Chloro-Furanocembranolides from Leptogorgia sp. Improve Pancreatic Beta-Cell Proliferation
Abstract
:1. Introduction
2. Results
Activation of Pancreatic Beta-Cell Proliferation
3. Experimental Section
3.1. General Experimental Procedures
3.2. Collection, Extraction and Isolation
3.3. Activation of Pancreatic Beta-Cell Proliferation
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dorta, E.; Díaz-Marrero, A.R.; Brito, I.; Cueto, M.; D’Croz, L.; Darias, J. The oxidation profile at C-18 of furanocembranolides may provide a taxonomical marker for several genera of octocorals. Tetrahedron 2007, 63, 9057–9062. [Google Scholar] [CrossRef]
- Díaz-Marrero, A.R.; Porras, G.; Cueto, M.; D’Croz, L.; Lorenzo, M.; San-Martín, A.; Darias, J. Leptogorgolide, a biogenetically interesting 1,4-diketo-cembranoid that reinforces the oxidation profile of C-18 as a taxonomical marker for octocorals. Tetrahedron 2009, 65, 6029–6033. [Google Scholar] [CrossRef]
- Wang, P.; Fiaschi-Taesch, N.M.; Vasavada, R.C.; Scott, D.K.; García-Ocaña, A.; Stewart, A.F. Diabetes mellitus—Advances and challenges in human β-cell proliferation. Nat. Rev. Endocrinol. 2015, 11, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Acosta, J.F.; Moreno-Amador, J.L.; Jimenez-Palomares, M.; Diaz-Marrero, A.R.; Cueto, M.; Perdomo, G.; Cozar-Castellano, I. Epoxypukalide induces proliferation and protects against cytokine-mediated apoptosis in primary cultures of pancreatic beta-cells. PLoS ONE 2013, 8, e52862. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Acosta, J.F.; Villa-Perez, P.; Fernandez-Diaz, C.M.; Roman Dde, L.; Diaz-Marrero, A.R.; Cueto, M.; Perdomo, G.; Cozar-Castellano, I. Protective effects of epoxypukalide on pancreatic beta-cells and glucose metabolism in STZ-induced diabetic mice. Islets 2015, 7, e1078053. [Google Scholar] [CrossRef] [PubMed]
- Villa-Perez, P.; Cueto, M.; Diaz-Marrero, A.R.; Lobatón, CD.; Moreno, A.; Perdomo, G.; Cozar-Castellano, I. Leptolide improves insulin resistance in diet-induced obese mice. Mar. Drugs 2017, 15, 289. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; Andersen, R.J.; Van Duyne, G.D.; Clardy, J. Cembrane and pseudopterane diterpenes from the soft coral Gersemia rubiformis. J. Org. Chem. 1987, 52, 332–335. [Google Scholar] [CrossRef]
- Bandurraga, M.M.; McKittrick, B.; Fenical, W.; Arnold, E.; Clardy, J. Diketone cembrenolides from the pacific gorgonian Lophogorgia alba. Tetrahedron 1982, 38, 305–310. [Google Scholar] [CrossRef]
- Rodríguez, A.D.; Shi, Y.-P. New Metabolites from the West Indian Sea Feather Pseudopterogorgia bipinnata. J. Nat. Prod. 2000, 63, 1548–1550. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.-H.; Ahmed, A.F.; Shiue, R.-T.; Dai, C.-F.; Kuo, Y.-H. Scabrolides A–D, Four New Norditerpenoids Isolated from the Soft Coral Sinularia scabra. J. Nat. Prod. 2002, 65, 1904–1908. [Google Scholar] [CrossRef] [PubMed]
- PCModel, version 9.2; Serena Software: Bloomington, IN, USA, 2007.
- Sánchez, C.M.; Ortega, M.J.; Zubía, E.; Carballo, J.L. Cembrane Diterpenes from the Gorgonian Lophogorgia peruana. J. Nat. Prod. 2006, 69, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Thao, N.P.; Nam, N.H.; Cuong, N.X.; Quang, T.H.; Tung, P.T.; Tai, B.H.; Luyen, B.T.T.; Chae, D.; Kim, S.; Koh, Y.-S.; et al. Diterpenoids from the Soft Coral Sinularia maxima and Their Inhibitory Effects on Lipopolysaccharide-Stimulated Production of Pro-inflammatory Cytokines in Bone Marrow-Derived Dendritic Cells. Chem. Pharm. Bull. 2012, 60, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
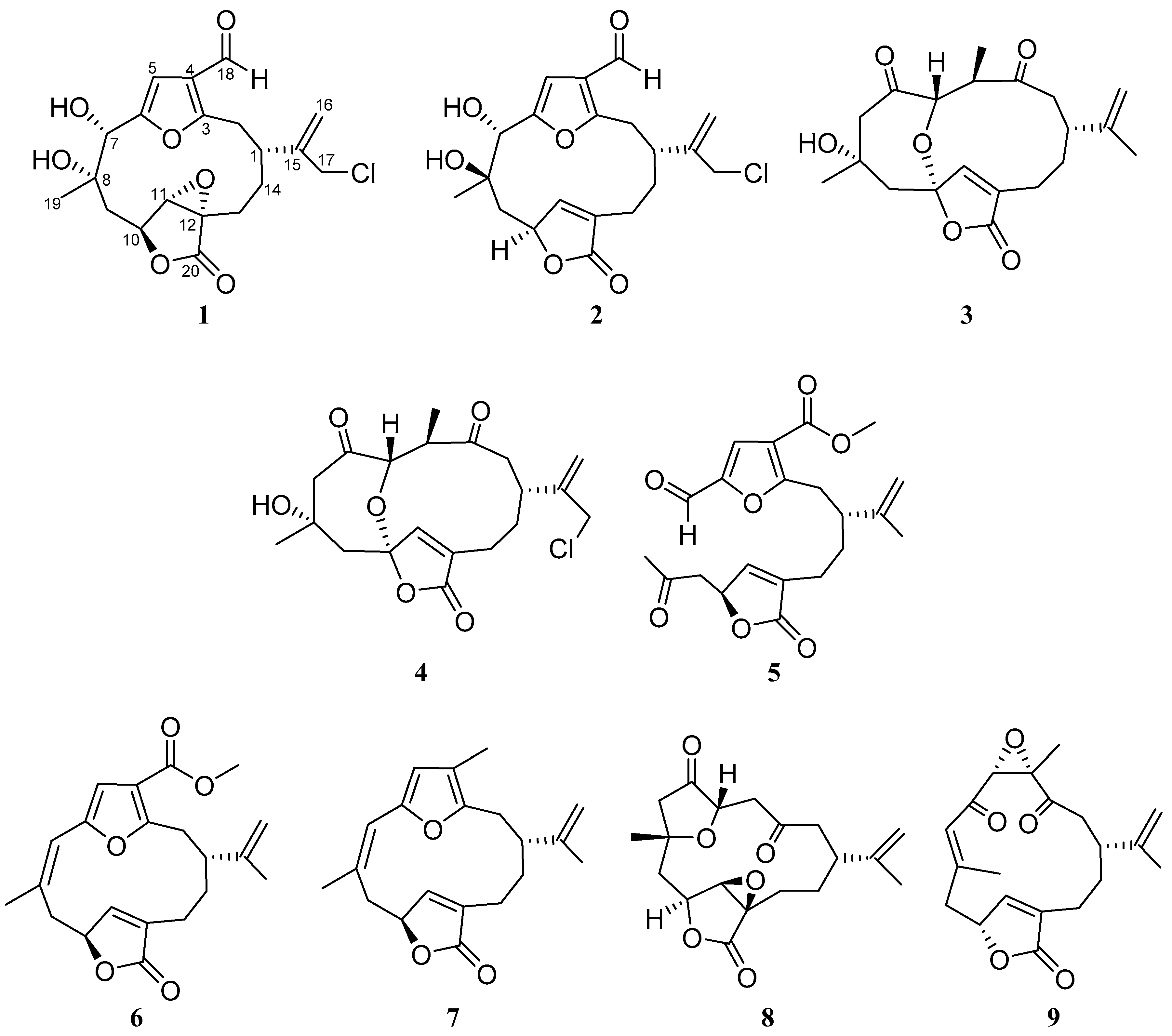
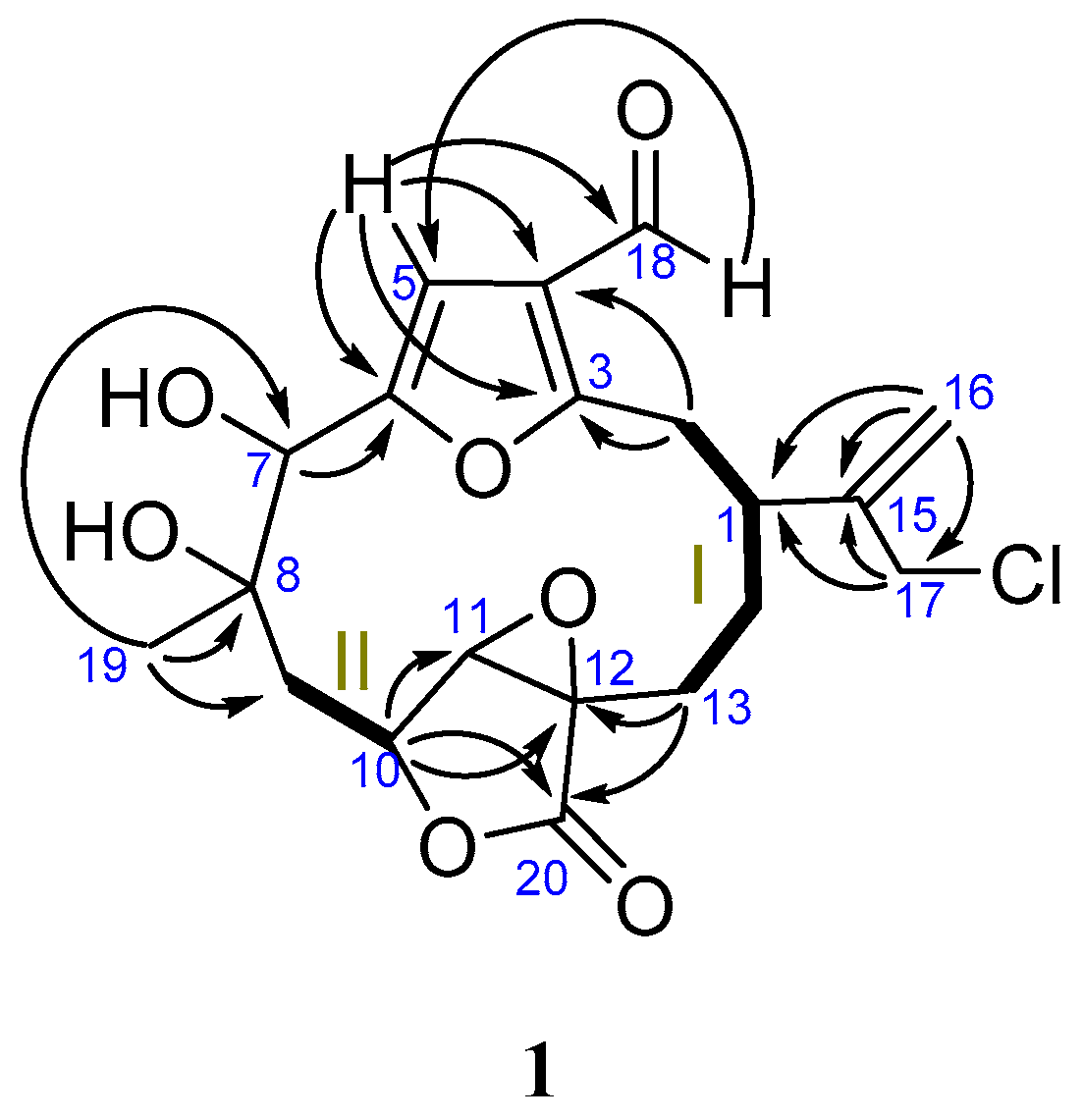

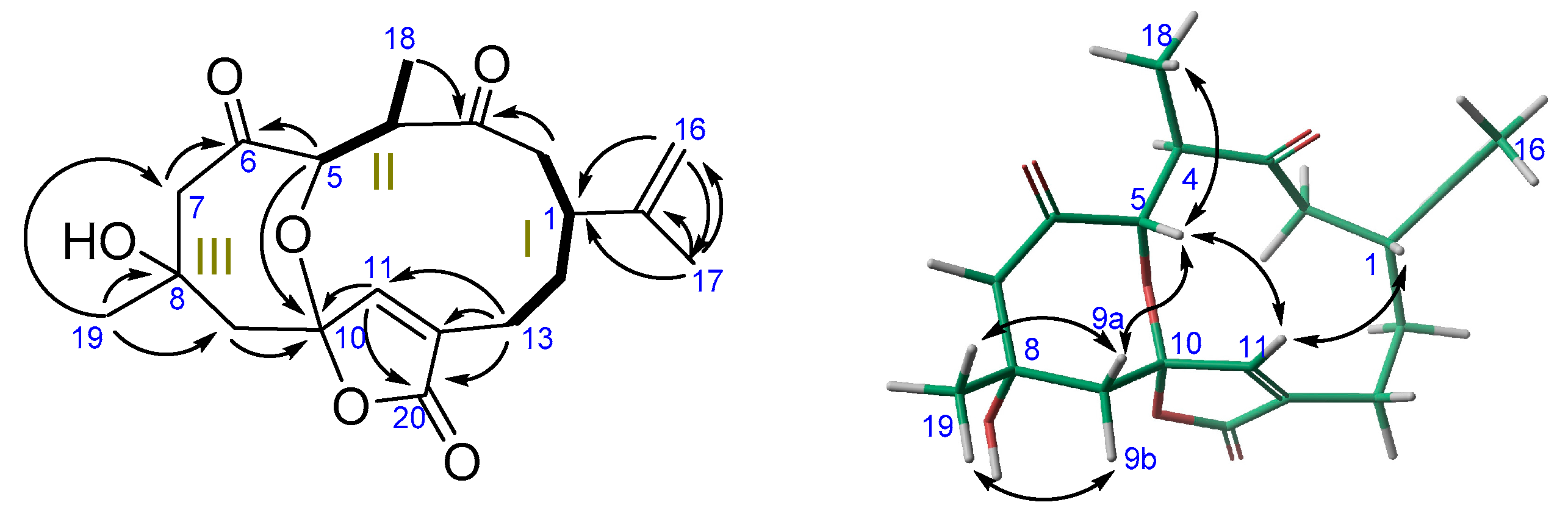
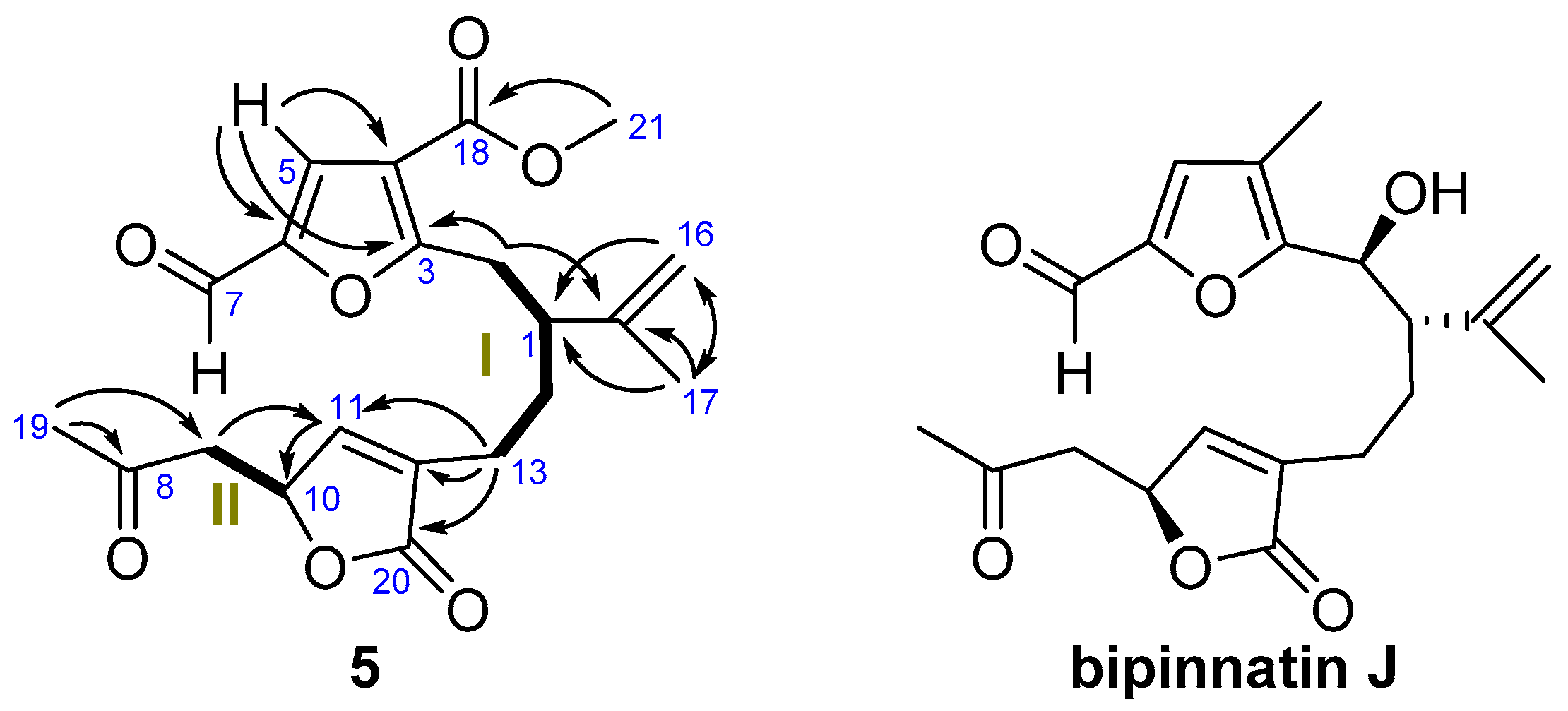
| No. | 1 | 2 | ||
|---|---|---|---|---|
| δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | |
| 1 | 37.6, CH | 3.39, m | 39.7, CH | 2.46, dddd (2.2, 2.5, 9.5, 11.7) |
| 2 | 32.9, CH2 | 3.15, m | 32.7, CH2 | a: 2.97, dd (2.5, 14.8) b: 3.19, dd (11.9, 15.1) |
| 3 | 161.3, C | - | 162.1, C | - |
| 4 | 123.4, C | - | 122.7, C | - |
| 5 | 106.5, CH | 6.70, s | 106.5, CH | 6.80, s |
| 6 | 156.0, C | - | 154.3, C | - |
| 7 | 74.0, CH | 5.29, s | 75.6, CH | 4.59, s |
| 8 | 74.3, C | - | 73.6, C | - |
| 9 | 40.5, CH2 | 1.51, m 1.87, dd (5.2, 15.0) | 43.0, CH | b: 1.89, dd (11.7, 14.8) a: 2.59, dd (4.2, 14.8) |
| 10 | 74.3, CH | 4.85, dd (5.2, 11.3) | 78.4, CH | 4.97, m |
| 11 | 62.9, CH | 3.73, s | 148.6, C | 5.86, s |
| 12 | 60.6, C | - | 136.2, C | - |
| 13 | 22.5, CH2 | a: 1.45, m b: 2.44, dd (11.5, 14.7) | 21.7, CH2 | a: 2.14, m b: 2.35, ddd (2.8, 11.9, 15.1) |
| 14 | 30.4, CH2 | 1.42, m 2.04, m | 30.2, CH2 | 1.60, ddd (2.8, 2.8, 15.1) 2.02, m |
| 15 | 144.2, C | - | 146.9, C | - |
| 16 | 118.0, CH2 | 5.37, s; 5.47, s | 117.0, CH2 | 5.19, s; 5.36, s |
| 17 | 46.6, CH2 | 4.18, br s | 47.2, CH2 | 4.12, br s |
| 18 | 184.7, CH | 9.89, s | 184.3, CH | 9.97, s |
| 19 | 22.4, CH3 | 1.35, s | 19.6, CH3 | 1.41, s |
| 20 | 172.1, C | - | 173.5, C | - |
 |  | ||||
|---|---|---|---|---|---|
| No. | Leptodiol | Lophodiol A | 1 | Sinumaximol B | 2 |
| δH-7 | 5.12, br s | 5.24, s | 5.29, s | 4.52, s | 4.59, s |
| δH-9 | 1.61, dd (8.8, 14.5) 1.68, dd (6.9, 14.5) | 1.55, m 1.76, dd (6.4, 14.8) | 1.55, m 1.76, dd (6.4, 14.8) | 1.85, dd (11.5, 14.5) 2.55, dd (4.0, 14.5) | 1.89, dd (11.7, 14.8) 2.59, dd (4.2, 14.8) |
| δH-19 | 1.38, s | 1.40 s | 1.40, s | 1.38, s | 1.41, s |
| δC-19 | 22.7 | 22.7 | 22.4 | 19.8 | 19.6 |
| δC-7 | 73.5 | 73.4 | 74.0 | 76.1 | 75.6 |
| δC-9 | 41.1 | 40.9 | 40.5 | 43.2 | 43.0 |
| No. | 3 | 4 | 5 | |||
|---|---|---|---|---|---|---|
| δC, Mult. | δH (J in Hz) | δC, Mult. | δH (J in Hz) | δC, Mult. | δH (J in Hz) | |
| 1 | 39.7 CH | 2.64, ddd (4.2, 7.1, 7.1) | 35.4 CH | 2.90, m | 45.7, CH | 2.70, m |
| 2 | 49.7, CH2 | 2.44, m 2.46, m | 49.9, CH2 | 2.53 m 2.53, m | 32.3, CH2 | 3.12, dd (6.6, 14.2) 3.22, dd (8.5, 14.2) |
| 3 | 215.4, C | - | 215.3, C | - | 166.6, C | - |
| 4 | 41.9, CH | 2.89, ddd (6.6, 6.6, 10.6) | 41.7, CH | 2.90, m | 116.7, C | - |
| 5 | 60.8, CH | 3.31, d (10.4) | 60.9, CH | 3.31, d (10.3) | 122.0, CH | 7.44, s |
| 6 | 203.5, C | - | 205.8, C | - | 150.6, C | - |
| 7 | 55.9, CH2 | 2.68, m | 55.9, CH2 | 2.69, m | 177.1, CH | 9.54, s |
| 8 | 74.3, C | - | 74.3, C | - | 204.3, C | - |
| 9 | 45.6, CH2 | b: 1.92, m a: 2.34, d (15.1) | 45.6, CH2 | b: 1.90, dd (2.6, 15.4) a: 2.32, d (15.1) | 46.5, CH2 | 2.63, dd (7.3, 17.7) 2.99, dd (6.7, 17.7) |
| 10 | 90.5, C | - | 90.3, C | - | 76.6, CH | 5.27, m |
| 11 | 155.9, CH | 6.76, s | 156.0, CH | 6.79, s | 147.7, CH | 7.11, m |
| 12 | 131.4, C | - | 131.1, C | - | 134.3, C | - |
| 13 | 24.0, CH2 | a: 2.27, ddd (5.4, 9.3, 14.3) b: 2.50, m | 22.8, CH2 | a: 2.28, m b: 2.49, m | 23.1, CH2 | 2.16, m 2.25, m |
| 14 | 31.7, CH2 | 1.89, m | 31.5, CH2 | 2.00, m | 30.1, CH2 | 1.64, m 1.72, m |
| 15 | 149.4, CH | - | 149.3, C | - | 144.8, C | - |
| 16 | 109.7, CH2 | 4.68, br s; 4.70, br s | 114.7, CH2 | 5.00, s; 5.20, dd (0.9, 0.9) | 113.6, CH2 | 4.64, br s 4.73, dd (1.6, 1.6) |
| 17 | 20.9, CH3 | 1.69, s | 47.6, CH2 | 4.03, m | 18.0, CH3 | 1.68, s |
| 18 | 17.2, CH3 | 1.04, d (6.6) | 17.3, CH3 | 1.04, d (6.6) | 162.9,C | - |
| 19 | 30.1, CH3 | 1.37, s | 30.1, CH3 | 1.37, s | 30.5, CH3 | 2.21, s |
| 20 | 170.6, C | - | 171.6, C | - | 172.9, C | - |
| OMe | - | - | - | - | 51.8, CH3 | 3.86, s |
| Compound (0.1 μM) | Proliferation Ratio a |
|---|---|
| 1 | 2.5 ± 0.65 |
| 2 | 2.0 ± 0.61 |
| Rubifolide (7) | 3.3 ± 0.80 b |
| Scabrolide D (8) | 2.8 ± 0.69 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallardo, A.B.; Díaz-Marrero, A.R.; De la Rosa, J.M.; D’Croz, L.; Perdomo, G.; Cózar-Castellano, I.; Darias, J.; Cueto, M. Chloro-Furanocembranolides from Leptogorgia sp. Improve Pancreatic Beta-Cell Proliferation. Mar. Drugs 2018, 16, 49. https://doi.org/10.3390/md16020049
Gallardo AB, Díaz-Marrero AR, De la Rosa JM, D’Croz L, Perdomo G, Cózar-Castellano I, Darias J, Cueto M. Chloro-Furanocembranolides from Leptogorgia sp. Improve Pancreatic Beta-Cell Proliferation. Marine Drugs. 2018; 16(2):49. https://doi.org/10.3390/md16020049
Chicago/Turabian StyleGallardo, Amalia B., Ana R. Díaz-Marrero, José M. De la Rosa, Luis D’Croz, Germán Perdomo, Irene Cózar-Castellano, José Darias, and Mercedes Cueto. 2018. "Chloro-Furanocembranolides from Leptogorgia sp. Improve Pancreatic Beta-Cell Proliferation" Marine Drugs 16, no. 2: 49. https://doi.org/10.3390/md16020049






