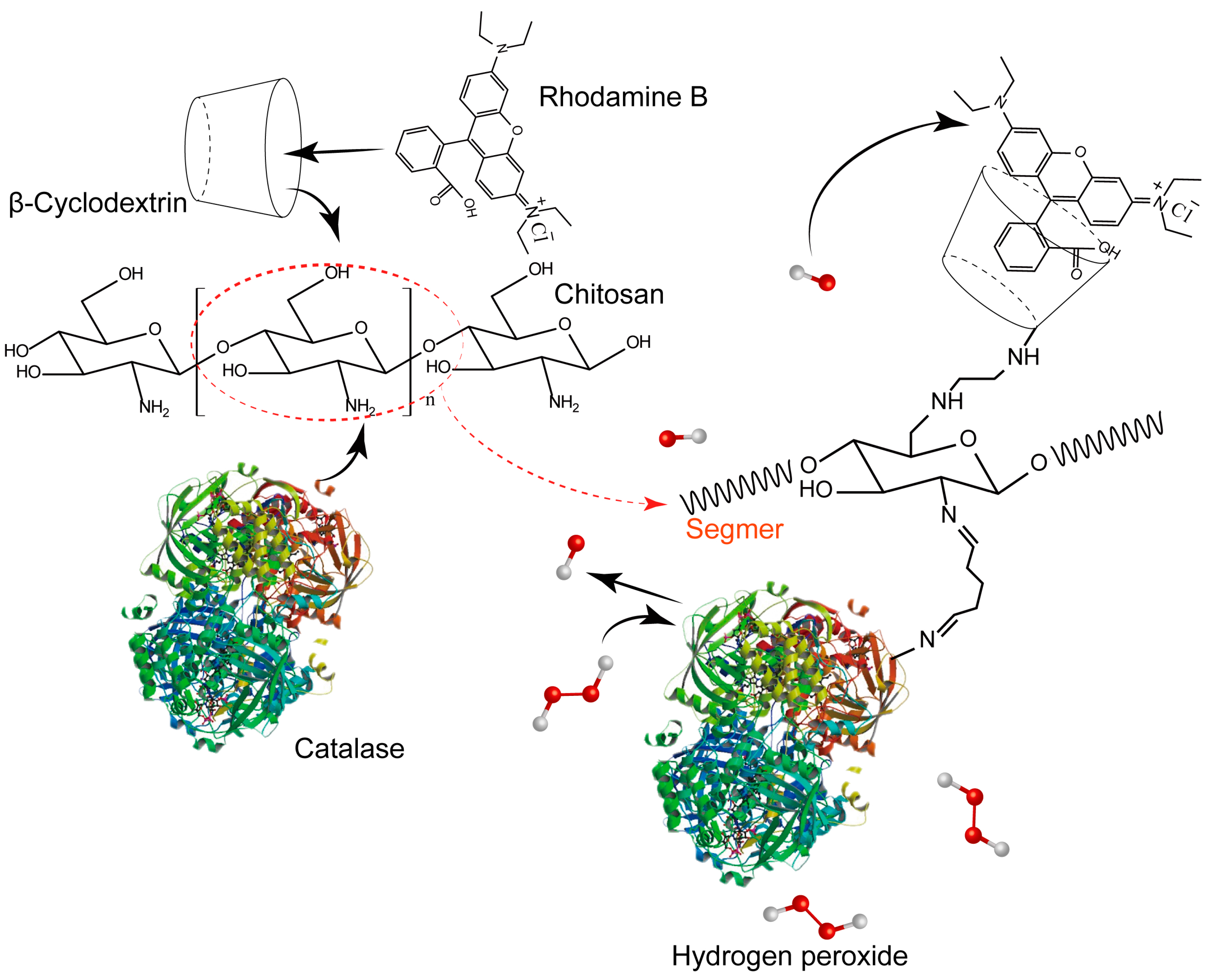
:1. Introduction
2. Results and Discussion
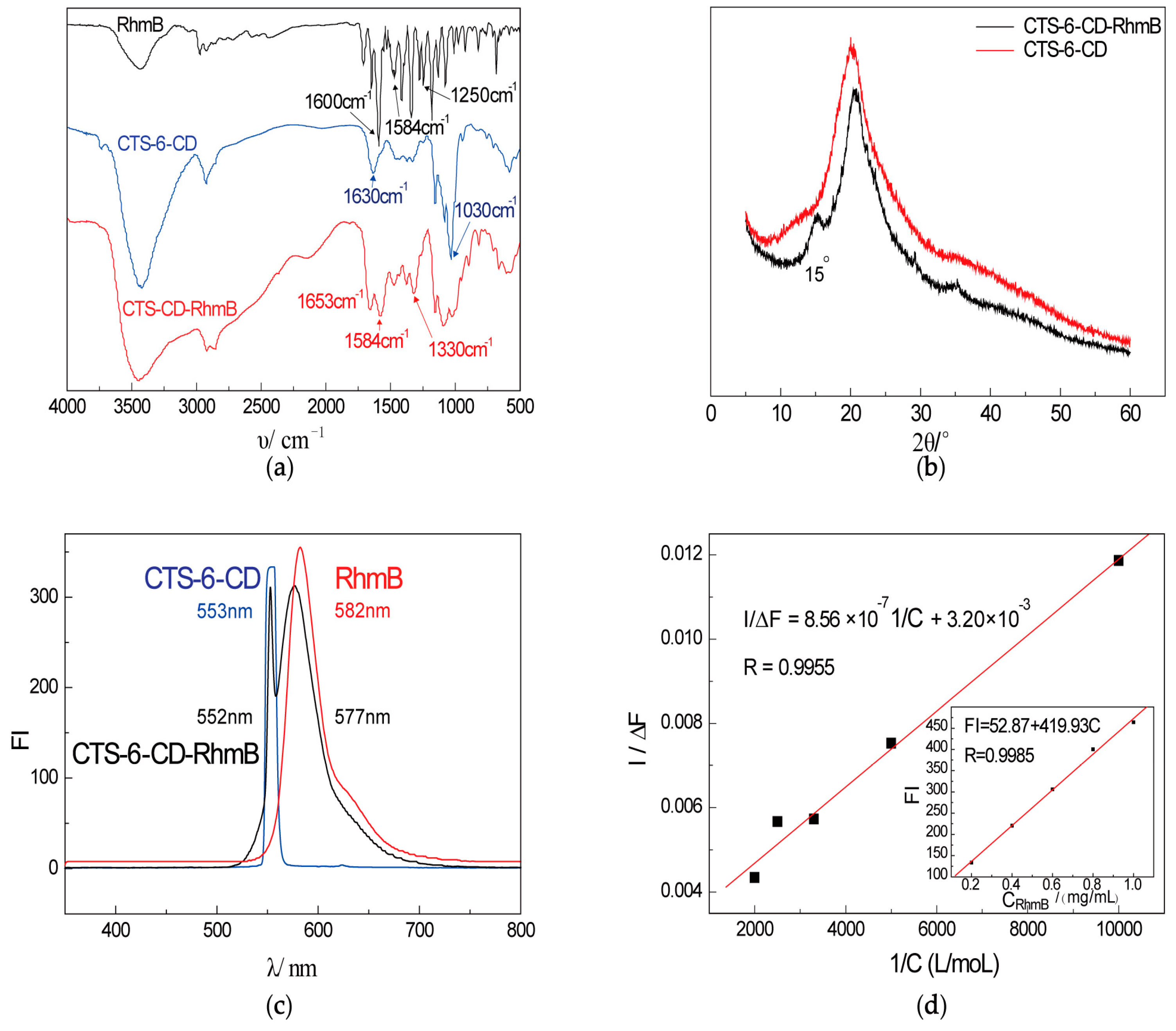
2.1. Characterization of CTS-6-CD-RhmB
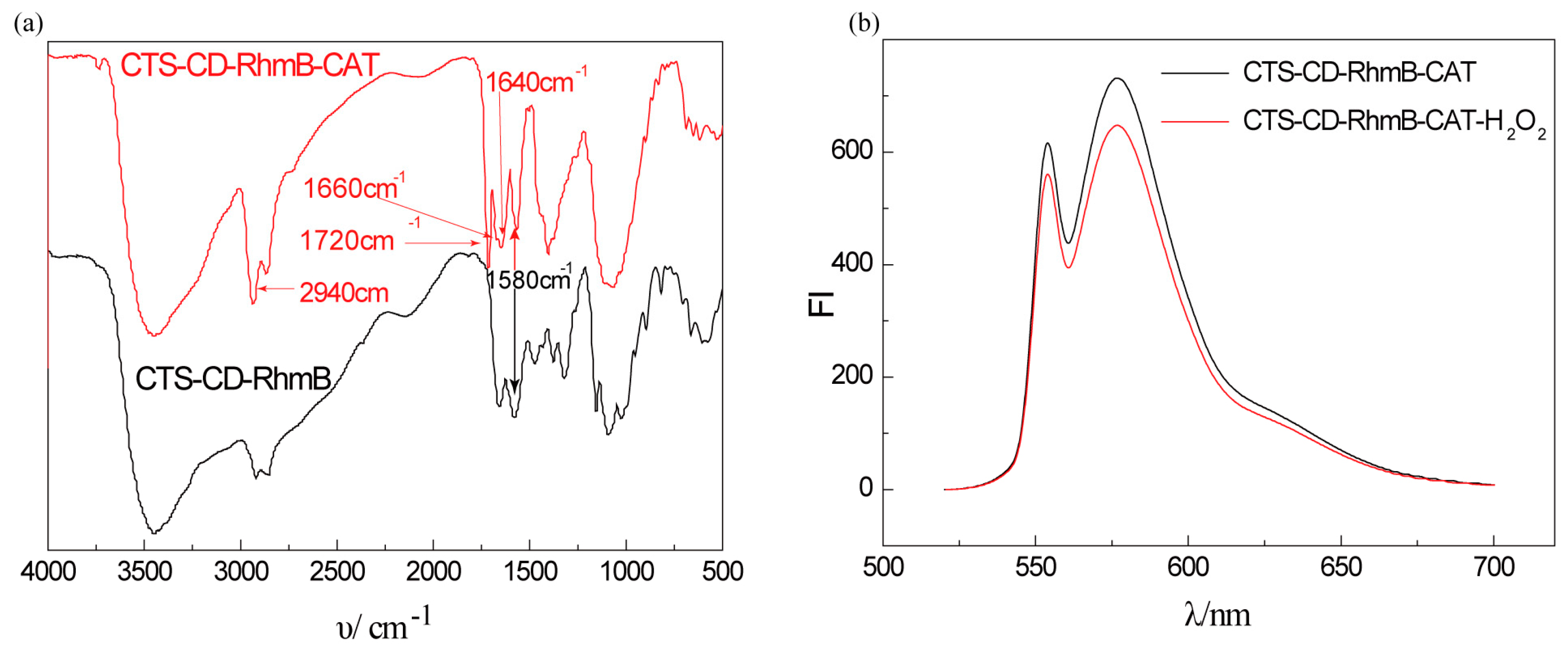
2.2. Characterization of CTS-6-CD-RhmB-CAT
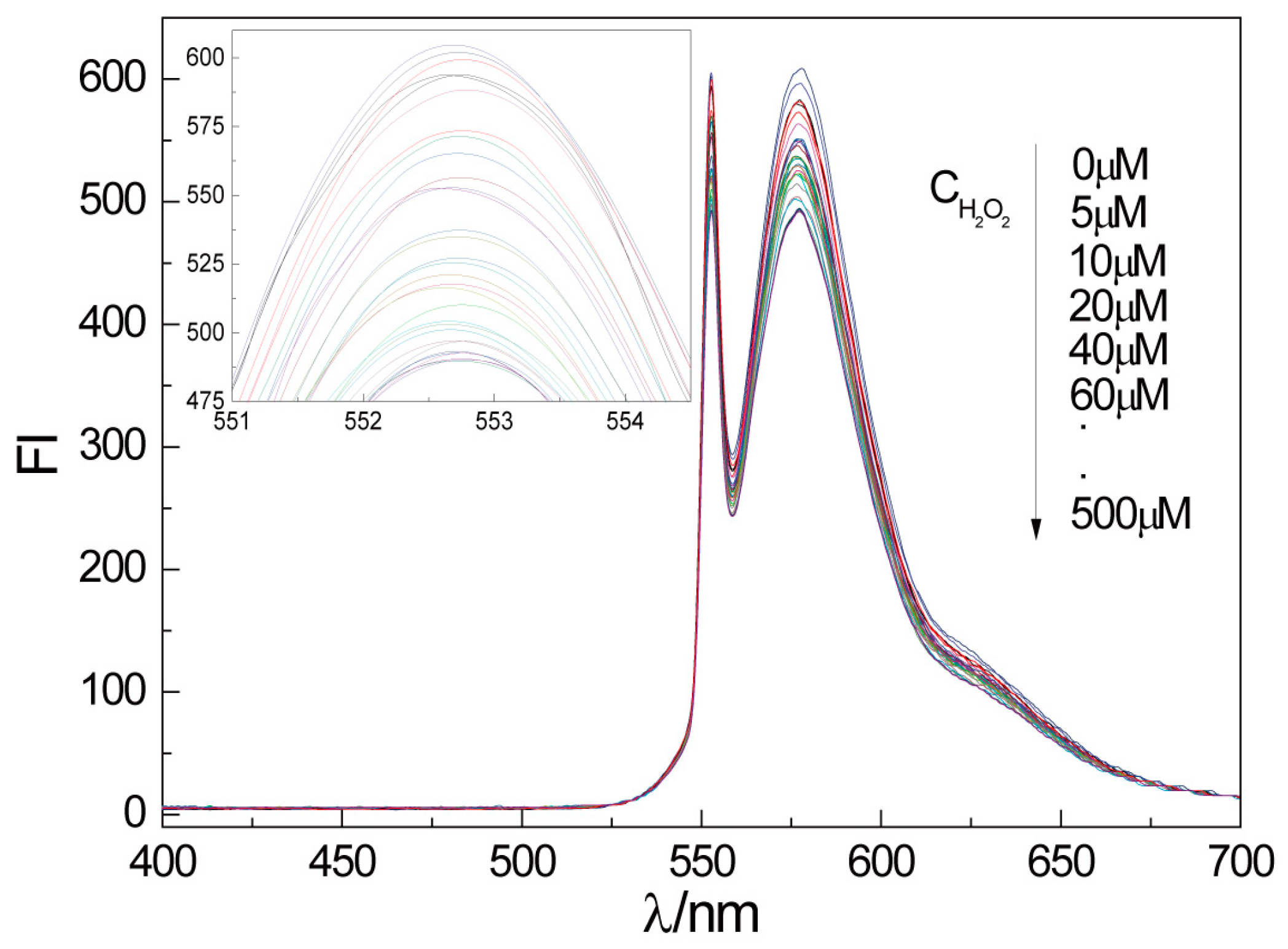
2.3. Fluorescence Detection of H2O2 by CTS-6-CD-RhmB-CAT
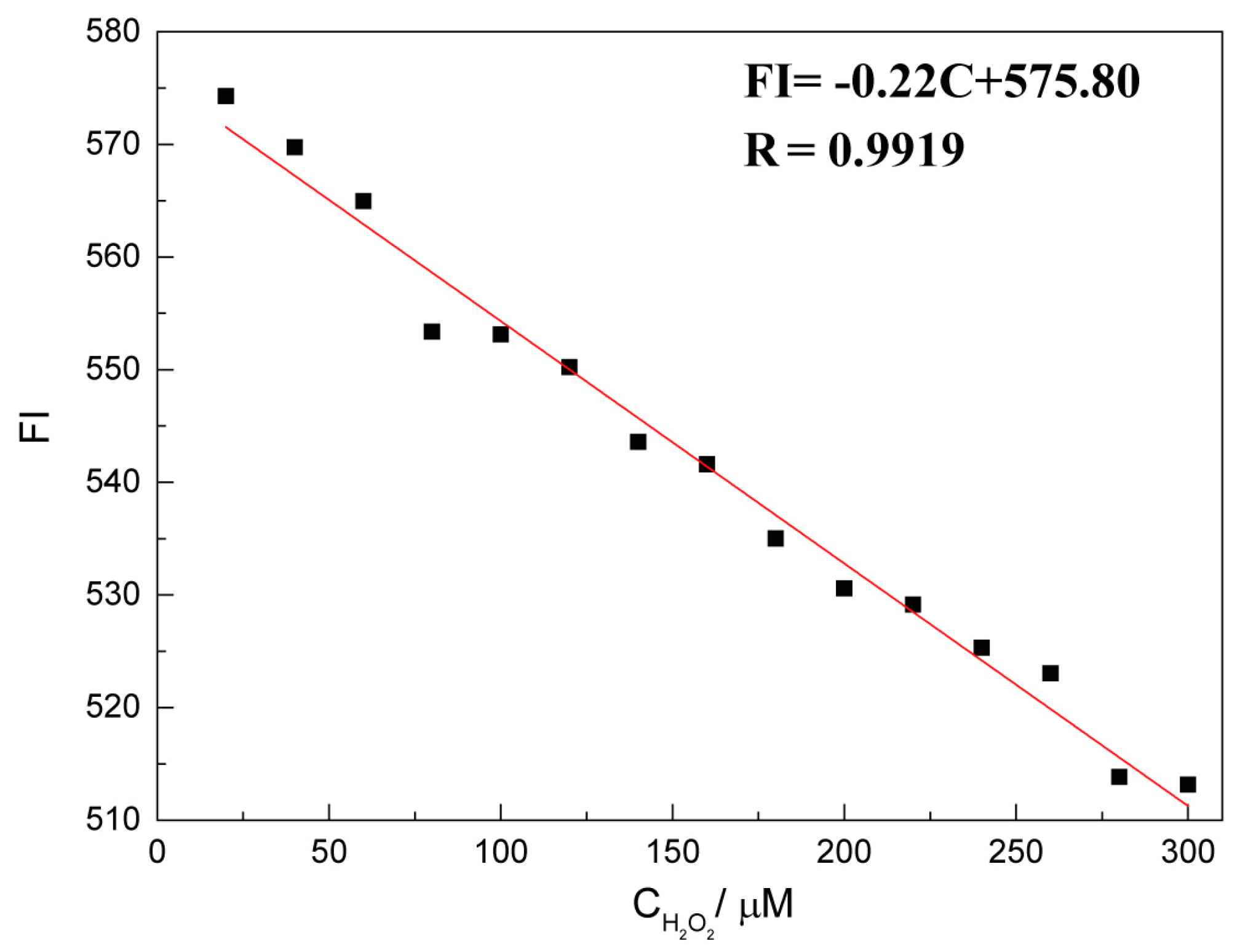
2.4. Quantitative Detection of H2O2
3. Materials and Methods
3.1. Materials
3.2. Synthesis of CTS-6-CD-RhmB
3.3. Synthesis of CTS-6-CD-RhmB-CAT
3.4. Characterization of CTS-6-CD-RhmB and CTS-6-CD-RhmB-CAT
3.5. Determination of Inclusion Constant, Inclusion Amount, and Inclusion Efficiency
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Villareal, T.A.; Altabet, M.A.; Culverrymsza, K. Nitrogen transport by vertically migrating diatom mats in the north pacific ocean. Nature 1993, 363, 709–712. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, C.; Tan, W.; Gu, G.; Guo, Z. Novel amino-pyridine functionalized chitosan quaternary ammonium derivatives: Design, synthesis, and antioxidant activity. Molecules 2017, 22, 156. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cai, H.; Yin, B.; Yao, P. Cholic acid modified N-(2-hydroxy)-propyl-3-trimethylammonium chitosan chloride for superoxide dismutase delivery. Int. J. Pharm. 2013, 454, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Cai, Z.; Gao, Y.; Zhang, H.; Cai, C. Enhancing the electrochemical reduction of hydrogen peroxide based on nitrogen-doped graphene for measurement of its releasing process from living cells. Chem. Commun. 2011, 47, 11327–11329. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wu, S.; Li, Y.; Wan, G. Sensing hydrogen peroxide using a glassy carbon electrode modified with in-situ electrodeposited platinum-gold bimetallic nanoclusters on a graphene surface. Microchim. Acta 2015, 182, 265–272. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, R.; Chai, Y.; Hu, F. Electrochemical sensing of hydrogen peroxide using metal nanoparticles: A review. Microchim. Acta 2013, 180, 15–32. [Google Scholar] [CrossRef]
- You, S.; Li, Z.; Yuan, F.L.; Li, Q.C.; Jia, L.X.; Cheng, Z.H. A visual detection of hydrogen peroxide on the basis of fenton reaction with gold nanoparticles. Anal. Chim. Acta 2010, 659, 224–228. [Google Scholar]
- Han, H.X. Detection of Hydrogen Peroxide and Glucose by Fluorescence Biosensor Based on Silver Nanoparticles and Nucleic Acid Dyes. Master’s Thesis, Hunan University, Changsha, China, 2016. [Google Scholar]
- Grigoras, A.G. Catalase immobilization—A review. Biochem. Eng. J. 2017, 117, 1–20. [Google Scholar] [CrossRef]
- Sajomsang, W.; Nuchuchua, O.; Gonil, P.; Saesoo, S.; Sramala, I.; Soottitantawat, A.; Puttipipatkhachorn, S.; Ruktanonchai, U.R. Water-soluble β-cyclodextrin grafted with chitosan and its inclusion complex as a mucoadhesive eugenol carrier. Carbohydr. Polym. 2012, 89, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Dhyani, H.; Ali, A.; Pal, S.P.; Srivastava, S.; Solanki, P.R.; Malhotra, B.D.; Sen, P. Mediator-free biosensor using chitosan capped cds quantum dots for detection of total cholesterol. Rsc Adv. 2015, 5, 45928–45934. [Google Scholar] [CrossRef]
- Chen, G.; Mi, J.; Wu, X.; Luo, C.; Li, J.; Tang, Y.; Li, J. Structural features and bioactivities of the chitosan. Int. J. Biol. Macromol. 2011, 49, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.P.; Ilayaraja, M.; Muthukrishnan, P.; Kannan, S. Preparation and application of β-cyclodextrin polymerized with phenol-formaldehyde as an adsorbent for the removal of pb(ii) and hg(ii) from aqueous media. J. Clin. Investig. 2016, 92, 2230–2239. [Google Scholar]
- Yan, J.; Tang, B.; Wu, D.; Li, S.; Xu, K.; Ma, X.; Wang, Q.; Li, H. Synthesis and characterization of β-cyclodextrin/fraxinellone inclusion complex and its influence on interaction with human serum albumin. Spectrosc. Lett. 2016, 49, 542–550. [Google Scholar] [CrossRef]
- Bakkialakshmi, S.; Menaka, T. Fluorescence enhancement of rhodamine 6G by forming inclusion complexes with β-cyclodextrin. J. Mol. Liq. 2011, 158, 117–123. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, H.; Wang, Y.; Zou, G.; Zhang, Q. β-cyclodextrin-induced fluorescence enhancement of a thermal-responsive azobenzene modified polydiacetylene vesicles for a temperature sensor. Mater. Chem. Phys. 2009, 124, 36–40. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.P.; Ye, Y.C.; Guo, Y.W.; Wang, T.W.; Xing, Y.F. The novel route to prepare immobilized macrocyclic compound on 6-oh of chitosan with good solubility and prime study on its applications. Mol. Cryst. Liq. Cryst. 2014, 604, 202–212. [Google Scholar]
- Sheng, L.; Hu, X.J.; Wang, H.X.; Lu, J.F.; Liu, G.L.; Su, B.Q. Study on the Inclusion Interaction of β-Cyclodextrin with Rhodamine B and Safranine T. Chem. Reag. 2009, 31, 883–886. [Google Scholar]
- Liu, H.Q. Study on Infrared Spectroscopic Absorption of β-Cyclodextrin Inclusion Molecule Molecules. In Proceedings of the 15th National Symposium on Molecular Spectroscopy, Beijing, China, 17 October 2008. [Google Scholar]
- Zhang, Y.L.; Ruan, W.J.; Hu, G.H.; Zhu, Z.A. Preparation and Spectroscopic Properties of Novel Dual-Core Schiff Base Complexes. Chin. J. Inorg. Chem. 2002, 18, 902–906. [Google Scholar]
- Ren, J.Y.; Kang, X.Y.; Chen, Y.; Guo, C.F. Study on immobilization of xanthine oxidase and its enzymatic properties by magnetic chitosan microspheres. Mod. Food Sci. Technol. 2016, 12, 66–73. [Google Scholar]
- Liang, Z.P.; Sang, M.X.; Li, J.; Cao, C.Q.; Wang, G.J. Preparation of glutaraldehyde crosslinked chitosan ball immobilized urease. J. Qingdao Univ. Sci. Technol. 2006, 27, 123–126. [Google Scholar]
- Jiang, H.L.; Liu, C.L.; Song, Z.J.; Sun, M.J.; Du, Z.H. Study on Different Catalytic Mechanism of Hydrogen Peroxide by Infrared Spectroscopy. In Proceedings of the 9th National Symposium on Molecular Spectroscopy, Guiyang, China, 10 September 1996. [Google Scholar]
- Su, R.; Wei, L.; Wei, Q.; He, Z. Fluorescence detection of alkaline protease based on protein-coated gold nanoclusters. J. Tianjin Univ. 2015, 48, 620–624. [Google Scholar]
- Hashemnia, S.; Eskanari, M. Preparation and electrochemical characterization of an enzyme electrode based on catalase immobilized onto a multiwall carbon nanotube-thionine film. J. Chin. Chem. Soc. 2015, 61, 903–909. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, W.; Li, W.; Chen, Y.; Ye, Y.; Jin, S. Construction of a Fluorescent H2O2 Biosensor with Chitosan 6-OH Immobilized β-Cyclodextrin Derivatives. Mar. Drugs 2017, 15, 284. https://doi.org/10.3390/md15090284
Dong W, Li W, Chen Y, Ye Y, Jin S. Construction of a Fluorescent H2O2 Biosensor with Chitosan 6-OH Immobilized β-Cyclodextrin Derivatives. Marine Drugs. 2017; 15(9):284. https://doi.org/10.3390/md15090284
Chicago/Turabian StyleDong, Wenbo, Weiping Li, Yu Chen, Yanchun Ye, and Shaohua Jin. 2017. "Construction of a Fluorescent H2O2 Biosensor with Chitosan 6-OH Immobilized β-Cyclodextrin Derivatives" Marine Drugs 15, no. 9: 284. https://doi.org/10.3390/md15090284



