Treated Wastewater Effluent as a Source of Microbial Pollution of Surface Water Resources
Abstract
:1. Introduction
2. Sources of Domestic and Industrial Wastewater
3. Impact of Improperly Treated Wastewater Effluent
3.1. Effect on the Environment, Micro- and Macrofauna
3.2. Effect on Human Health
4. Overview of Steps Involved in Wastewater Treatment
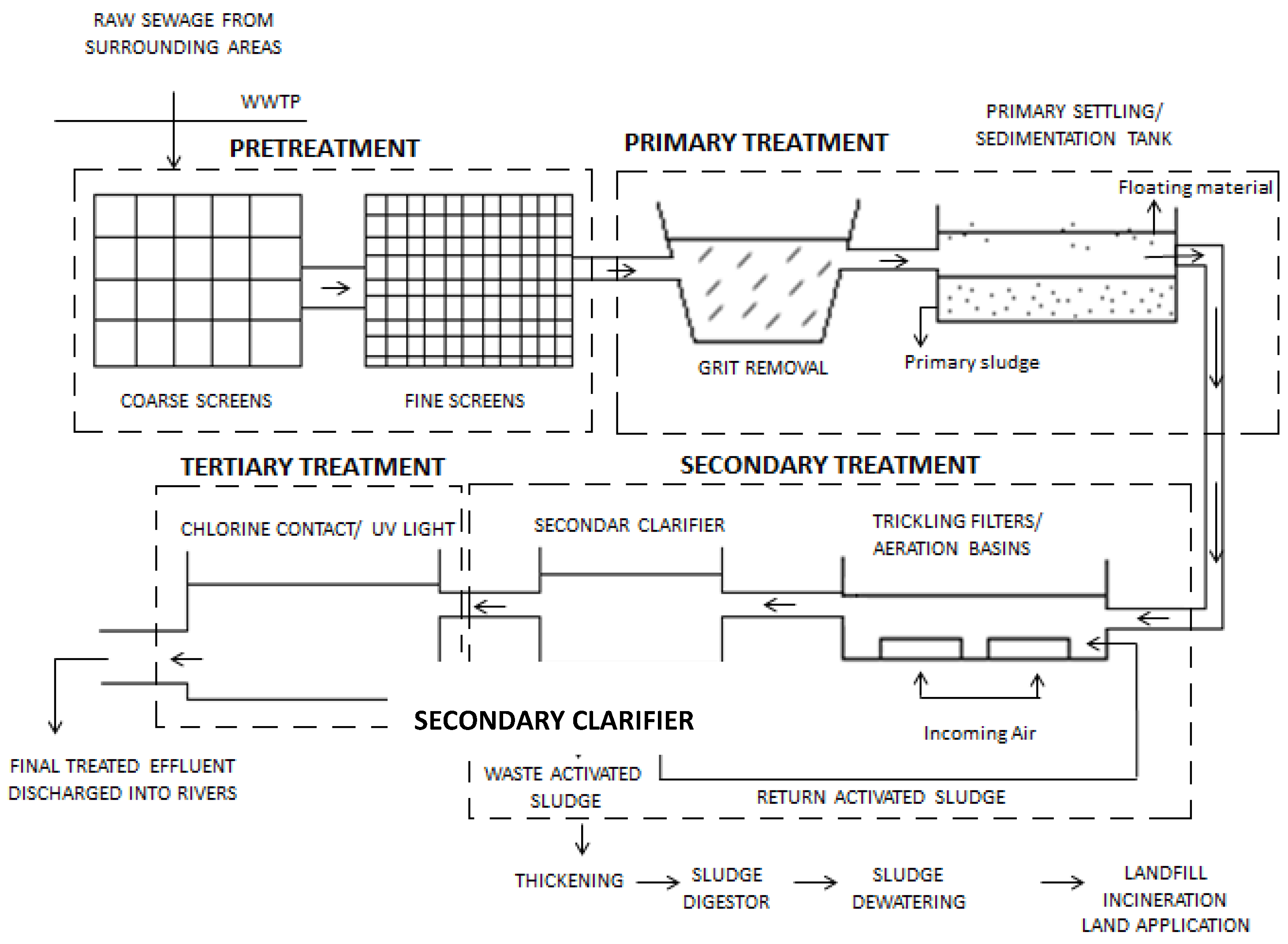
4.1. Pretreatment
4.2. Primary Treatment
| Treatment | Design criteria | Effluent quality | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|
| WASTE STABILISATION PONDS | |||||
| Anaerobic ponds | 2–5 m deep, pH usually below 6.5; less surface area; covered either by gravel, plants, steel, and plastic. Loaded at high rates to prevent inlet of any oxygen | BOD Removal of 60%–85% | Low cost, little excess sludge produced, Small pond volume needed; Low nutrient requirements; Low operating costs; no electricity required; Methane by-product | Requires more land; Long start-up period; Post treatment always required, can produce an unpleasant odour; Requires sludge removal more often; Operates optimally at warmer temperatures (>25 °C) | [10,19] |
| Facultative ponds | Shallow—1–3 m deep; Length to breadth ratio should be a minimum of 2:1; lined with compact clay (minimum thickness 0.3 m) or polyethylene; formation of two layers—aerobic at surface and anaerobic at bottom | BOD removal of 70%–85% | Efficient BOD reduction; Nutrient reduction by aerobic and anaerobic bacterial processes as well as by surrounding plants; Natural aeration of the upper layer via movement of air; Low energy consumption | Significant space requirements; Efficiency is strongly affected by environmental factors; continuous maintenance required | [10] |
| Maturation ponds (polishing ponds) | Shallow—0.9–1 m deep; allows for light penetration; completely aerobic; high pH and high concentration of dissolved oxygen due to algal activity; little biological stratification; size and number depends on required effluent pathogen concentration | Little BOD removal because most has been removed in previous stages | Removes excess nutrients and pathogens such as faecal coliforms | Small BOD removal; additional costs; additional land requirements | [10] |
| SUSPENDED GROWTH SYSTEMS | |||||
| Activated sludge | oxygen supplied for initial sludge decomposition and provide agitation to promote flocculation; 85% sludge removed whilst 15% recirculated | BOD removal of 90%–98% | Production of high quality effluent; reasonable operational and maintenance costs | High capital costs; high energy consumption; regular monitoring required; back washing needed | [20] |
| Batch reactor | Equalization, biological treatment and secondary clarification are performed in a single reactor vessel using a timed control sequence; aeration may be provided by bubble diffusers/floating aerators | BOD removal of 89%–98% | Initial capital cost savings; all processes carried out in a single reactor vessel; timed cycles; requires limited land; equalization of processes | Higher level of sophistication and maintenance required as timing must be controlled; may discharge settled or floating sludge; clogging of aeration devices; requires oversized outfalls as effluent discharge is timed | [21,22] |
| SUSPENDED GROWTH SYSTEMS | |||||
| Aerated lagoons | Should be lined with clay or some natural source, 1.8–6 m depth, 10–30 day retention time, oxygen supplied by additional mechanical means | BOD removal of up to 95% | Low cost, low maintenance and energy requirements, can be well integrated into surrounding landscapes, reliable treatment even at high loads | Nutrient removal is less efficient due to short retention times | [23,24] |
| FIXED FILM SYSTEMS | |||||
| Conventional biofilters (trickling filters) | Bed with supportive media such as stones, plastic, wood; 0.9–2.4 m deep; oxygen supplied via natural flow of air | BOD Removal of between 80%–90% | Low land requirement Moderate level of skill required for operation and maintenance Suitable for small to medium communities | Accumulation of excess biomass will affect performance; high level of clogging thus regular backwashing is required; if suddenly shut down–anaerobic conditions result in reduced effluent quality; odour and snail problems | [25,26] |
| Rotating biological contactors | High contact time; high effluent quality; resistant to shock hydraulic or organic loading; short contact periods; large active surface area; silent; low sludge production; easy transfer of oxygen from air | Continuous power supply required; oxygen may be a limiting substrate | [27] | ||
| Biological aerated filters | Consists of a reactor container, media for supporting biofilm growth, influent distribution and effluent collection system;Optimal conditions—pH 6.5–7.5 with mixing; Media should be chemically stable, high surface area and low weight e.g., sunken clay, floating polystyrene beads | High nutrient removal (80%–100%) | Environmental factors such as pH, temperature will aid microbial growth; high removal efficiencies; can combine ammonia oxidation and solids removal in a single unit | Media may become clogged due to biomass growth and accumulation—may create resistance to air and flow of liquid; regular back washing is required to remove excess biomass and particles | [28,29] |
4.3. Secondary Treatment
4.4. Disinfection and Tertiary Treatment Processes
4.4.1. Disinfection
Chlorination
Ultraviolet Light
Ozonation
4.4.2. Tertiary Treatment
Nutrient Removal
Filtration
Activated Carbon
5. Methods of Effluent Disposal
| Destination | Preliminary | Primary | Secondary | Tertiary |
|---|---|---|---|---|
| Irrigation | ||||
| Produce Eaten Raw | YES | YES | YES | YES |
| Other Produce | YES | YES | YES | NO |
| GroundWater | YES | YES | YES | YES |
| Surface Waters | YES | YES | YES | NO |
| Sea Outfalls | YES | YES | YES | NO |
6. Commonly Detected Microbial Indicators in Treated Wastewater Effluent
| Microorganisms | Diseases | Source | Numbers * | |
|---|---|---|---|---|
| Bacteria | Salmonella enterica subsp. enterica serovar Typhi | Thyphoid fever | Human faeces | 0.2–8,000 |
| Salmonella enterica subsp. enterica serovar Paratyphi | Paratyphoid fever | Human faeces | ||
| Salmonella enterica subsp. enterica serovar Enteritidis and Salmonella enterica subsp. enterica serovar Typhimurium | Salmonellosis/gastroenteritis | Human/animal | ||
| Shigella sp. (Shigella dysenteriae, Shigella flexneri, Shigella boydii, Shigella sonnei) | Dysentery | Human faeces | 0.1–1,000 | |
| Vibrio cholera | Cholera | Human faeces | ||
| Vibrio parahaemolyticus | Gastroenteritis | Human/animal | ||
| E. coli (E. coli O:148; O:157; O:124) | Gastroenteritis | Human faeces | 106–107 | |
| Campylobacter sp. | Gastroenteritis | Human/animal | 104–105 | |
| Clostridium perfringens | Human/animal | 6 × 104–8 × 104 | ||
| Faecal streptococci | Human/animal | 4.7 × 103–4 × 105 | ||
| Enterococci | Human/animal | |||
| Viruses | Poliovirus | Poliomyelitis | Human faeces | 180–500,000 |
| Rotavirus | Diahorrea, vomiting | Human faeces | 400–85,000 | |
| Adenovirus | Gastroenteritis | Human faeces | ||
| Norwalk virus | Diahorrea, vomiting | Human faeces | ||
| Hepatitis A Virus | Hepatitis | Human faeces | ||
| Protozoa | Cryptosporidium parvum | Diahorrea | 0.1–39 | |
| Entamoeba histolytica | Amoeba dysentery | 0.4 | ||
| Giardia lamblia cysts | Diahorrea | 12.5–20,000 | ||
6.1. Total and Faecal Coliforms
6.2. E. coli
6.3. Faecal Streptococci and Enterococci
6.4. Salmonella sp.
6.5. Shigella sp.
6.6. Vibrio sp.
6.7. Coliphages
6.7.1. Somatic Coliphages
6.7.2. Male Specific F-RNA Coliphages
6.7.3. Phages that Infect Bacteroides fragilis
7. Current Guidelines for Treated Effluent
| Parameter | A | B |
|---|---|---|
| Colour/Odour/Taste | None | None |
| pH | 5.5–9.5 | 5.5–7.5 |
| Dissolved Oxygen (mg/L) | 75% saturation | 75% saturation |
| Faecal Coliforms (CFU/100 mL) | 0 | 0 |
| Temperature (°C) | 35 | 25 |
| Chemical Oxygen Demand (mg/L) | 75 | 30 |
| Electrical Conductivity (mS/m) | 75 | |
| Total Suspended Solids (mg/L) | 90 | 10 |
| Sodium Content (mg/L) | 90 | 50 |
| Soap/Oil/Grease (mg/L) | 2.5 | None |
| Residual Chlorine (mg/L) | 0.1 | 0 |
| Free/Saline Ammonia (mg/L) | 1 | 1 |
| Nitrate (mg/L) | None | 1.5 |
| Orthophosphate (mg/L) | 1 | 1 |
8. Conclusions
Acknowledgments
Conflicts of Interest
References
- United Nations Water Global Annual Assessment of Sanitation and Drinking Water (GLAAS). The Challenge of Extending and Sustaining Services; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- UNICEF and World Health Organization. Progress on Drinking Water and Sanitation. Joint Monitoring Programme for Water Supply and Sanitation; UNICEF: New York, NY, USA, 2012. [Google Scholar]
- World Water Assessment Programme (WWAP). The United Nations World Water Development Report 4: Managing Water under Uncertainty and Risk; UNESCO: Paris, France, 2012. [Google Scholar]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2011; ISBN:978-92-4-154815-1. [Google Scholar]
- Department of Water Affairs. Green Drop Handbook, Version 1; Department of Water Affairs: Pretoria, South Africa, 2011. [Google Scholar]
- CIDWT Decentralized Wastewater Glossary. 2009. Available online: http://www.onsiteconsortium.org/glossary.html (accessed on 17 December 2013).
- Mara, D. Domestic Wastewater Treatment in Developing Communities; Earthscan: Oxford, UK, 2004; ISBN: 1-84407-019-0, Chapter 1. [Google Scholar]
- Hamdy, A.; Shatanawi, M.; Smadi, H. Urban wastewater problems, risks and its potential use for irrigation. The use of non-conventional water resources. Bari: CIHEAM/EU-DG Res. 2005, pp. 15–44. Available online: http://om.ciheam.org/om/pdf/a66/00800299.pdf (accessed on 19 December 2013).
- Environmental Protection Administration, Taiwan. Water: Domestic Wastewater and Pollution. Available online: http://www.epa.gov.tw/en/epashow.aspx?list=102&path=135&guid=c4b6ad0f-13e5-4259-be98-8356037dc862&lang=en-us (accessed on 17 December 2013).
- Department of Water Affairs and Forestry. South African Water Quality Guidelines. Volume 1: Domestic Water Use, 2nd ed.; The Department of Water Affairs and Forestry: Pretoria, South Africa, 1996. [Google Scholar]
- Rosenwinkel, K.H.; Austermann-Haun, U.; Meyer, H. Industrial Wastewater Sources and Treatment Strategies. Environmental Biotechnology. Concepts and Applications; Jördening, H.-J., Winter, J., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; ISBN: 3-527-30585-8. [Google Scholar]
- Wakelin, S.A.; Colloff, M.J.; Kookanal, R.S. Effect of wastewater treatment plant effluent on microbial function and community structure in the sediment of a freshwater stream with variable seasonal flow. Appl. Environ. Microbiol. 2008, 74, 2659–2668. [Google Scholar] [CrossRef]
- Coetzee, M.A.S. Water Pollution in South Africa: Its Impact on Wetland Biota; Centre for Water in the Environment; Department of Botany, University of the Witwatersrand: Wits, South Africa, 2003. [Google Scholar]
- Okoh, A.I.; Sibanda, T.; Gusha, S.S. Inadequately treated wastewater as a source of human enteric viruses in the environment. Int. J. Environ. Res. Public Health. 2010, 7, 2620–2637. [Google Scholar] [CrossRef]
- USEPA. Primer for Municipal Wastewater Treatment Systems. Office of Water and Wastewater Management. 2004. Available online: http://www.epa.gov.owm (accessed on 17 December 2013).
- Environmental Protection Agency. Wastewater Treatment Manuals—Primary, Secondary and Tertiary Treatment; Environmental Protection Agency: Ireland, 1997. [Google Scholar]
- United Nations Environmental Programme. Division of Technology, Industry and Economics. Biosolids, Management and Environmentally Sound Approach for Managing Sewage Treatment Plant Sludge. 2012. Available online: http://www.unep.or.jp/ietc/publications/freshwater/fms1/2.asp (accessed on 17 December 2013).
- Sonune, A.; Ghate, R. Developments in wastewater treatment methods. Desalination 2004, 167, 55–63. [Google Scholar] [CrossRef]
- Alexiou, G.E.; Mara, D.D. Anaerobic wastewater stabilization ponds: A low cost contribution to a sustainable wastewater reuse cycle. Appl. Biochem. Biotechnol. 2003, 109, 241–252. [Google Scholar] [CrossRef]
- Norton, A.; Björnberg, S.; Kibirige, D.; Raja, A.B. Wastewater Treatment–Treatment Ponds. Available online: http://www.chemeng.lth.se/vvan01/Arkiv/Report_2012_Treatment_Ponds.pdf (accessed on 17 December 2013).
- USEPA. Wastewater Technology Fact Sheet–Sequencing Batch Reactors; Office of Water: Washington, DC, USA, 1999; EPA-832-F-99-073. [Google Scholar]
- Mahvi, A.H. Sequencing batch reactor: A promising technology in wastewater treatment. Iran. J. Environ. Health Sci. Eng. 2008, 5, 79–90. [Google Scholar]
- USEPA. Wastewater Technology Fact Sheet–Aerated, Partial Mix Lagoons; Office of Water: Washington, DC, USA, 2002; EPA-832-F02-008. [Google Scholar]
- FUCHS Clean Solutions. Aerated Lagoons for the Treatment of Municipal Wastewater. 2011. Available online: http://www.fuchs-germany.com (accessed on 17 December 2013).
- Chaudhary, D.S.; Vigneswaran, S.; Ngo, H.; Shim, W.G.; Moon, H. Biofilter in water and wastewater treatment. Korean J. Chem. Eng. 2003, 20, 1054–1065. [Google Scholar] [CrossRef]
- USEPA. Wastewater Technology Fact Sheet–Trickling Filters; Office of Water: Washington, DC, USA, 2000; EPA-832-F-00-014. [Google Scholar]
- Kadu, P.A.; Rao, Y.R.M. A review of rotating biological contactors system. IJERA 2012, 2, 2149–2153. [Google Scholar]
- Mendoza-Espinosa, L.; Stephenson, T. A review of biological aerated filters (BAFs) for wastewater treatment. Environ. Eng. Sci. 1999, 16, 201–216. [Google Scholar] [CrossRef]
- Asiedu, K. Evaluating Biological Treatment Systems–Moving Bed Biofilm Reactor vs. Biological Aerated Filtration and Sulfide-induced Corrosion in Anaerobic Digester Gas Piping; Department of Civil and Environmental Engineering: Blacksburg, VA, USA, 2001. [Google Scholar]
- SOPAC. Small Scale Wastewater Treatment Plant Project. Report on Project Criteria, Guidelines and Technologies. Available online: http://ict.sopac.org/VirLib/TR0288.pdf (accessed on 17 December 2013).
- USEPA. Wastewater Technology Fact Sheet–Chlorine Disinfection; Office of Water: Washington DC, USA, 1999; EPA-832-F-99-062. [Google Scholar]
- University Curriculum Development for Decentralized Wastewater Management. 2004. Available online: http://www.onsiteconsortium.org/ed_curriculum/University/IV.%20B.%20Septic%20Tanks/uniseptictext.pdf (accessed on 19 December 2013).
- Syrian Lebanese Higher Council. Wastewater Treatment Guidance Manuel–Integrated Coastal Management between Jbeil/Amsheet (Lebanon) and Latakia (Syria); Integrated Coastal Management Project: Syria, Lebanon, 2012. [Google Scholar]
- Basu, S.; Page, J.; Wei, I.W. UV disinfection of treated wastewater effluent: Influence of colour, reactivation and regrowth of coliform bacteria. Environ. Eng.: Appl. Res. Pract. 2007, 4, 1–8. [Google Scholar]
- Lazarova, V.; Savoye, P.; Janex, M.L.; Blatchley, E.R., III; Pommepuy, M. Advanced wastewater disinfection technologies: State of the art and perspectives. Water Sci. Technol. 1999, 40, 203–213. [Google Scholar]
- USEPA. Wastewater Technology Fact Sheet–Ultraviolet Disinfection; Office of Water: Washington, DC, USA, 1999; EPA-832-F-99-064. [Google Scholar]
- Hartman, P.; Cleland, J. Wastewater Treatment Performance and Cost Data to Support an Affordability Analysis for Water Quality Standards; Montana Department of Environmental Quality: Helena, Montana, 2007. [Google Scholar]
- Bolong, N.; Ismail, A.F.; Salim, M.R.; Matsuura, T. A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination 2009, 239, 229–246. [Google Scholar]
- Cecen, F.; Aktas, O. Activated Carbon for Water and Wastewater Treatment: Integration of Adsorption and Biological Treatment, 1st ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011. [Google Scholar]
- USEPA. United States. In Wastewater Technology Fact Sheet–Granular Activated Carbon Adsorption and Regeneration; Office of Water: Washington, DC, USA, 2000; EPA-832-F-00-017. [Google Scholar]
- Minnesota Water Sustainability Framework. Wastewater Treatment Best Practices. Wastewater Treatment Best Practices. 2011. Available online: http://wrc.umn.edu/prod/groups/cfans/@pub/@cfans/@wrc/documents/asset/cfans_asset_292046.pdf (accessed on 19 December 2013).
- Department of Water Affairs and Forestry. Water Quality Management Series. Sub-Series No. MS11. Towards a Strategy for a Waste Discharge Charge System, 1st ed.; Department of Water Affairs and Forestry: Pretoria, South Africa, 2003. [Google Scholar]
- Guidelines for Drinking-water Quality (Electronic Resource): Incorporating 1st and 2nd Addenda, Volume 1, Recommendations, 3rd ed.; World Health Organization: Geneva, Switzerland, 2008.
- Barrell, R.A.E.; Hunter, P.R.; Nichols, G. Microbiological standards for water and their relationship to health risk. Commun. Dis. Public Health. 2000, 3, 8–13. [Google Scholar]
- Ashbolt, N.J. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology 2004, 198, 229–238. [Google Scholar]
- Grabow, W. Bacteriophages: Update on application as models for viruses in water. Water Sa 2001, 27, 251–268. [Google Scholar]
- Leclerc, H.; Mossel, D.A.A.; Edberg, S.C.; Struijk, C.B. Advances in the bacteriology of the coliform group: Their suitability as markers of microbial water safety. Annu. Rev. Microbiol. 2001, 55, 201–234. [Google Scholar]
- NHMRC; NRMMC. Australian Drinking Water Guidelines Paper 6 National Water Quality Management Strategy; National Health and Medical Research Council, National Resource Management Ministerial Council, Commonwealth of Australia: Canberra, Australia, 2011. [Google Scholar]
- Nataro, J.P.; Kaper, J.B. Diarrhaegenic Escherichia coli. Clin. Microbiol. Rev. 1998, 11, 142–201. [Google Scholar]
- Rice, E.W.; Johnson, C.H.; Reasoner, D.J. Detection of Escherichia coli O157:H7 in water from coliform enrichment cultures. Lett. Appl. Microbiol. 1996, 23, 179–182. [Google Scholar]
- Wu, C.J.; Hsueh, P.R.; Ko, W.C. A new health threat in Europe: Shiga toxin—Producing Escherichia coli 0104:H4 infections. J. Microbiol. Immunol. Infect. 2011, 44, 390–393. [Google Scholar]
- Soon, J.M.; Seaman, P.; Baines, R.N. Escherichia coli O104:H4 outbreak from sprouted seeds. Int. J. Hyg. Environ. Heal. 2013, 216, 346–354. [Google Scholar]
- Wheeler, A.L.; Hartel, P.G.; Godfrey, D.G.; Hill, J.L.; Segars, W.I. Potential of Enterococcus faecalis as a human fecal indicator for microbial source tracking. J. Environ. Qual. 2002, 31, 1286–1293. [Google Scholar]
- Layton, B.A.; Walters, S.P.; Lam, L.H.; Boehm, A.B. Enterococcus species distribution among human and animal hosts using multiplex PCR. J. Appl. Microbiol. 2009, 109, 539–547. [Google Scholar]
- Cabral, J.P.S. Water microbiology. Bacterial pathogens and water. Int. J. Environ. Res. Public Health 2010, 7, 3657–3703. [Google Scholar]
- Angulo, F.J.; Tippen, S.; Sharp, D.J.; Payne, B.J.; Collier, C.; Hill, J.E.; Barett, T.J.; Clark, R.M.; Geldrich, E.E.; Donnell, H.D., Jr.; et al. A community waterborne outbreak of salmonellosis and the effectiveness of a boil water order. Amer. J. Public Health 1997, 87, 580–584. [Google Scholar]
- World Health Organization. Guidelines for Drinking-Water Quality, 2nd ed.; World Health Organization: Geneva, Switzerland, 2002; pp. 119–142. [Google Scholar]
- Kott, Y. Viruses and bacteriophages. Sci. Total Environ. 1981, 18, 13–23. [Google Scholar]
- Grabow, W.O.K. Indicator systems for assessment of the virological safety of treated drinking water. Water Sci. Technol. 1986, 18, 159–165. [Google Scholar]
- Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2003; Volume 1.
- Government Gazette. No. 9225. Requirements for the Purification of Waste Water or Effluent. General and Special Standards; Minister of Environment Affairs and Fisheries: South Africa, 1984.
- Tchobanoglous, G.; Burton, F.L.; Stensel, H.D. Wastewater Engineering Treatment and Reuse, 4th ed.; Metcalf & Eddy, Inc.: Wakefield, MA, USA, 2002. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Naidoo, S.; Olaniran, A.O. Treated Wastewater Effluent as a Source of Microbial Pollution of Surface Water Resources. Int. J. Environ. Res. Public Health 2014, 11, 249-270. https://doi.org/10.3390/ijerph110100249
Naidoo S, Olaniran AO. Treated Wastewater Effluent as a Source of Microbial Pollution of Surface Water Resources. International Journal of Environmental Research and Public Health. 2014; 11(1):249-270. https://doi.org/10.3390/ijerph110100249
Chicago/Turabian StyleNaidoo, Shalinee, and Ademola O. Olaniran. 2014. "Treated Wastewater Effluent as a Source of Microbial Pollution of Surface Water Resources" International Journal of Environmental Research and Public Health 11, no. 1: 249-270. https://doi.org/10.3390/ijerph110100249




