The Influence of Urban Natural and Built Environments on Physiological and Psychological Measures of Stress— A Pilot Study
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Experimental Design
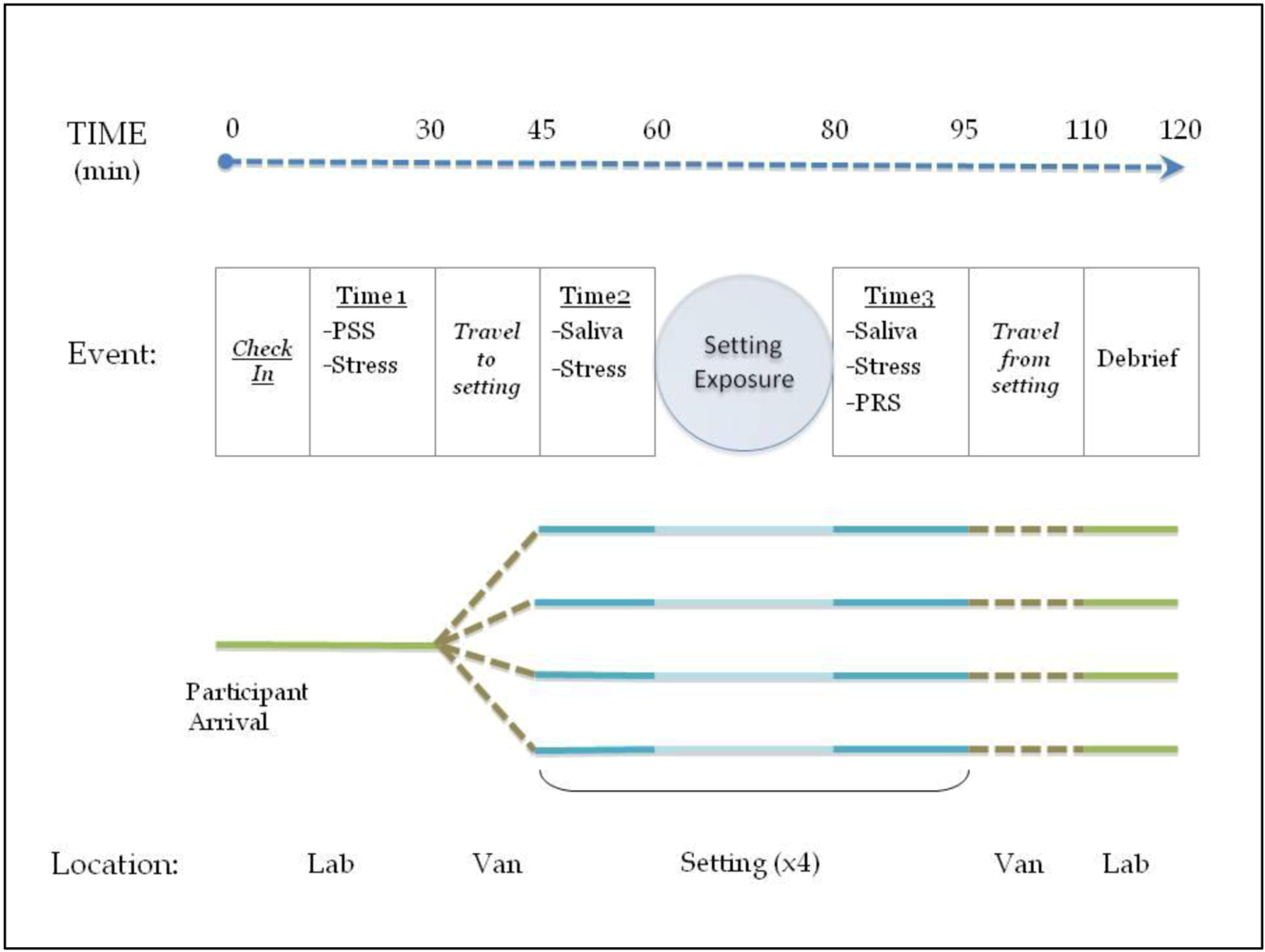
Environmental Settings
- √
- Very Natural: Trees, shrubs, and other natural elements with minimal evidence of human influence. Study setting was a 187-acre forested urban nature reserve
- √
- Mostly Natural: Presence of significant amounts of vegetation and some human influence such as walkways and buildings. Study setting was a 8.76-acre tree-lined urban park
- √
- Mostly Built: Majority of viewable landscape is due to human influence, with some natural elements such as trees. Study setting was a 0.92-acre urban plaza
- √
- Very Built: Entirety of viewable landscape is due to human influence, with minimal presence of natural elements. Study settings was a 3.46-acre outdoor shopping mall

2.3. Measures
2.3.1. Outcome Measures
Saliva (sCort and sAA)
Subjective Stress Scale (Stress)
2.3.2. Exploratory Co-Variates (Pre-Exposure)
Environmental Identity (EID) Scale
Perceived Stress Scale (PSS)
2.3.3. Exploratory Co-Variates (Post-Exposure)
Perceived Restorativeness Scale (PRS)
2.4. Statistical Analysis
3. Results
3.1. Salivary Measures
3.1.1. Cortisol (sCort)
3.1.2. Amylase (sAA)
 = 6.31 vs. 45.05 U/mL, respectively; p = 0.033), suggesting some difference in activation of the SAM pathway between these two built urban settings. Participant reporting during the debriefing revealed strong dislike and feelings of unease in the Very Built setting, which likely contributed to the elevation of sAA.
= 6.31 vs. 45.05 U/mL, respectively; p = 0.033), suggesting some difference in activation of the SAM pathway between these two built urban settings. Participant reporting during the debriefing revealed strong dislike and feelings of unease in the Very Built setting, which likely contributed to the elevation of sAA. 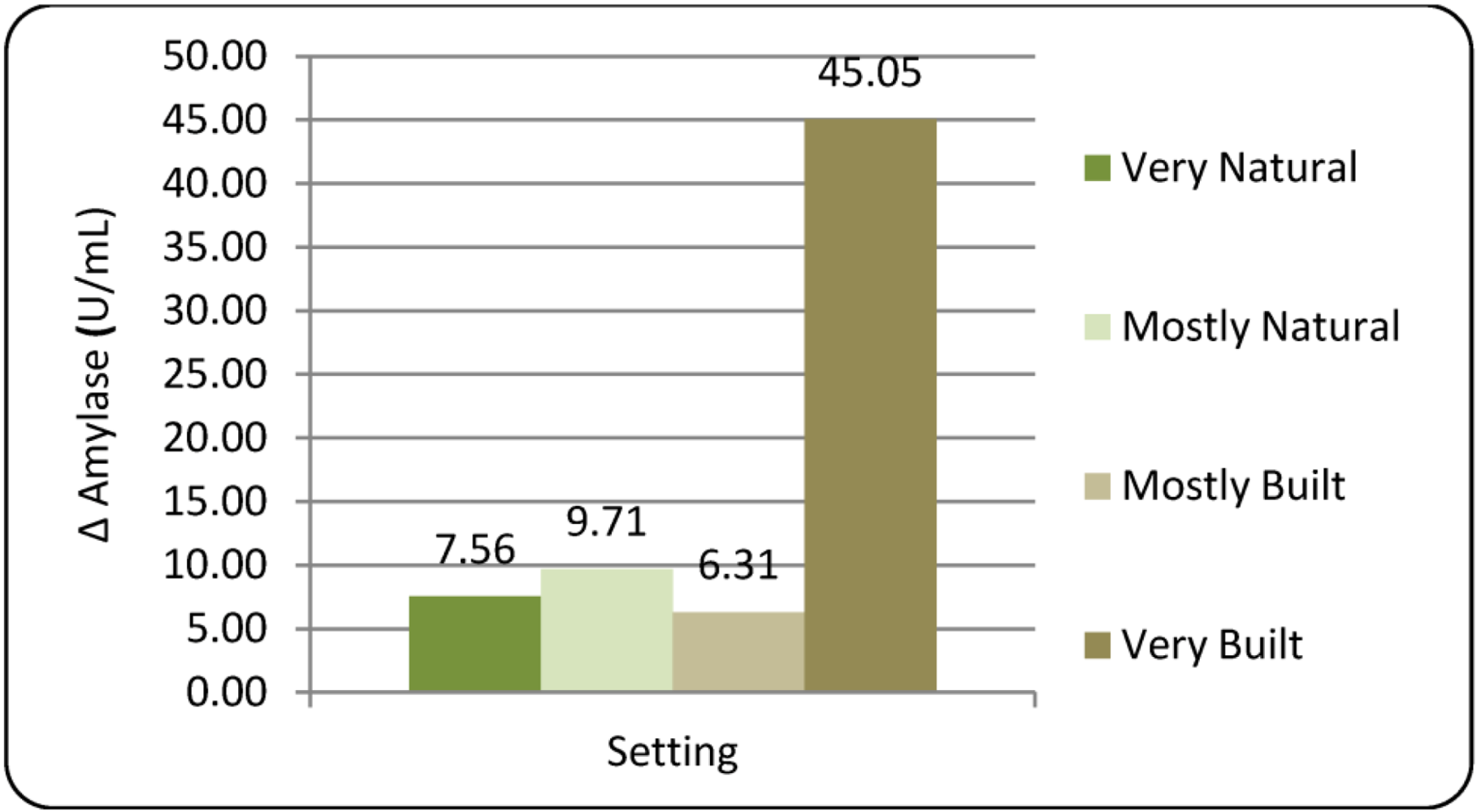
3.2. Subjective Stress Measure
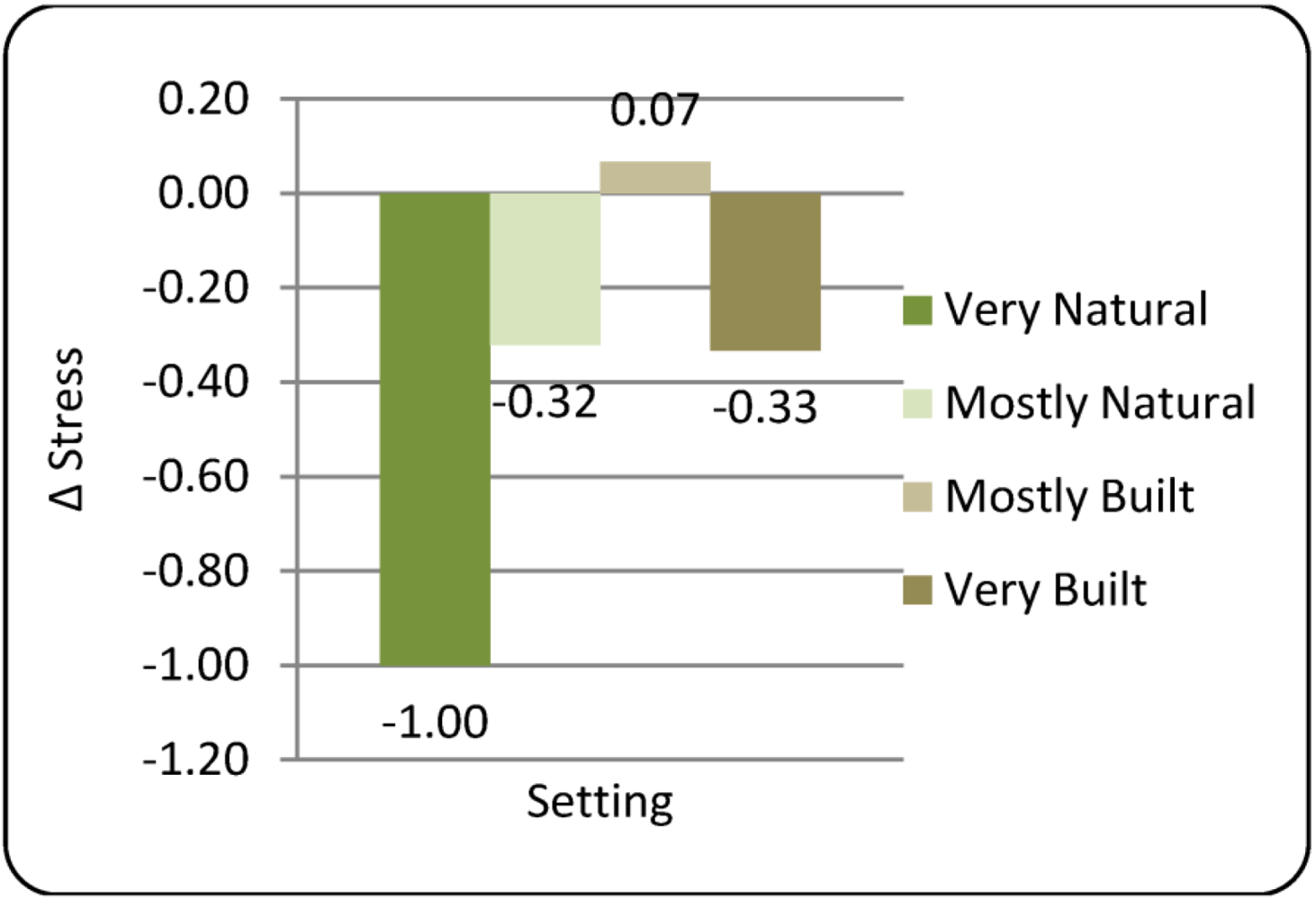
 = −1.00 vs. +0.07, respectively; p = 0.008), suggesting that while these two settings did have different effects on stress status, these may have been obscured by the four-way design of this study. It is interesting to note that comments made during debriefing were mixed for the Mostly Built setting, with many participants enjoying the physical setting but disliking the noise and activity of some non-study personnel. Over-all these comments were more positive than the negative comments about the Very Built setting in which there was no statistical effect on ΔStress. Comments about the Very Natural setting were overwhelmingly positive.
= −1.00 vs. +0.07, respectively; p = 0.008), suggesting that while these two settings did have different effects on stress status, these may have been obscured by the four-way design of this study. It is interesting to note that comments made during debriefing were mixed for the Mostly Built setting, with many participants enjoying the physical setting but disliking the noise and activity of some non-study personnel. Over-all these comments were more positive than the negative comments about the Very Built setting in which there was no statistical effect on ΔStress. Comments about the Very Natural setting were overwhelmingly positive. | Setting | Gender | Mean ΔStress | 95% Confidence Interval | |
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| Very natural | Male | −0.88 | −1.73 | −0.04 |
| Female | −1.26 | −2.17 | −0.34 | |
| Mostly Natural | Male | −0.33 | −1.30 | 0.64 |
| Female | −0.48 | −1.41 | 0.46 | |
| Mostly Built | Male | −0.60 | −1.48 | 0.28 |
| Female | 0.89 | −0.07 | 1.84 | |
| Very Built | Male | −0.02 | −0.87 | 0.83 |
| Female | −0.47 | −1.39 | 0.45 | |
3.3. Co-Variate Measures
3.3.1. Environmental Identity Scale (EID)
3.3.2. Perceived Stress Scale (PSS)
3.3.3. Perceived Restorativeness Scale (PRS)
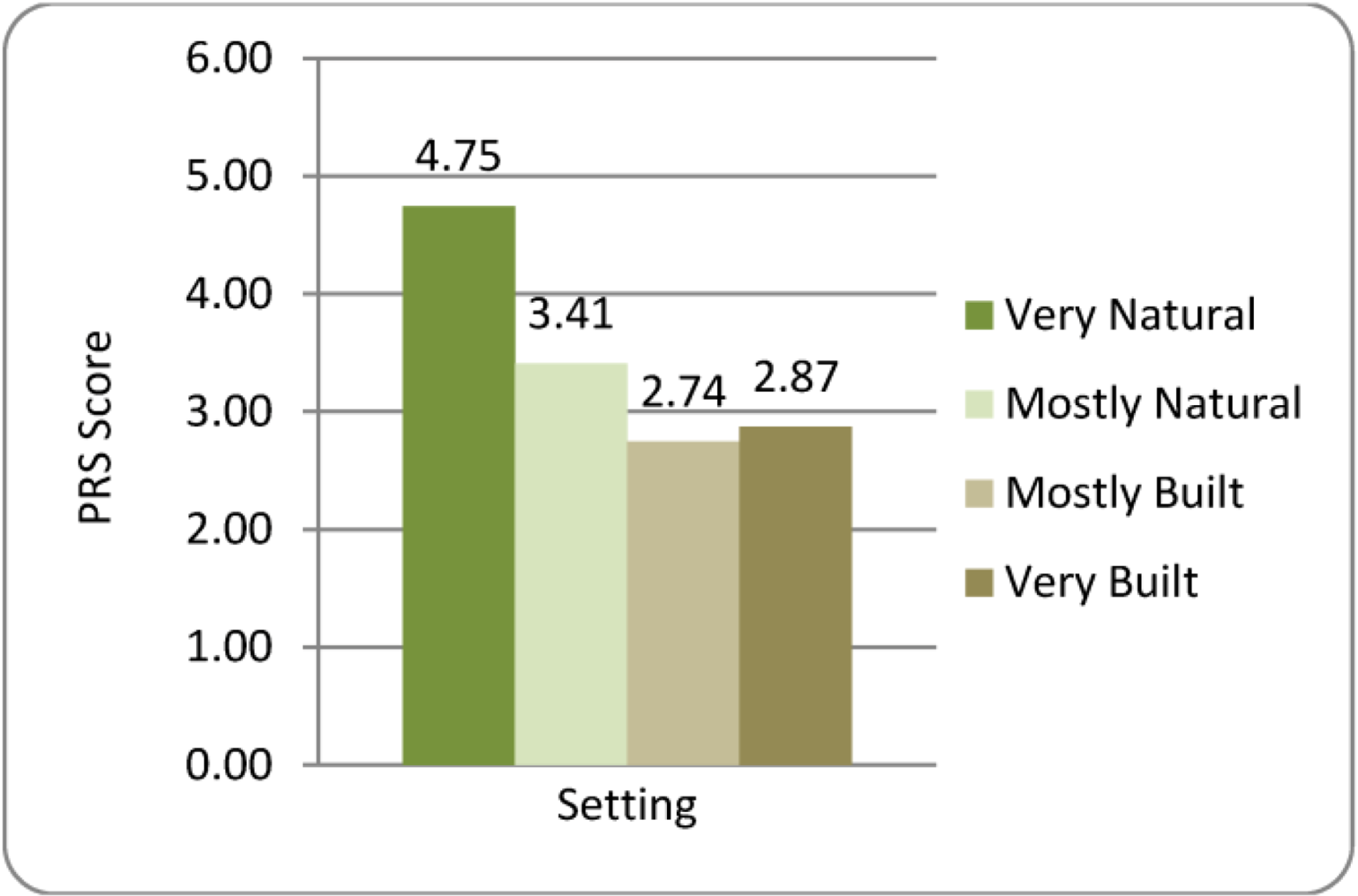
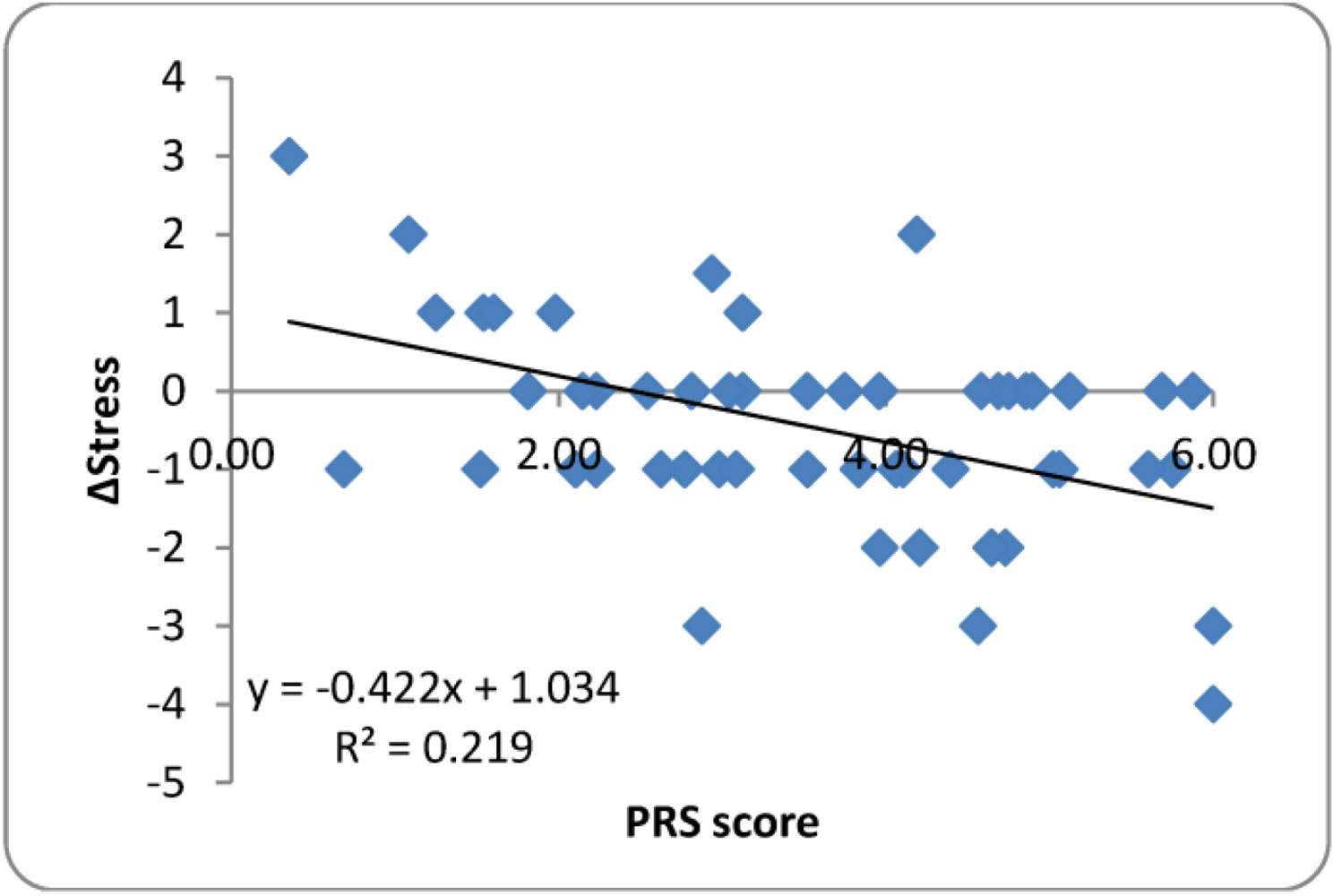
4. Discussion
 = 2.39, SD = 1.71; Scale 0–10) indicates a near-floor effect regarding baseline stress level. The likelihood of detecting measurable changes in stress, particularly after exposure to a mild and passive activity, was minimal. Therefore, the common use of an initiating, pre-exposure stressor may be warranted in future studies so that more robust physiological and psychological stress changes can be measured.
= 2.39, SD = 1.71; Scale 0–10) indicates a near-floor effect regarding baseline stress level. The likelihood of detecting measurable changes in stress, particularly after exposure to a mild and passive activity, was minimal. Therefore, the common use of an initiating, pre-exposure stressor may be warranted in future studies so that more robust physiological and psychological stress changes can be measured. Limitations
5. Conclusions
Acknowledgements
Conflict of Interest
References
- Dooris, M. Expert voices for change: Bridging the silos-towards healthy and sustainable settings for the 21st century. Health Place 2013, 20, 39–50. [Google Scholar] [CrossRef]
- WHO, Ottawa Charter for Health Promotion. In First International Conference on Health Promotion; World Health Organization: Ottawa, Canada, 1986; pp. 1–4.
- Dannenberg, A.L.; Jackson, R.; Frumkin, H.; Schieber, R.A.; Pratt, M.; Kochtitzky, C.; Tilson, H.H. The impact of community design and land-use choices on public health: A scientific research agenda. Am. J. Public Health 2003, 93, 1500–1508. [Google Scholar] [CrossRef]
- Hancock, D.; Duhl, L. WHO Healthy Cities Project: Promoting Health in the Urban Context; World Health Organization: Geneva, Switzerland, 1988; pp. 1–54. [Google Scholar]
- CDC. Designing and Building Healthy Places. Available online: http://www.cdc.gov/healthyplaces/default.htm (accessed on 29 October 2012).
- State of World Population 2007: Unleashing the Potential of Urban Growth. United Nations Population Fund (UNFPA): New York, NY, USA, 2007.
- Peen, J.; Schoevers, R.A.; Beekman, A.T.; Dekker, J. The current status of urban-rural differences in psychiatric disorders. Acta Psychiatr. Scand. 2010, 121, 84–93. [Google Scholar]
- Dhingra, S.S.; Strine, T.W.; Holt, J.B.; Berry, J.T.; Mokdad, A. H. Rural-urban variations in psychological distress: Findings from the behavioral risk factor surveillance system, 2007. Int. J. Public Health 2009, 54, 16–22. [Google Scholar] [CrossRef]
- Verheij, R.A.; Maas, J.; Groenewegen, P.P. Urban-rural health differences and the availability of green space. Eur. Urban Reg. Stud. 2008, 15, 307–316. [Google Scholar]
- Kuo, F.E. Parks and other green environments: Essential components of a healthy human habitat. Australas. Parks Leisure 2010, 14, 1–48. [Google Scholar]
- Maller, C.; Townsend, M.; Pryor, A.; Brown, P.; St Leger, L. Healthy nature healthy people: “Contact with nature” as an upstream health promotion intervention for populations. Health Promot. Int. 2006, 21, 45–54. [Google Scholar] [CrossRef]
- Frumkin, H. Beyond toxicity: Human health and the natural environment. Am. J. Prev. Med. 2001, 20, 234–240. [Google Scholar] [CrossRef]
- Maas, J.; Verheij, R.A.; De Vries, S.; Spreeuwenberg, P.; Schellevis, F.G.; Groenewegen, P.P. Morbidity is related to a green living environment. J. Epidemiol. Community Health 2009, 63, 967–973. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Popham, F. Effect of exposure to natural environment on health inequalities: An observational population study. Lancet 2008, 372, 1655–1660. [Google Scholar] [CrossRef]
- Richardson, E.A.; Mitchell, R.J.; Hartig, T.; de Vries, S.; Astell-Burt, T.; Frumkin, H. Green cities and health: A question of scale? J. Epidemiol. Community Health 2012, 66, 160–165. [Google Scholar] [CrossRef]
- Thompson, C.W.; Roe, J.J.; Aspinall, P.; Mitchell, R.J.; Clow, A.; Miller, D. More green space is linked to less stress in deprived communities: Evidence from salivary cortisol patterns. Landsc. Urban Plan. 2012, 105, 221–229. [Google Scholar] [CrossRef]
- Van den Berg, A.E.; Maas, J.; Verheij, R.A.; Groenewegen, P.P. Green space as a buffer between stressful life events and health. Soc. Sci. Med. 2010, 70, 1203–1210. [Google Scholar] [CrossRef]
- Lottrup, L.; Grahn, P.; Stigsdotter, U.K. Workplace greenery and perceived level of stress: Benefits of access to a green outdoor environment at the workplace. Landsc. Urban Plan. 2013, 110, 5–11. [Google Scholar] [CrossRef]
- Largo-Wight, E.; Chen, W.W.; Dodd, V.; Weiler, R. Healthy workplaces: The effects of nature contact at work on employee stress and health. Public Health Rep. 2011, 126, 124–130. [Google Scholar]
- Lohr, V.I.; Pearson-Mims, C.H.; Goodwin, G.K. Interior plants may improve worker productivity and reduce stress in a windowless environment. J. Environ. Hortic. 1996, 14, 97–100. [Google Scholar]
- Beukeboom, C.J.; Langeveld, D.; Tanja-Dijkstra, K. Stress-reducing effects of real and artificial nature in a hospital waiting room. J. Altern. Complement. Med. 2012, 18, 329–333. [Google Scholar] [CrossRef]
- Alvarsson, J.; Wiens, S.; Nilsson, M.E. Stress recovery during exposure to nature sound and environmental noise. Int. J. Environ. Res. Public Health 2010, 7, 1036–1046. [Google Scholar] [CrossRef]
- Ulrich, R.S. View through a window may help recovery from surgery. Science 1984, 224, 420–421. [Google Scholar]
- Ulrich, R.S.; Simons, R.F.; Losito, B.D.; Fiorito, E.; Miles, M.A.; Zelson, M. Stress recovery during exposure to natural and urban environments. J. Environ. Psychol. 1991, 11, 201–230. [Google Scholar]
- Ulrich, R.S. Natural versus urban scenes: Some psychophysiological effects. Environ. Behav. 1981, 13, 523–556. [Google Scholar] [CrossRef]
- Wilson, E.O. Biophilia; Harvard University Press: Cambridge, MA, USA, 1984. [Google Scholar]
- Grinde, B.; Patil, G.G. Biophilia: Does visual contact with nature impact on health and well-being? Int. J. Environ. Res. Public Health 2009, 6, 2332–2343. [Google Scholar] [CrossRef]
- Stress in America: Our Health at Risk; American Psychological Association: Washington, DC, USA, 2012; pp. 1–78.
- Larzelere, M.M.; Jones, G.N. Stress and health. Prim. Care 2008, 35, 839–856. [Google Scholar] [CrossRef]
- Engert, V.; Vogel, S.; Efanov, S.I.; Duchesne, A.; Corbo, V.; Ali, N.; Pruessner, J.C. Investigation into the cross-correlation of salivary cortisol and alpha-amylase responses to psychological stress. Psychoneuroendocrinology 2011, 9, 1294–1302. [Google Scholar]
- Park, B.-J.; Tsunetsugu, Y.; Kasetani, T.; Kagawa, T.; Miyazaki, Y. The physiological effects of Shinrin-Yoku (taking in the forest atmosphere or forest bathing): Evidence from field experiments in 24 forests across Japan. Environ. Health Prev. Med. 2010, 15, 18–26. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Deguchi, M.; Miyazaki, Y. The effects of exercise in forest and urban environments on sympathetic nervous activity of normal young adults. J. Int. Med. Res. 2006, 34, 152–159. [Google Scholar]
- Van den Berg, A.E.; Custers, M.H.G. Gardening promotes neuroendocrine and affective restoration from stress. J. Health Psychol. 2010, 16, 3–11. [Google Scholar] [CrossRef]
- Hartig, T.; Evans, G.W.; Jamner, L.D.; Davis, D.S.; Gärling, T. Tracking restoration in natural and urban field settings. J. Environ. Psychol. 2003, 23, 109–123. [Google Scholar] [CrossRef]
- Matsuoka, R.H. Student performance and high school landscapes: Examining the links. Landsc. Urban Plan. 2010, 97, 273–282. [Google Scholar] [CrossRef]
- Nater, U.M.; Rohleder, N.; Gaab, J.; Berger, S.; Jud, A.; Kirschbaum, C.; Ehlert, U. Human salivary alpha-amylase reactivity in a psychosocial stress paradigm. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2005, 55, 333–342. [Google Scholar] [CrossRef]
- Clayton, S. Environmental identity: A conceptual and an operational definition. In Identity and the Natural Environment: The Psychological Significance of Nature; Clayton, S., Opotow, S., Eds.; MIT Press: Cambridge, MA, USA, 2003; pp. 45–65. [Google Scholar]
- Hinds, J.; Sparks, P. Engaging with the natural environment: The role of affective connection and identity. J. Environ. Psychol. 2008, 28, 109–120. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Hartig, T.; Korpela, K.M.; Evans, G.W.; Garling, T. A Measure of restorative quality in environments. Scand. Hous. Plan. Res. 1997, 14, 175–194. [Google Scholar] [CrossRef]
- Kaplan, S. The restorative benefits of nature: Toward an integrative framework. J. Environ. Psychol. 1995, 15, 169–182. [Google Scholar] [CrossRef]
- Chang, C.Y.; Hammitt, W.E.; Chen, P.; Machnik, L.; Su, W. Psychophysiological responses and restorative values of natural environments in Taiwan. Landsc. Urban Plan. 2008, 85, 79–84. [Google Scholar] [CrossRef]
- Berto, R. Exposure to restorative environments helps restore attentional capacity. J. Environ. Psychol. 2005, 25, 249–259. [Google Scholar] [CrossRef]
- Richardson, E.A.; Mitchell, R.J. Gender differences in relationships between urban green space and health in the United Kingdom. Soc. Sci. Med. 2010, 71, 568–575. [Google Scholar] [CrossRef]
- Mair, C.; Cutchin, M.; Peek, M.K. Allostatic load in an environmental riskscape: The role of stressors and gender. Health Place 2011, 17, 978–987. [Google Scholar]
- Takai, N.; Yamaguchi, M.; Aragaki, T.; Eto, K.; Uchihashi, K.; Nishikawa, Y. Gender-specific differences in salivary biomarker responses to acute psychological stress. Ann. New York Acad. Sci. 2007, 1098, 510–515. [Google Scholar] [CrossRef]
- Cohen, S.; Janicki-Deverts, D. Who’s stressed? Distributions of psychological stress in the United States in probability samples from 1983, 2006, and 2009. J. Appl. Soc. Psychol. 2012, 42, 1320–1334. [Google Scholar] [CrossRef]
- Van den Berg, A.E.; Hartig, T.; Staats, H. Preference for nature in urbanized societies: Stress, restoration, and the pursuit of sustainability. J. Soc. Issues 2007, 63, 79–96. [Google Scholar] [CrossRef]
- Karmanov, D.; Hamel, R. Assessing the restorative potential of contemporary urban environment(s): Beyond the nature versus urban dicotomy. Landsc. Urban Plan. 2008, 86, 115–125. [Google Scholar] [CrossRef]
- Hauru, K.; Lehvävirta, S.; Korpela, K.M.; Kotze, D.J. Closure of view to the urban matrix has positive effects on perceived restorativeness in urban forests in Helsinki, Finland. Landsc. Urban Plan. 2012, 107, 361–369. [Google Scholar] [CrossRef]
- Tenngart Ivarsson, C.; Hagerhall, C.M. The perceived restorativeness of gardens-assessing the restorativeness of a mixed built and natural scene type. Urban Forest Urban Green. 2008, 7, 107–118. [Google Scholar] [CrossRef]
- Takai, N.; Yamaguchi, M.; Aragaki, T.; Eto, K.; Uchihashi, K.; Nishikawa, Y. Effect of psychological stress on the salivary cortisol and amylase levels in healthy young adults. Arch. Oral Biol. 2004, 49, 963–968. [Google Scholar] [CrossRef]
- Martimportugués-Goyenechea, C.; Gómez-Jacinto, L. Simultaneous multiple stressors in the environment: Physiological stress reactions, performance, and stress evaluationitle. Psychol. Rep. 2005, 97, 867–874. [Google Scholar]
- Stone, A.A.; Schwartz, J.E.; Smyth, J.; Kirschbaum, C.; Cohen, S.; Hellhammer, D.; Grossman, S. Individual differences in the diurnal cycle of salivary free cortisol: A replication of flattened cycles for some individuals. Psychoneuroendocrinology 2001, 26, 295–306. [Google Scholar] [CrossRef]
- Fries, E.; Dettenborn, L.; Kirschbaum, C. The cortisol awakening response (CAR): Facts and future directions. Int. J. Psychophysiol. 2009, 72, 67–73. [Google Scholar] [CrossRef]
- Dijkstra, K.; Pieterse, M.E.; Pruyn, A.T.H. Stress-reducing effects of indoor plants in the built healthcare environment: The mediating role of perceived attractiveness. Prev. Med. 2008, 47, 279–283. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Beil, K.; Hanes, D. The Influence of Urban Natural and Built Environments on Physiological and Psychological Measures of Stress— A Pilot Study. Int. J. Environ. Res. Public Health 2013, 10, 1250-1267. https://doi.org/10.3390/ijerph10041250
Beil K, Hanes D. The Influence of Urban Natural and Built Environments on Physiological and Psychological Measures of Stress— A Pilot Study. International Journal of Environmental Research and Public Health. 2013; 10(4):1250-1267. https://doi.org/10.3390/ijerph10041250
Chicago/Turabian StyleBeil, Kurt, and Douglas Hanes. 2013. "The Influence of Urban Natural and Built Environments on Physiological and Psychological Measures of Stress— A Pilot Study" International Journal of Environmental Research and Public Health 10, no. 4: 1250-1267. https://doi.org/10.3390/ijerph10041250




