Freshwater Microcosms-Based Assessment of Eco-toxicological Effects of a Chemical Effluent from the Pilcam Industry in Cameroon
Abstract
:Introduction
Materials and Methods
Data Analysis
Results and Discussion
Effect of Pilcam Effluent on Fish
Effect of Pilcam Effluent on Microcosm Water Quality
| TAXA | MICROCOSM | |||
|---|---|---|---|---|
| Control | 1.6% | 8% | 16% | |
| (v/v} | (v/v} | (v/v} | ||
| Phytoplancton (Cells/ml ) | ||||
| CyAnophytA | ||||
| Oscillatoria tenuis | 105490 | 7988 | 58242 | 83876 |
| Oscillatoria putrida | 308 | 50 | 142 | 98 |
| CHLOROPHYTA | ||||
| Monographidium contortum | 77 | 202 | / | / |
| Monographidium griffithii | 154 | / | 77 | / |
| Scenedesmus sp. | / | / | / | 308 |
| CHROMOPHYTA | ||||
| Cyclotella meneghiniani | 77 | 10 | 142 | 308 |
| Zooplancton (Cells /100ml) | ||||
| CILIATES | ||||
| Strombidium gyrans | 5500 | 630 | 10588 | 12374 |
| Coleps hirtus | 725 | 256 | 2751 | 595 |
| Disematostoma sp. | 25 | 59 | 2497 | 127 |
| Uronema sp. | 125 | 98 | 393 | 64 |
| Urocentrum turbo | 725 | 20 | 259 | 535 |
| Prorodon ovalis | 150 | 20 | / | / |
| Pleurotricha lanceolata | / | 20 | 46 | / |
| Litonotus sp. | / | / | / | / |
| Paramecium africanum | / | 20 | / | / |
| Euplotes amieti | / | / | 139 | / |
| Dileptus sp. | / | / | 23 | / |
| ROTIFERS | ||||
| Dicranophorus caudatus | 25 | / | 23 | / |
| Brachionus angularis | / | 39 | 92 | / |
| Rotaria rotaria | / | 39 | 23 | / |
| Anureopsis fissa | / | 20 | / | 85 |
| Macrotrachella sp. | / | / | 46 | 21 |
| Asplanchna sp. | / | / | / | 85 |
| COPEPODA | 75 | / | / | 85 |
| OSTRACODA | 100 | / | 46 | 340 |
| BENTHIC MACRO-INVERTÉBRATES (n/100ml) | ||||
| PLANARIA | 25 | / | 116 | 127 |
| NEMATODS | 75 | 25 | 116 | 20 |
| ANNELIDS | ||||
| Tubifex sp1. | 350 | 216 | 162 | 85 |
| Tubifex sp2. | 225 | 59 | 92 | / |
| SNAILS (n/ 40 liters ) | ||||
| Lymnea sp. | 84 | 69 | 77 | 98 |
| Bulinus sp. | / | 4 | 1 | 1 |
| Physa sp. | / | / | 2 | 9 |
| Biomphalaria sp. | 4 | / | 5 | 3 |
| Effluent concentration (%v/v) | No. of fish exposed | No. a of fish dead at | |
|---|---|---|---|
| 48h | 96h | ||
| 20 | 20 | 8 | 18 |
| 18 | 20 | 4 | 16 |
| 16 | 20 | 4 | 12 |
| 17 | 20 | 2 | 8 |
| 14 | 20 | 0 | 6 |
| 0 | 20 | 0 | 0 |
| LC50 (%v/v) estimated by probit analysis. | 20.8 | 16.0 | |
| 95% Confidence limits | 18.9 - 49.8 | 13.4 - 17.2 | |
| LC90 (%v/v) estimated by probit analysis. | 26.6 | 20.7 | |
| 95% Confidence limits | 21.9 - 237.2 | 18.6 - 34.3 | |
| Slope of probit line | 12 | 11 | |
| Effluent concentration (% v/v) | ||||
|---|---|---|---|---|
| Control | 1.6 | 8.0 | 16.0 | |
| Temperature ( oC) | 24.24 ± 1.79 | 24.24 ± 1.79 | 24.23 ± 1.87 | 24.24 ± 1.79 |
| pH (Std Units) | 7.68 ± 0.35 | 7.39 ± 0.48 | 7.25 ± 0.16* | 6.93 ± 0.68* |
| Dissolved oxygen (mg/L) | 4.02 ± 1.26 | 3.86 ± 1.17 | 3.87 ± 1.49* | 3.45± 1.23* |
| Conductivity (µS/Cm) | 221.6 ± 14.2 | 255.4 ± 9.7* | 319.5 ± 27.7* | 392.3 ± 60.2* |
| TSS (g/L) | 87.4 ± 50.6 | 4.7 ± 2.9 | 51.1 ± 32.3* | 58.4± 36.9* |
| T. hardness (mg/L as CaCO3) | 36.4 ± 15.3 | 30.9 ± 5.0 | 49.7 ± 12.2 | 61.5 ± 17.3 |
| T. alk. (mg/L as CaCO3) | 69.5 ± 24.0 | 53.5 ± 10.6 | 70.5 ± 11.3 | 52.5 ± 20.5 |
| Turbidity (FTU) | 89.7 ± 43.9 | 11.5 ± 4.7 | 50.6 ± 29.6* | 58.7 ± 36.0* |
| Colour (PtCo) | 415.5 ± 171.9 | 63.7 ± 22.1 | 271.0 ± 142.3* | 299.6 ± 152.6* |
| Ammonia-N (mg/L) | 0.56 ± 0.26 | 0.08 ± 0.03 | 0.33 ± 0.20* | 0.41 ± 0.25* |
| Chlorophyll a (µg/mL) | 128.5 ± 117.9 | 172.1 ± 176.0 | 202.5 ± 305.2 | 55.8 ± 136.9 |
| Total protein (µg/mL) | 6.6 ± 3.0 | 4.3 ± 1.8 | 4.4 ± 2.6 | 1.8 ± 1.4 |
| Alk phos. (µg PNP/mL/h) | 0.1 ± 0.1 | 0.4 ± 1.0* | 0.4 ± 1.3* | 0.1 ± 0.1* |
Effect of Pilcam Effluent on the Plankton Community Function
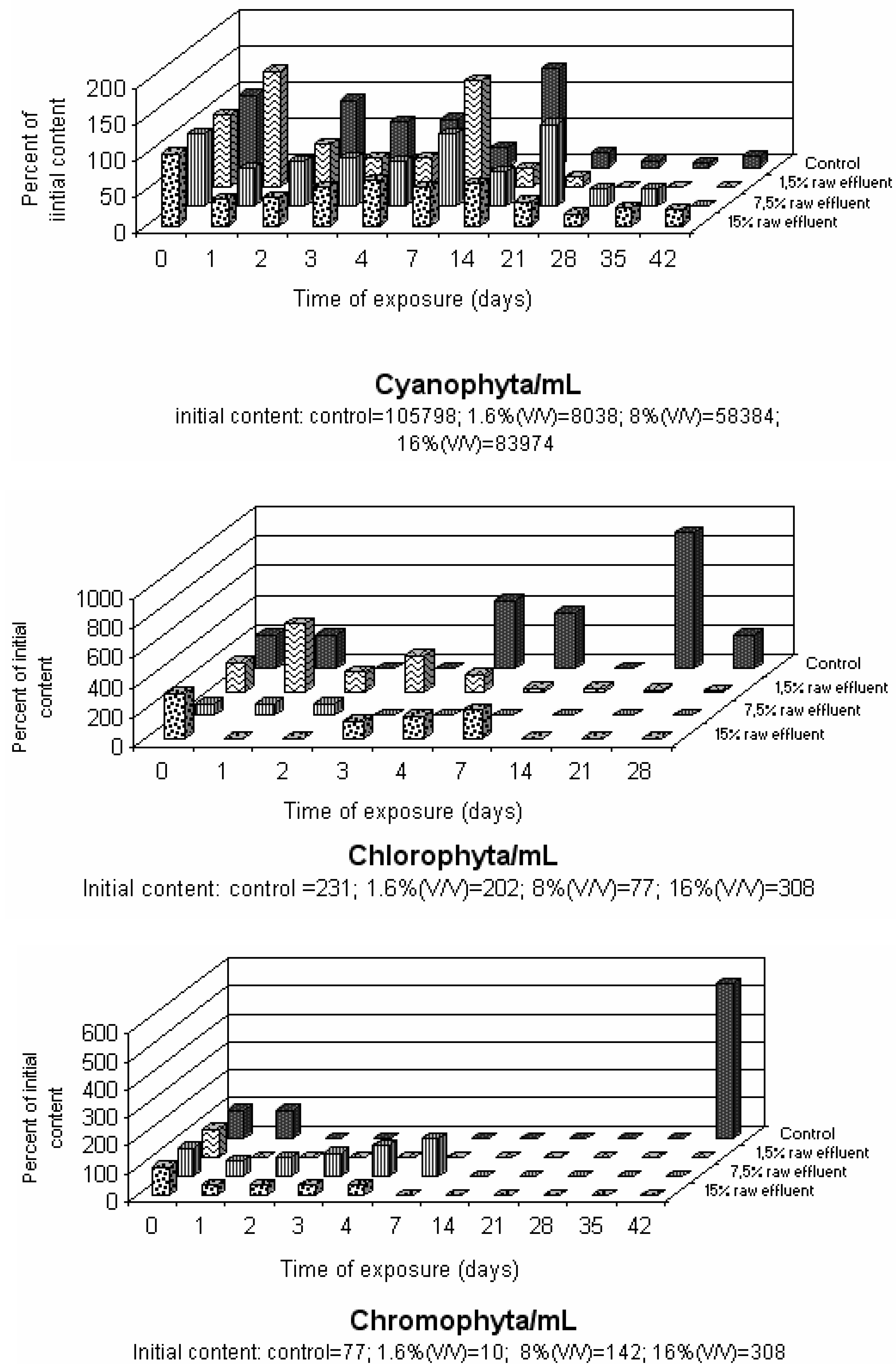
Effect of Pilcam Effluent on the Phytoplankton Structure
Effect of Pilcam Effluent on the Zooplankton Structure





Effect of Pilcam Effluent on the Benthic Macro-Invertebrates
Conclusions
Acknowledgements
References
- Dieter, H.; Manfred, H. Actions de l’homme sur le paysage. In Atlas de l’écologie; Dieter, H., Manfred, H., Eds.; Livre de poche: Paris, 1996; Chapter 7; pp. 198–208. [Google Scholar]
- Connell, D.W. Water pollution. Causes and effects in Australia and New Zealand, 2nd editionUniversity of Queensland Press: St Lucia, Qld, 1981; pp. 169–192. [Google Scholar]
- Alabaster, J.S.; Llyod, R. Water Quality Criteria for freshwater fish. FAO and Butterwotrhs: London, 1982; pp. 319–354. [Google Scholar]
- Lorenz, S.; Francese, M.; Smith, V.J.; Ferrero, E.A. Heavy metals affect the circulating haemocyte in the shrimps, Palaemon elegans. Fish Shellfish Immunol. 2001, 11, 459–472. [Google Scholar] [CrossRef]
- Dejoux, C. Les pollutions liees a l’essor démographique et économique de l’Afrique. In La pollution des eaux continentales africaines : Expérience acquise, situation actuelle et perspectives; Orstom: Paris, 1988; pp. 458–484. [Google Scholar]
- Wattras, C.J.; Back, R.C.; Halvorsen, S.; Hudson, R.J.; Morrison, K.A.; Wente, S.P. Bio-accumulation of mercury in pelagic freshwater good webs. Sci. Total. Environ. Contam. Toxicol 1998, 19, 183–208. [Google Scholar]
- Gray, J.S. Biomagnification in marine systems: the perspective of an ecologist. Mar. Pollut. Bull. 2002, 45, 46–52. [Google Scholar] [CrossRef]
- Spicer, J.I.; Weber, R.E. Respiratory impairment in crustaceans and molluscs due to exposure to heavy metals. Comp. Biochem. Physiol. 1991, 100, 339–42. [Google Scholar]
- Sama, D.A. The Constraints in Managing the Pathways of Persistent Organic Pollutants into the Large Marine Ecosystem of the Gulf of Guinea-The Case of Cameroon. In Intergovernmental Forum on Chemical Safety (WHO/IFCS), EXPERTS MEETING ON Persistent Organic Pollutants, Manila, Philippines, 19 June 1996.
- Cairns, J., Jr.; Pratt, J.R.; Niederlehner, B.R.; McCormick, P.V. A simple cost-effective multispecies toxicity test using organisms with a cosmopolitan distribution. Env. Monit. Assess. 1986, 6, 207–220. [Google Scholar] [CrossRef]
- Niederlehner, B.R.; Pratt, J.R.; Buikema, A.L., Jr.; Cairns, J., Jr. Laboratory tests evaluating the effects of cadmium on freshwater protozoan communities. Env. Toxic. chem. 1985, 4, 155–165. [Google Scholar] [CrossRef]
- Pratt, J.R.; Bowers, N.; Niederlehner, B.R.; Cairns, J., Jr. Effects of atrazine on freshwater microbial communities. Arch. Environ. Contam. Toxicol. 1988, 17, 449–457. [Google Scholar] [CrossRef]
- Elder, J.F.; Collins, J.J. Fresh water molluscs as indication of bio-availability and toxicity of metals in surface-water systems. Rev. Environ. Contam. Toxicol. 1991, 122, 37–79. [Google Scholar]
- Neveu, A. Les invertébrés aquatiques, bio-indicateurs de perturbations. In L’eau dans l’espace rural : vie et milieux aquatiques; Neveu, A., Riou, C., Bonhomme, R., Chassin, P., Papy, F., Eds.; INRA Editions: Paris, 2001; pp. 175–196. [Google Scholar]
- Wells, F.; Metzeling, L.; Newall, P. Macro-invertebrates regionalization for use in the management of aquatic ecosystems in Victoria, Australia. Environ. Monit. Assess. 2002, 74, 271–274. [Google Scholar] [CrossRef]
- Monod, G. Le poisson cible et révélateur de la pollution chimique. In L’eau dans l’espace rural: vie et milieux aquatiques; Neveu, A., Riou, C., Bonhomme, R., Chassin, P., Papy, F., Eds.; INRA Editions: Paris, 2001; pp. 135–156. [Google Scholar]
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Wastewater, 18th EditionAPHA–AWWA, WPCF, Eds.; Washington D.C., 1998; p. 1268. [Google Scholar]
- USEPA. Methods for Acute Toxicity Tests with Fish, Macro-invertebrates and Amphibians. Ecological Research Series; 1972; EPA/660/4-75/009. [Google Scholar]
- Rausch, P. The estimation of microalgae protein content and ist mining to the evaluation of algae biomass, v: comparison of methods for extraction protein. Hydrobiologia 1981, 78, 237–251. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantification of microgram quantities of proteins, utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar]
- Sayler, G.; Puziss, M. Alkaline phosphatase assay for freshwater sediments: application to perturbed sediment system. Environ. Microbiol 1979, 38, 922–927. [Google Scholar]
- Cairns, J., Jr.; Kuhn, D.; Plafkin, J. Protozoan colonization of artificial substrates. In Methods and Measurements of attached microcommunities; Weitzel, R.L., Ed.; American Society for Testing and Materials: Philadelphia, 1979; pp. 39–54. [Google Scholar]
- Kemka, N. Evaluation du degré de trophie du lac municipal de Yaoundé: Etude du milieu, dynamique et structure du peuplement phytoplanctonique. These de doctorat troisieme cycle, Universite de Yaounde I, 2000; p. 186. [Google Scholar]
- Anonymous. Metals. 2004. Available online: http://www.liv.ac.uk/~preston/metals.htm.
- Onwumere, B.G.; Oladimeji, A.A. Accumulation of metal and histopathology in Oreochromis niloticus exposed to treated NNPC Kaduna (Nigeria) petroleum refinery effluents. Ecotoxicol. Environ. Safety 1990, 19, 123–34. [Google Scholar] [CrossRef]
- Alam, K.; Maughan, M.O.; Van Ert, M.D. Physicochemical characterization of industrial effluents and their effect on fish survival. Environ. Sci. Health. 1991, 26, 683–96. [Google Scholar] [CrossRef]
- Bu-olayan, A.H.; Al-Hassan, R.; Thomas, B.V. Trace metal toxicity to phytoplankton of Kuwait coastal waters. Ecotoxicol. 2001, 10, 185–9. [Google Scholar] [CrossRef]
- Stirling, H.P. Chemical and biological methods of water analysis for aquaculturists. Institute of Aquaculture, University of Stirling: UK, 1985; p. 117. [Google Scholar]
- Sudhakar, G.; Jyothi, B.; Venkateswarlu, V. Metal pollution and its impacts on algae in flowing waters in India. Arch. Environ. Contam. Toxicol 1991, 21, 556–66. [Google Scholar] [CrossRef]
- Paerl, H.W. Growth and reproductive strategies of freshwater blue-green algae (cyanobacteria). In Growth and reproductive strategies of freshwater phytoplankton; Sandgren, C.D., Ed.; Cambridge University press: Cambridge, 1988; pp. 261–315. [Google Scholar]
- Eva, W.; Gunnel, A.; Anna-Carin, S. Toxic cyanophyta in three Swedish lakes. Verh. Interntl. Verein. Limnol. 2000, 27, 560–564. [Google Scholar]
- Sant’Anna, Celia L.; de Azevelo, Theresa Maria. Contribution to the knowledge of potentially toxic cyanobacteria from Brazil. nova hedwigia 2000, 71, 359–385. [Google Scholar]
© 2004 MDPI. All rights reserved.
Share and Cite
Monkiedje, A.; Njine, T.; Meyabeme Elono, A.L.; Zebaze, S.H.; Kemka, N.; Tchounwou, P.B.; Djomo, J.E. Freshwater Microcosms-Based Assessment of Eco-toxicological Effects of a Chemical Effluent from the Pilcam Industry in Cameroon. Int. J. Environ. Res. Public Health 2004, 1, 111-123. https://doi.org/10.3390/ijerph2004020111
Monkiedje A, Njine T, Meyabeme Elono AL, Zebaze SH, Kemka N, Tchounwou PB, Djomo JE. Freshwater Microcosms-Based Assessment of Eco-toxicological Effects of a Chemical Effluent from the Pilcam Industry in Cameroon. International Journal of Environmental Research and Public Health. 2004; 1(2):111-123. https://doi.org/10.3390/ijerph2004020111
Chicago/Turabian StyleMonkiedje, A ., T. Njine, A. L. Meyabeme Elono, S. H. Zebaze, N. Kemka, P. B. Tchounwou, and J. E. Djomo. 2004. "Freshwater Microcosms-Based Assessment of Eco-toxicological Effects of a Chemical Effluent from the Pilcam Industry in Cameroon" International Journal of Environmental Research and Public Health 1, no. 2: 111-123. https://doi.org/10.3390/ijerph2004020111




