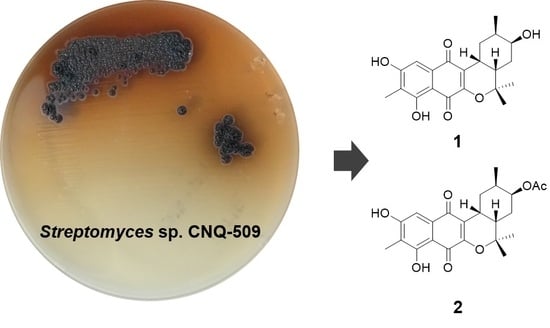
New Naphthoquinone Terpenoids from Marine Actinobacterium, Streptomyces sp. CNQ-509
Abstract
:1. Introduction
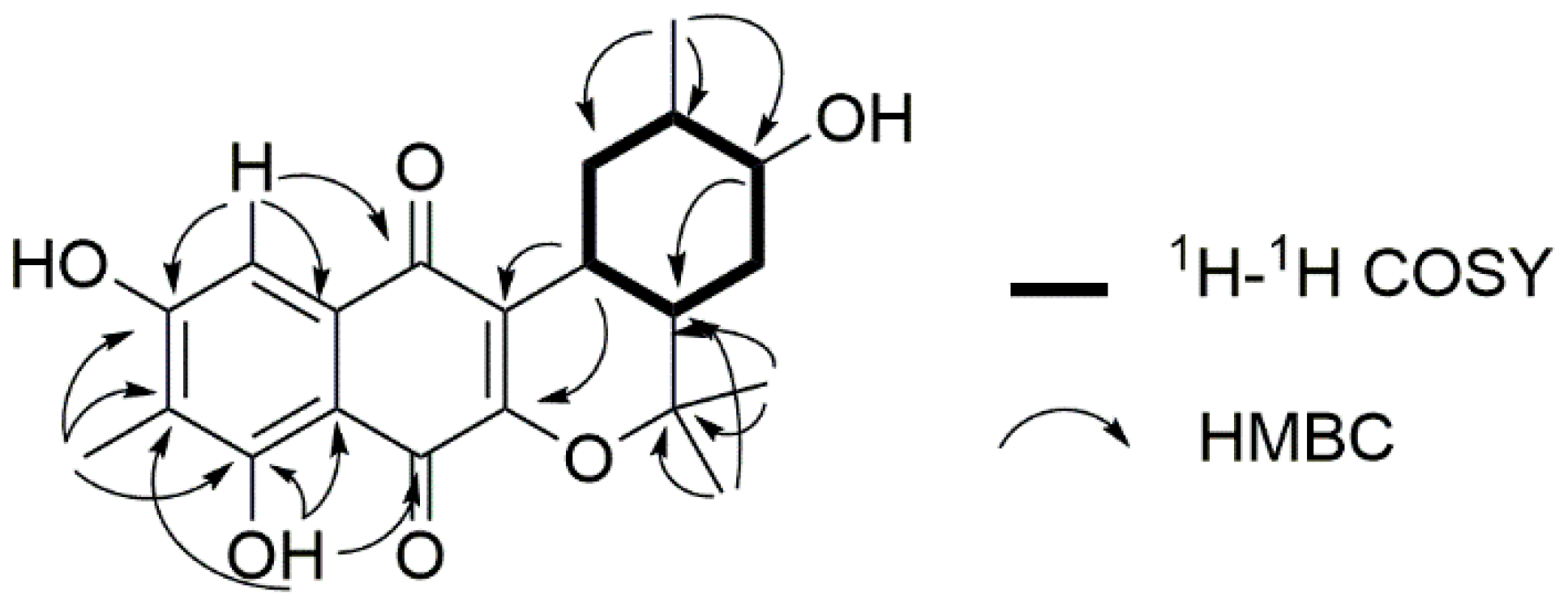
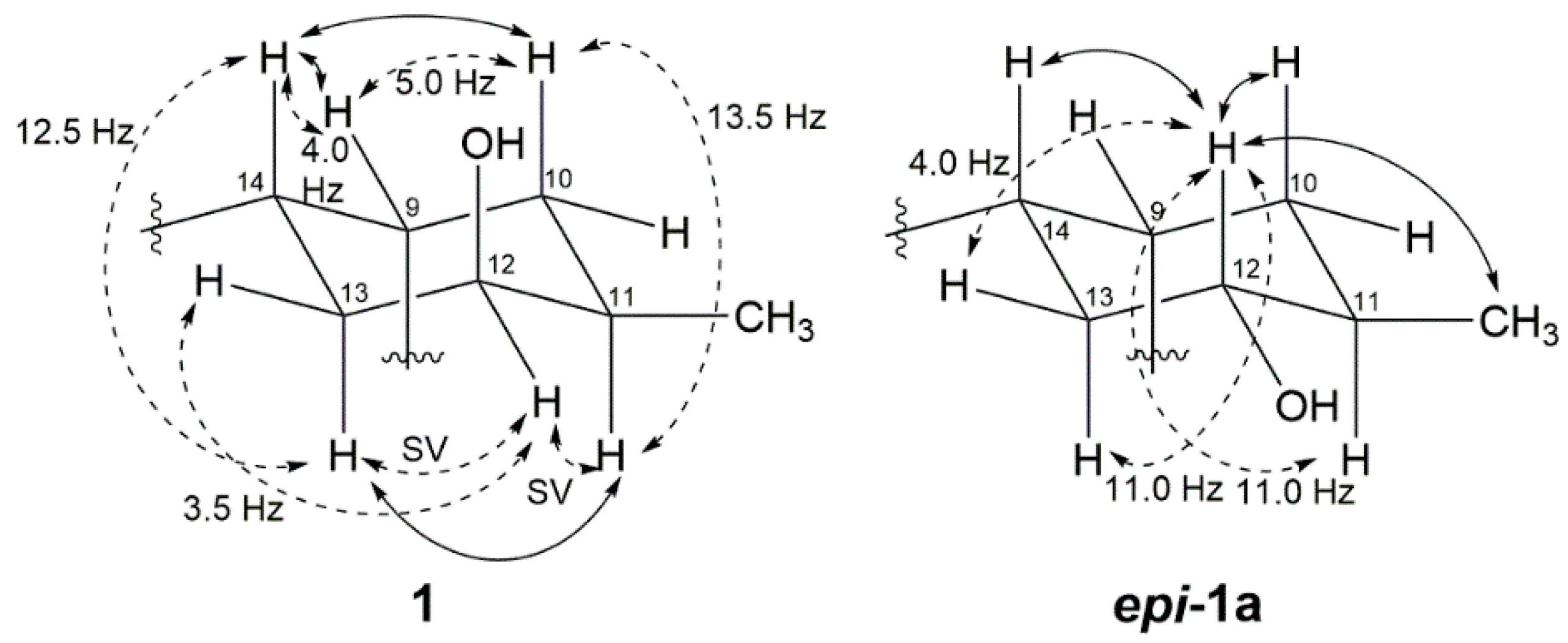
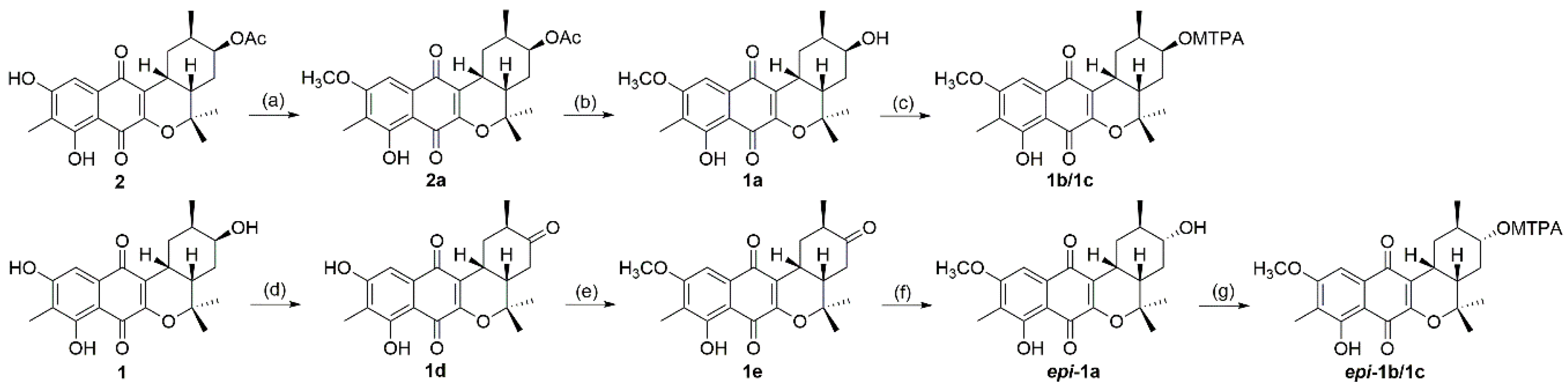
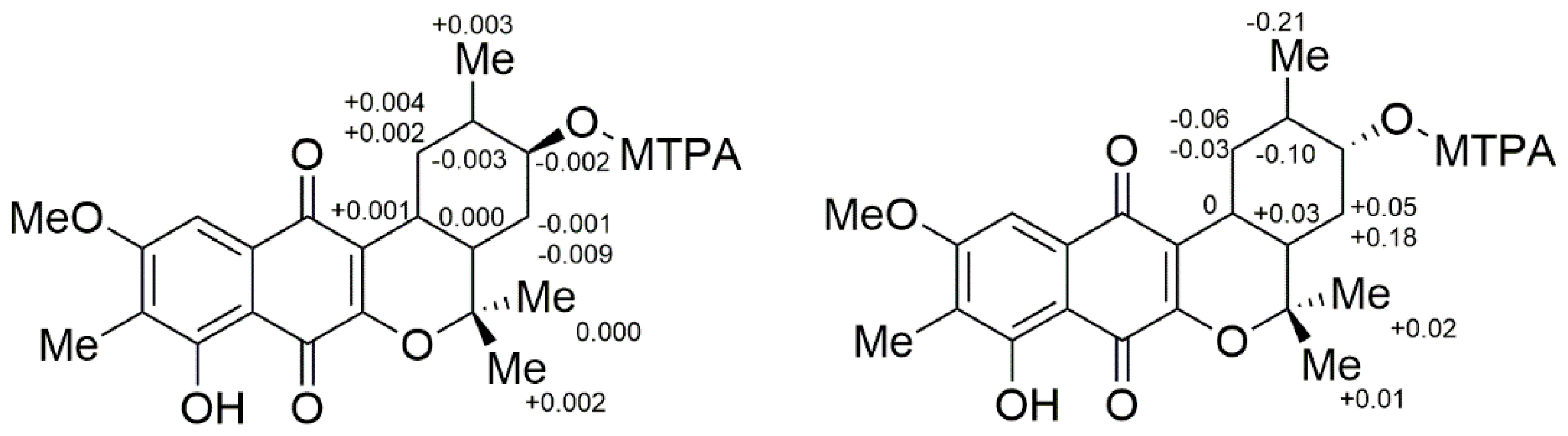
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Cultivation and Extraction of Strain CNQ-509
3.3. Isolation and Purification of Naphterpins D and E (1, 2)
3.4. Methylation of 2 (2a) and Deacetylation of 2a (1a)
3.5. Oxidation of 1 (1d) and Methylation of 1d (1e)
3.6. Reduction of Compound 1e (epi-1a)
3.7. Preparation of MTPA Esters
3.8. On-Line Detection of Radical Scavenging Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mincer, T.J.; Jensen, P.R.; Kauffman, C.A.; Fenical, W. Widespread and persistent populations of a major new marine actinomycete taxon in ocean sediments. Appl. Environ. Microbiol. 2002, 68, 5005–5011. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.S. Discovery of novel metabolites from marine actinomycetes. Curr. Opin. Microbiol. 2006, 9, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Kuzuyama, T. Biosynthetic studies on terpenoids produced by Streptomyces. J. Antibiot. 2017, 70, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, K.A.; Fenical, W.; Jensen, P.R. Hybrid isoprenoid secondary metabolite production in terrestrial and marine actinomycetes. Curr. Opin. Biotechnol. 2010, 21, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.C.; Espindola, A.P.; Park, J.S.; Prieto-Davo, A.; Rose, M.; Jensen, P.R.; Fenical, W. Nitropyrrolins A–E, cytotoxic farnesyl-alpha-nitropyrroles from a marine-derived bacterium within the actinomycete family Streptomycetaceae. J. Nat. Prod. 2010, 73, 2047–2052. [Google Scholar] [CrossRef] [PubMed]
- Pathirana, C.; Jensen, P.R.; Fenical, W. Marinone and debromomarinone—Antibiotic sesquiterpenoid naphthoquinones of a new structure class from a marine bacterium. Tetrahedron Lett. 1992, 33, 7663–7666. [Google Scholar] [CrossRef]
- Izumikawa, M.; Nagai, A.; Hashimoto, J.; Takagi, M.; Shin-ya, K. Isolation of 2 new naphthablin analogs, JBIR-79 and JBIR-80, from Streptomyces sp. Ri24. J. Antibiot. 2010, 63, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Seco, J.M.; Quinoa, E.; Riguera, R. The assignment of absolute configuration by NMR. Chem. Rev. 2004, 104, 17–117. [Google Scholar] [CrossRef]
- DAmbrosio, M.; Guerriero, A.; Debitus, C.; Pietra, F. Leucascandrolide a, a new type of macrolide: The first powerfully bioactive metabolite of calcareous sponges (Leucascandra caveolata, a new genus from the coral sea). Helv. Chim. Acta 1996, 79, 51–60. [Google Scholar] [CrossRef]
- Klotz, L.O.; Hou, X.Q.; Jacob, C. 1,4-naphthoquinones: From oxidative damage to cellular and inter-cellular signaling. Molecules 2014, 19, 14902–14918. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, Y.; Shinkai, Y.; Miura, T.; Cho, A.K. The chemical biology of naphthoquinones and its environmental implications. Annu. Rev. Pharmacol. 2012, 52, 221–247. [Google Scholar] [CrossRef] [PubMed]
- Shin-ya, K.; Imai, S.; Furihata, K.; Hayakawa, Y.; Kato, Y.; Vanduyne, G.D.; Clardy, J.; Seto, H. Isolation and structural elucidation of an antioxidative agent, naphterpin. J. Antibiot. 1990, 43, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Abe, Y.; Shigematsu, N.; Goto, T.; Okuhara, M.; Kohsaka, M. Napyradiomycin A and B1: Nonsteroidal estrogen-receptor antagonists produced by a Streptomyces. J. Antibiot. 1993, 46, 1890–1893. [Google Scholar] [CrossRef] [PubMed]
- Sakoulas, G.; Nam, S.J.; Loesgen, S.; Fenical, W.; Jensen, P.R.; Nizet, V.; Hensler, M. Novel bacterial metabolite merochlorin A demonstrates in vitro activity against multi-drug resistant methicillin-resistant Staphylococcus aureus. PLoS ONE 2012, 7, e29439. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, K.; Masuoka, S.; Ohse, T.; Naganawa, H.; Kondo, S.; Ikeda, Y.; Kinoshita, N.; Hamada, M.; Sawa, T.; Takeuchi, T. Isolation from Streptomyces of a novel naphthoquinone compound, naphthablin, that inhibits Abl oncogene functions. J. Antibiot. 1995, 48, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Kuzuyama, T.; Noel, J.P.; Richard, S.B. Structural basis for the promiscuous biosynthetic prenylation of aromatic natural products. Nature 2005, 435, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.M.; Moffitt, M.C.; Zazopoulos, E.; McAlpine, J.B.; Dorrestein, P.C.; Moore, B.S. Molecular basis for chloronium-mediated meroterpene cyclization—Cloning, sequencing, and heterologous expression of the napyradiomycin biosynthetic gene cluster. J. Biol. Chem. 2007, 282, 16362–16368. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Hayashi, Y.; Kuzuyama, T.; Furihata, K.; Itoh, N.; Seto, H.; Dairi, T. Biosynthesis of a natural polyketide-isoprenoid hybrid compound, furaquinocin A: Identification and heterologous expression of the gene cluster. J. Bacteriol. 2006, 188, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Miao, Y.P.; Wang, B.; Cui, G.L.; Merz, K.M. Catalytic mechanism of aromatic prenylation by NphB. Biochemistry 2012, 51, 2606–2618. [Google Scholar] [CrossRef] [PubMed]
- Miles, Z.D.; Diethelm, S.; Pepper, H.P.; Huang, D.M.; George, J.H.; Moore, B.S. A unifying paradigm for naphthoquinone-based meroterpenoid (bio)synthesis. Nat. Chem. 2017, 9, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Zeyhle, P.; Bauer, J.S.; Steimle, M.; Leipoldt, F.; Rosch, M.; Kalinowski, J.; Gross, H.; Heide, L. A membrane-bound prenyltransferase catalyzes the O-prenylation of 1,6-dihydroxyphenazine in the marine bacterium Streptomyces sp. CNQ-509. Chembiochem 2014, 15, 2385–2392. [Google Scholar] [CrossRef] [PubMed]
- Ruckert, C.; Leipoldt, F.; Zeyhle, P.; Fenical, W.; Jensen, P.R.; Kalinowski, J.; Heide, L.; Kaysser, L. Complete genome sequence of Streptomyces sp. CNQ-509, a prolific producer of meroterpenoid chemistry. J. Biotechnol. 2015, 216, 140–141. [Google Scholar] [CrossRef] [PubMed]
- Leipoldt, F.; Zeyhle, P.; Kulik, A.; Kalinowski, J.; Heide, L.; Kaysser, L. Diversity of abba prenyltransferases in marine Streptomyces sp. CNQ-509: Promiscuous enzymes for the biosynthesis of mixed terpenoid compounds. PLoS ONE 2015, 10, e0143237. [Google Scholar] [CrossRef] [PubMed]
- Cane, D.E.; Ikeda, H. Exploration and mining of the bacterial terpenome. Acc. Chem. Res. 2012, 45, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.R.; Kim, E.O.; Kang, K.; Oidoysambuu, S.; Jung, S.H.; Kim, B.S.; Nho, C.W.; Um, B.H. Antioxidant activity of phenolics in leaves of three red pepper (Capsicum annuum) cultivars. J. Agric. Food Chem. 2014, 62, 850–859. [Google Scholar] [CrossRef] [PubMed]
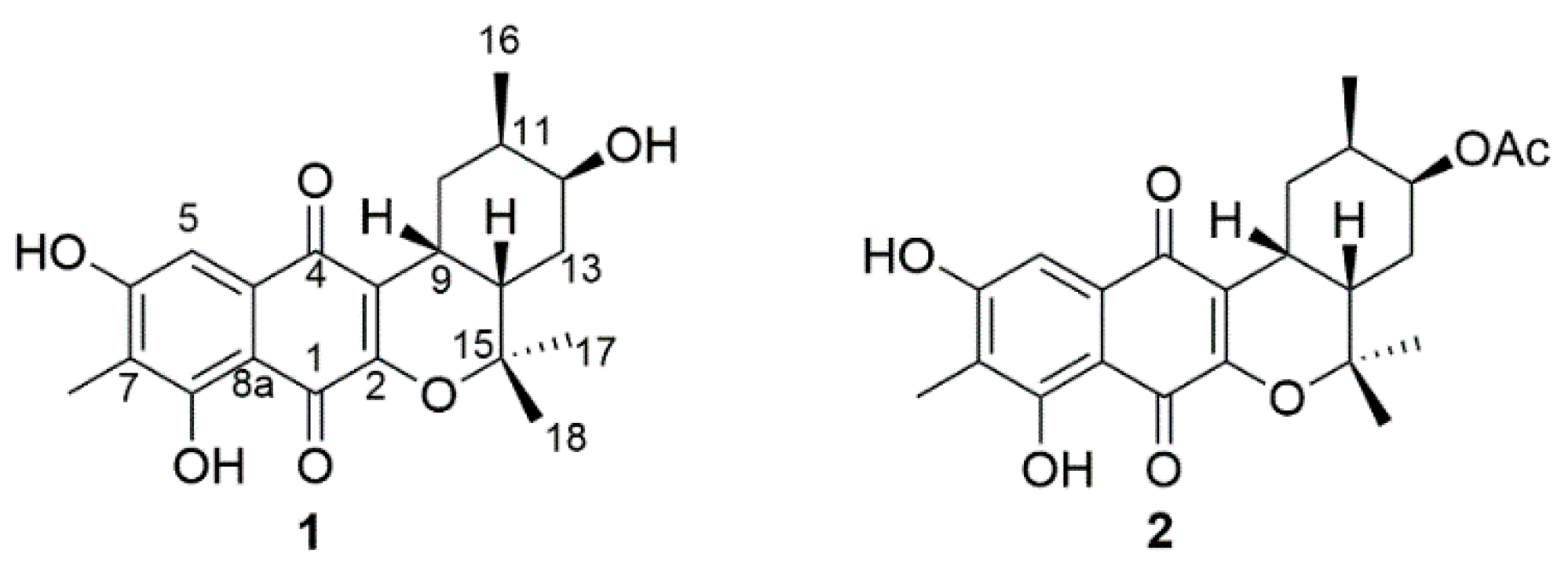
| Position | Compound 1 | Compound 2 | ||
|---|---|---|---|---|
| δH, mult (J in Hz) | δC | δH, mult (J in Hz) | δC | |
| 1 | 182.5 | 182.6 | ||
| 2 | 156.0 | 155.6 | ||
| 3 | 121.2 | 121.1 | ||
| 4 | 184.6 | 183.9 | ||
| 4a | 131.7 | 131.9 | ||
| 5 | 7.20 s | 108.1 | 7.06 s | 107.6 |
| 6 | 161.3 | 160.5 | ||
| 7 | 117.0 | 116.7 | ||
| 8 | 162.5 | 162.3 | ||
| 8a | 108.0 | 108.5 | ||
| 9 | 3.19 m | 30.5 | 3.21 m | 30.3 |
| 10 | 2.67 ddd (14.0, 2.5, 2.5) 1.78 ddd (13.5, 13.5, 5.0) | 27.5 | 2.79 dt (14.0, 2.5) 1.74 ddd (13.5, 13.5, 4.0) | 28.6 |
| 11 | 1.37 m | 31.4 | 1.43 m | 30.4 |
| 12 | 3.84 m | 69.8 | 5.00 ddd(2.0, 2.0, 2.0) | 72.2 |
| 13 | 2.01 dt (12.5, 3.5) 1.35 m | 30.4 | 2.04 m 1.34 m | 28.0 |
| 14 | 2.06 dt (12.5, 4.0) | 35.5 | 1.91 ddd(13.0, 5.0, 4.5) | 36.3 |
| 15 | 81.2 | 80.8 | ||
| 16 | 0.90 d (7.0) | 17.9 | 0.83 d (6.5) | 17.7 |
| 17 | 1.49 s | 25.7 | 1.49 s | 25.8 |
| 18 | 1.33 s | 25.3 | 1.33 s | 25.2 |
| 7-CH3 | 2.18 s | 7.8 | 2.18 s | 7.7 |
| 6-OH | 7.63 s | 6.02 s | ||
| 8-OH | 12.23 s | 12.3 s | ||
| 12-OH | 2.02 s | |||
| OAc | 2.10 s | 21.3 | ||
| -COO- | 170.8 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-S.; Kwon, H.C. New Naphthoquinone Terpenoids from Marine Actinobacterium, Streptomyces sp. CNQ-509. Mar. Drugs 2018, 16, 90. https://doi.org/10.3390/md16030090
Park J-S, Kwon HC. New Naphthoquinone Terpenoids from Marine Actinobacterium, Streptomyces sp. CNQ-509. Marine Drugs. 2018; 16(3):90. https://doi.org/10.3390/md16030090
Chicago/Turabian StylePark, Jin-Soo, and Hak Cheol Kwon. 2018. "New Naphthoquinone Terpenoids from Marine Actinobacterium, Streptomyces sp. CNQ-509" Marine Drugs 16, no. 3: 90. https://doi.org/10.3390/md16030090