Feasibility of Silver Doped TiO2/Glass Fiber Photocatalyst under Visible Irradiation as an Indoor Air Germicide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.3. Disinfection Experiment
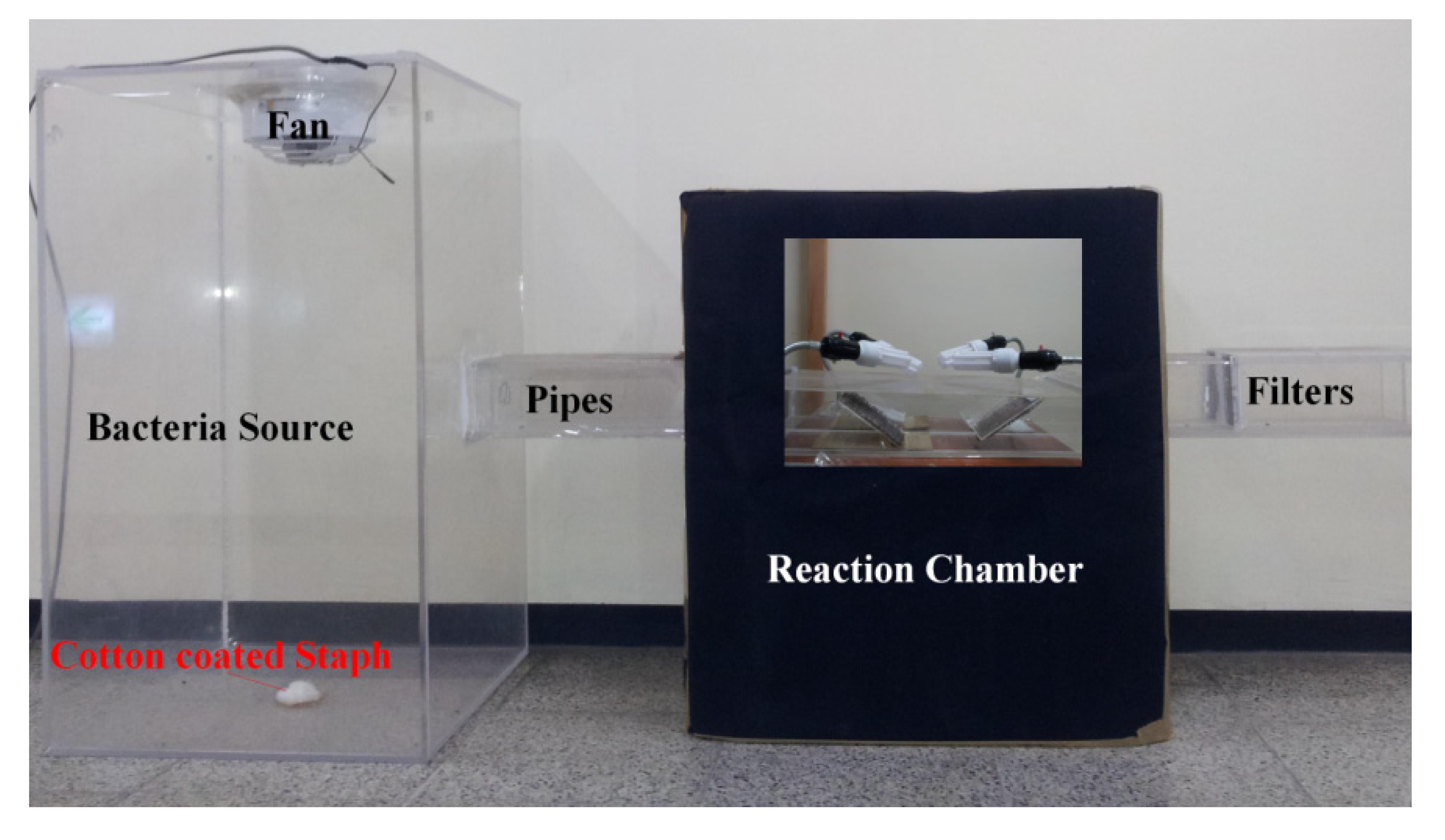
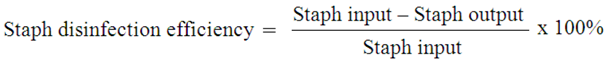
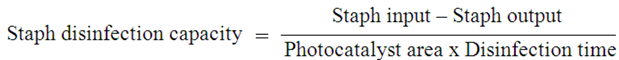
3. Results and Discussion
3.1. Photocatalyst Characterization
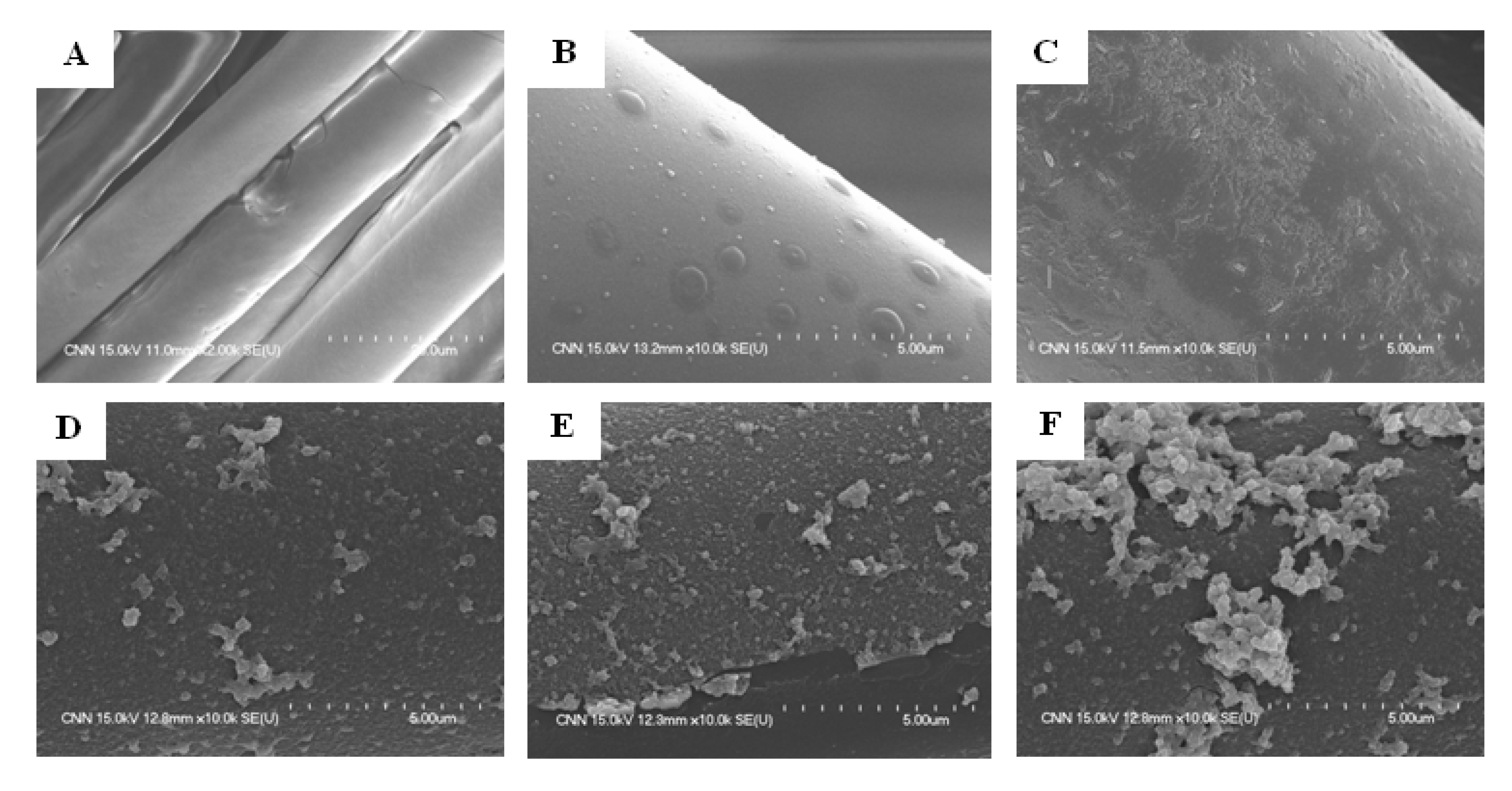
3.1.1. SEM Observation
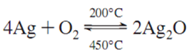
3.1.2. XRD Analysis
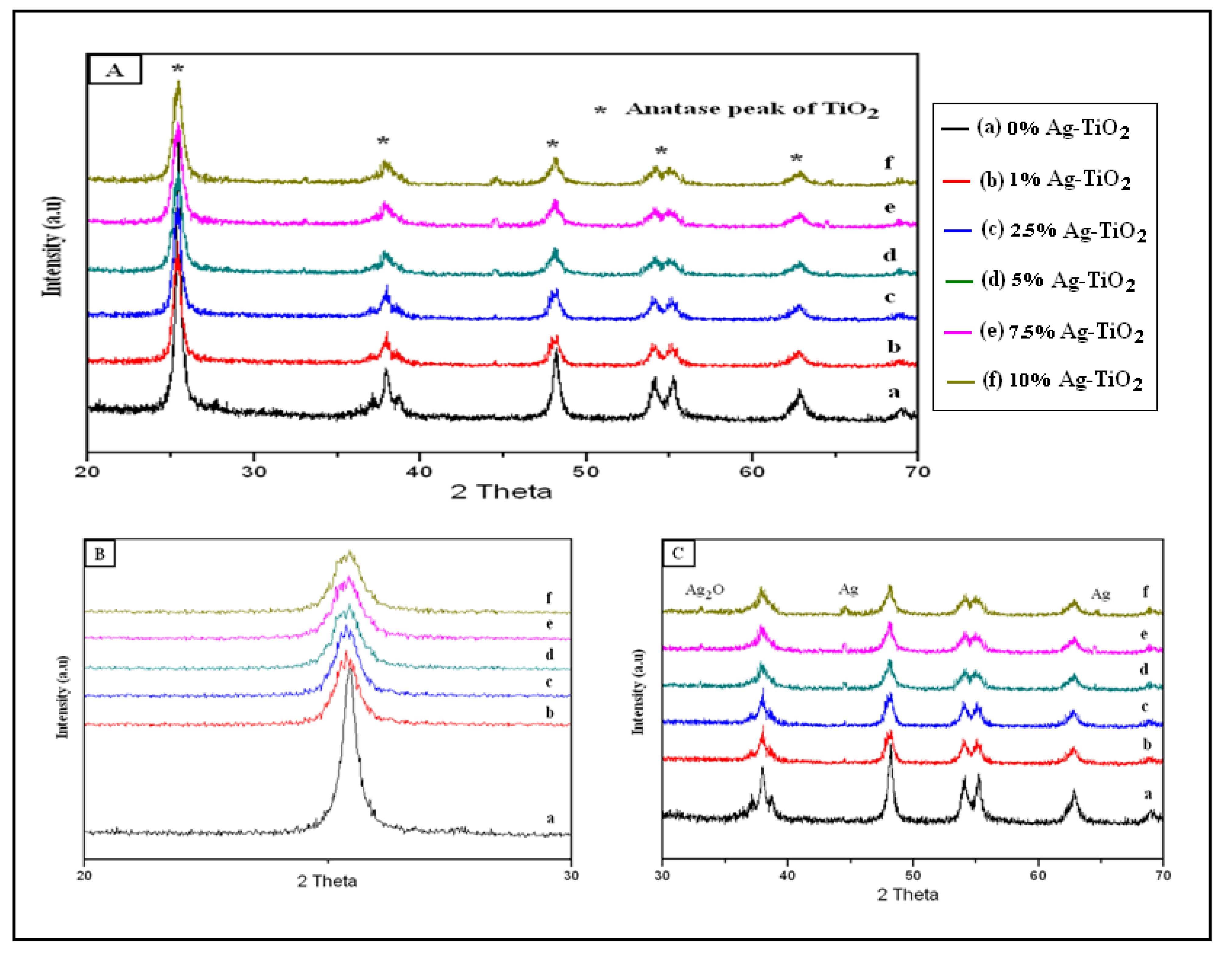
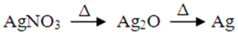
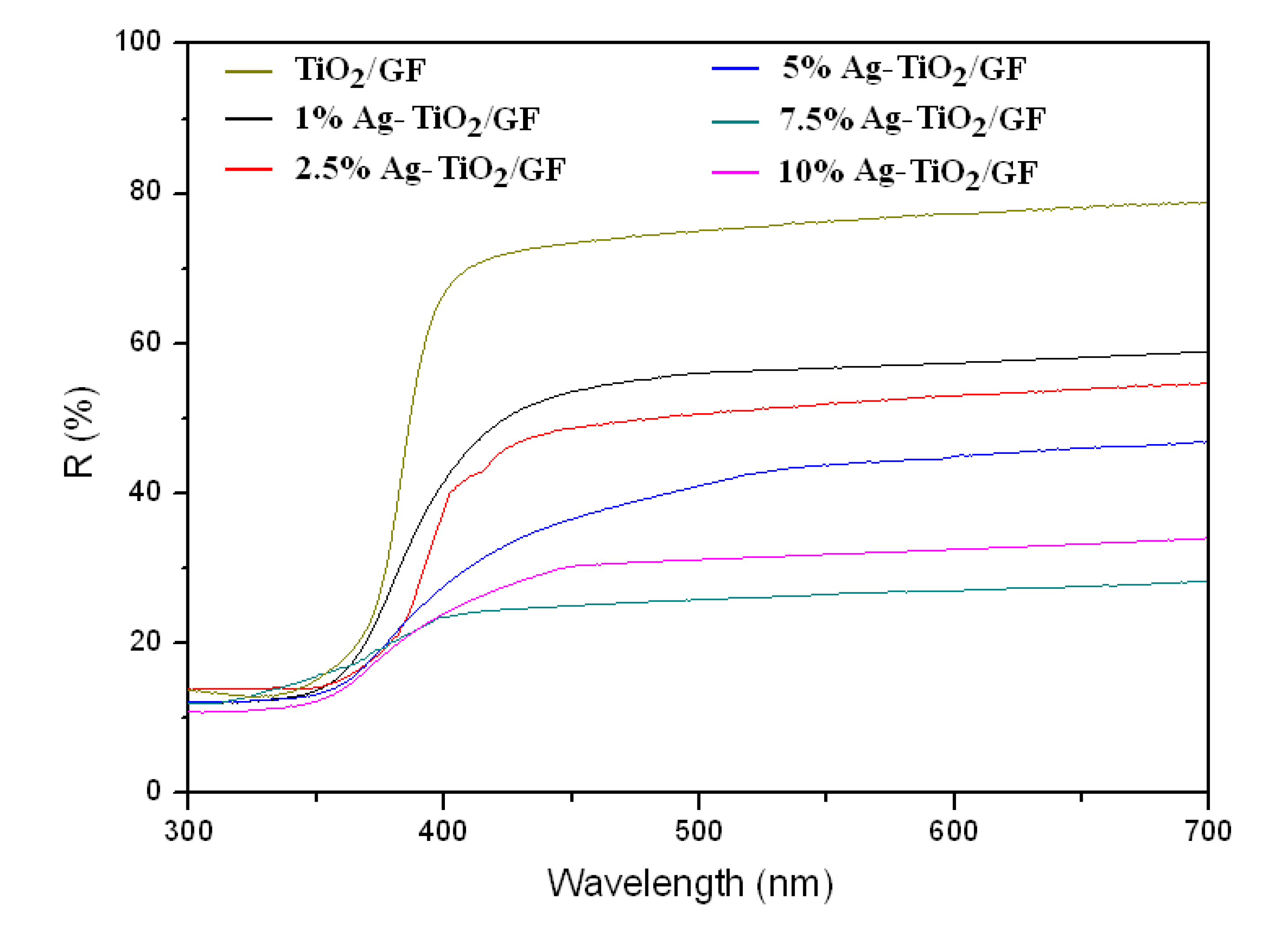
3.1.3. UV-Visible Spectra
3.1.4. XPS Studies
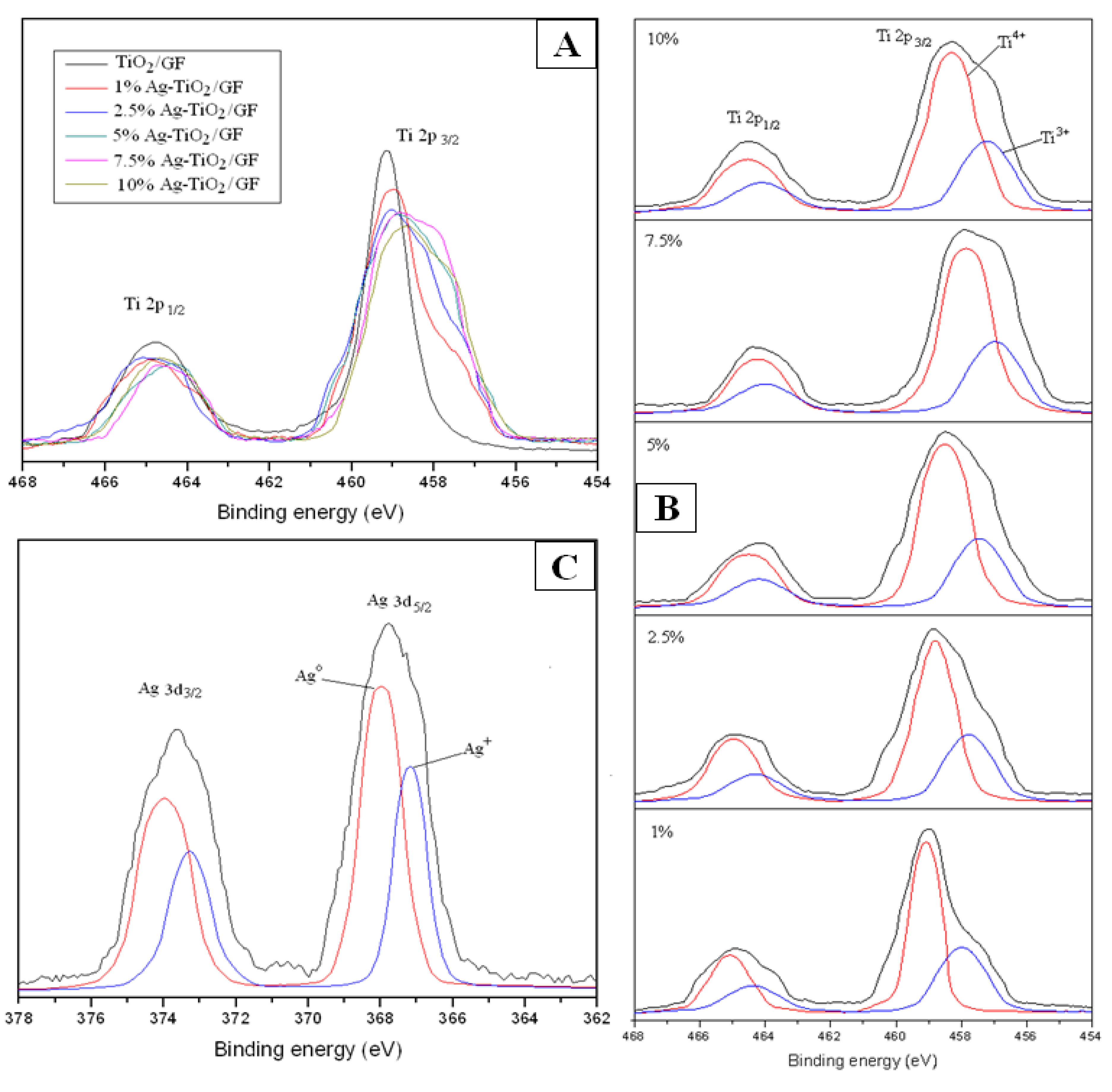
3.2. Disinfection Results
3.2.1 Optimum Ag Doping
| Ag Doping in TiO2 (%) | 0 | 1 | 2.5 | 5 | 7.5 | 10 |
|---|---|---|---|---|---|---|
| Staph input (CFU) | 2.14E + 07 | 2.11E + 07 | 2.08E + 07 | 2.03E + 07 | 2.16E + 07 | 2.06E + 07 |
| Staph output (CFU) | 2.08E + 07 | 9.75E + 06 | 7.90E + 06 | 6.50E + 06 | 5.35E + 06 | 5.95E + 06 |
| Disinfection efficiency (%) | 2.80 | 53.79 | 62.02 | 67.98 | 75.23 | 71.12 |
3.2.2. Humidity Effects
| Relative Humidity (%) | 40 ± 5 | 60 ± 5 | 80 ± 5 |
|---|---|---|---|
| Staph input (CFU) | 2.05E + 07 | 2.16E + 07 | 2.10E + 07 |
| Staph output (CFU) | 9.65E + 06 | 5.35E + 06 | 7.15E + 06 |
| Disinfection efficiency (%) | 52.93 | 75.23 | 65.95 |
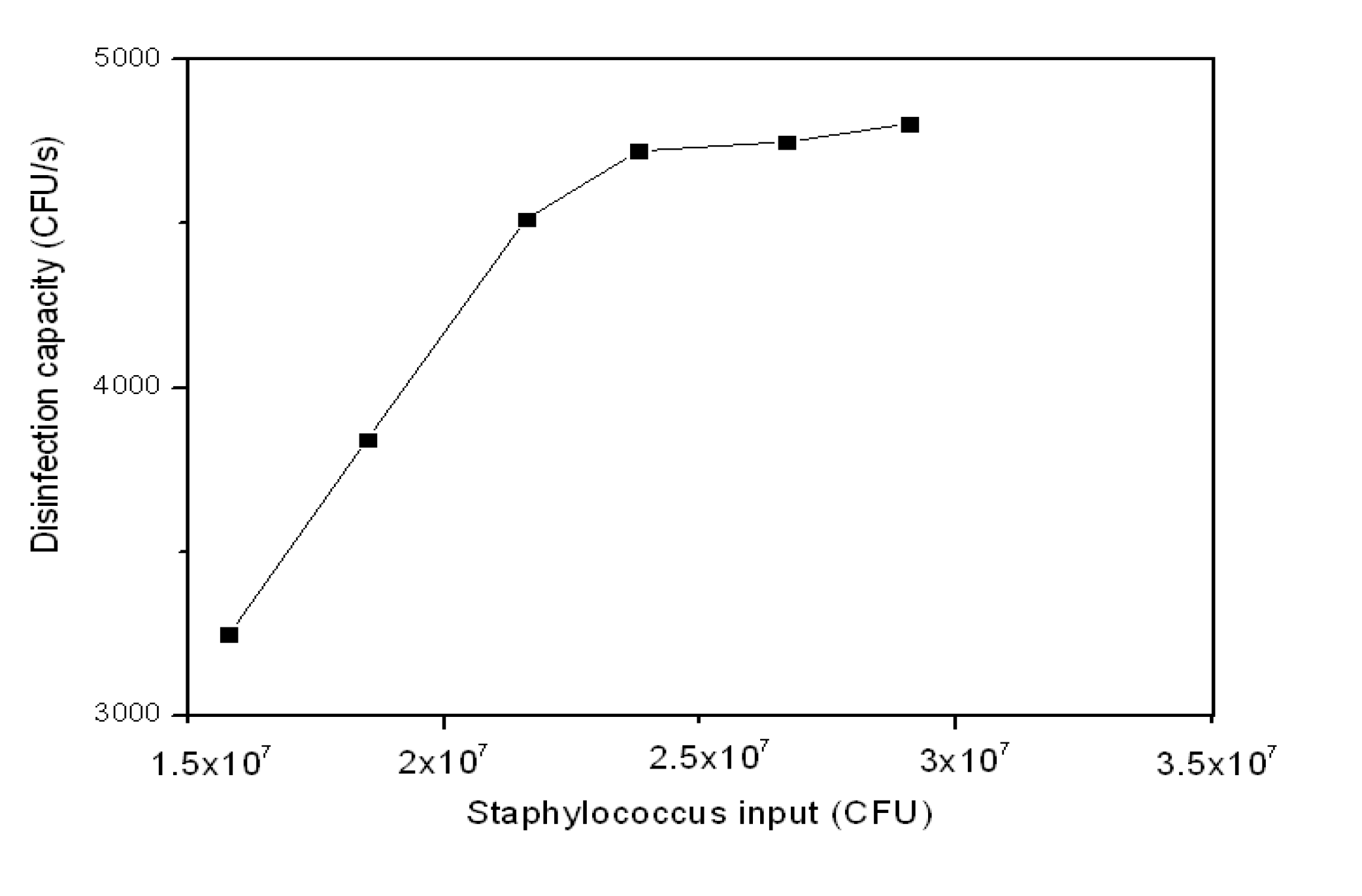
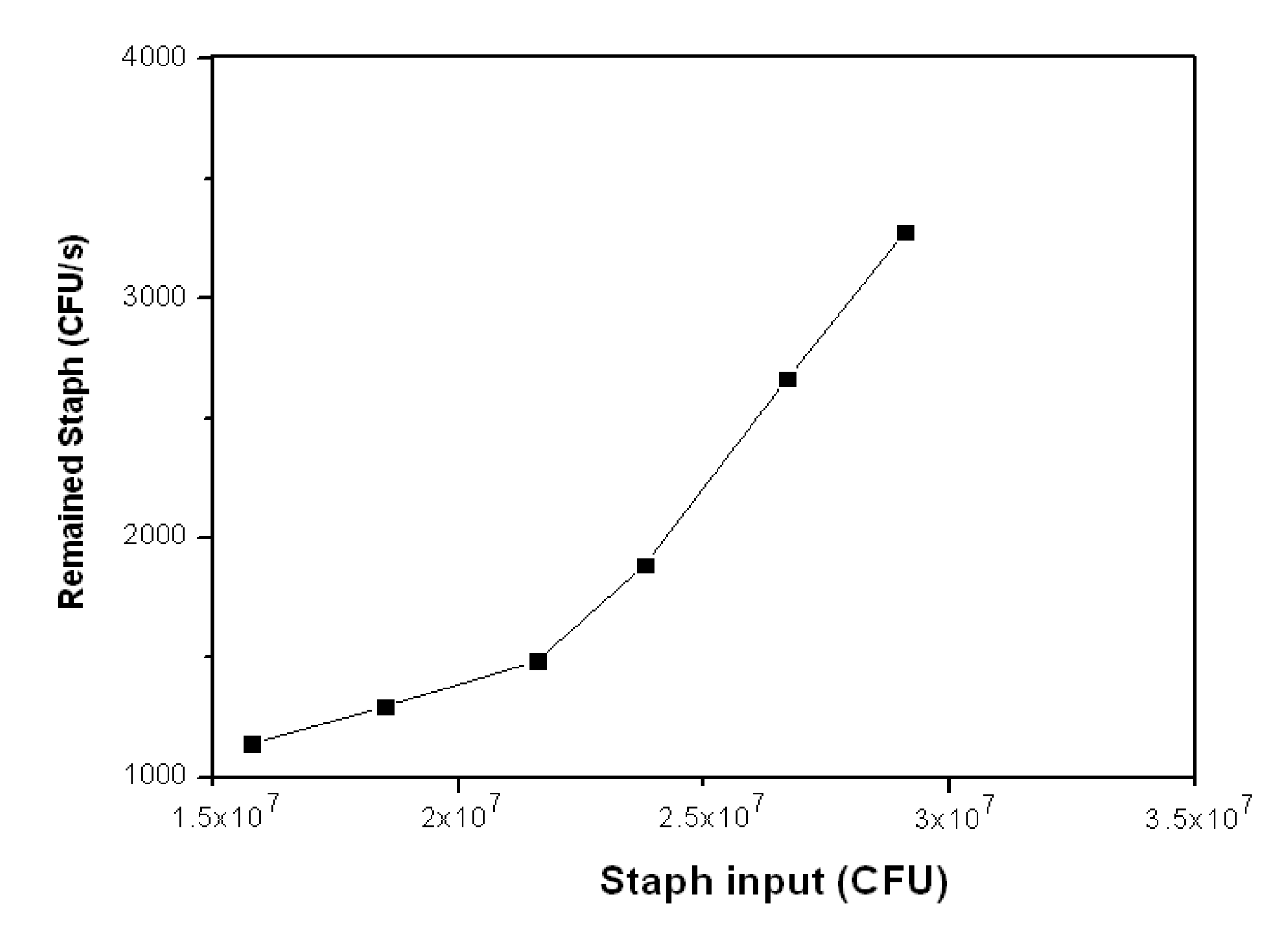
3.2.3. Staph Disinfection Capacity of Optimal Photocatalysis
| Staph Input (CFU) | Staph Output (CFU) | Disinfection Capacity | Staph Remained (CFU·s−1) | |
|---|---|---|---|---|
| (CFU·s−1) | (CFU·s−1·cm−2) | |||
| 1.58E + 07 | 4.11E + 06 | 3,247 | 14 | 1,142 |
| 1.85E + 07 | 4.67E + 06 | 3,842 | 16 | 1,297 |
| 2.16E + 07 | 5.35E + 06 | 4,514 | 19 | 1,486 |
| 2.38E + 07 | 6.80E + 06 | 4,722 | 20 | 1,889 |
| 2.67E + 07 | 9.60E +06 | 4,750 | 20 | 2,667 |
| 2.91E + 07 | 1.18E + 07 | 4,806 | 20 | 3,278 |
4. Conclusions
Acknowledgment
Author Contributions
Conflicts of Interest
References
- The World Health Report 2002, Annex Table 9. Available online: http://www.who.int/whr/2002/en/whr2002_annex9_10.pdf (accessed on 20 January 2014).
- Douwes, J.; Thorne, P.; Peace, N.; Heederik, D. Bioaerosol health effects and exposure assessment: Progress and prospects. Ann. Occup. Hyg. 2003, 47, 187–200. [Google Scholar]
- Christopher, S.C.; Wathes, C.M. Bioaerosols Handbook; Lewis Publishers: Chelsea, MI, USA, 1995. [Google Scholar]
- Ivnitski, D.; Hamid, I.A.; Atanasov, P.; Wilkins, E. Biosensors for detection of pathogenic bacteria. Biosens. Bioelectron. 1999, 14, 599–624. [Google Scholar] [CrossRef]
- Chuaybamroong, P.; Chotigawin, R.; Supothina, S.; Sribenjalux, P.; Larpkiattaworn, S.; Wu, C.Y. Efficacy of photocatalytic HEPA filter on microorganism removal. Indoor Air. 2010, 20, 246–254. [Google Scholar] [CrossRef]
- Sung, W.P.; Tsai, T.T.; Wu, M.J.; Wang, H.J.; Surampalli, R.Y. Removal of indoor airborne bacteria by nano-Ag/TiO2 as photocatalyst: Feasibility study in museum and nursing institutions. J. Environ. Eng. 2011, 137, 163–170. [Google Scholar]
- Zhao, J.; Yang, X. Photocatalytic oxidation for indoor air purification: A literature review. Build. Environ. 2003, 38, 645–654. [Google Scholar] [CrossRef]
- Shintani, H.; Kurosu, S.; Miki, A.; Hayashi, F.; Kato, S. Sterilization efficiency of the photocatalyst against environmental microorganisms in a health care facility. Biocontrol. Sci. 2006, 11, 17–26. [Google Scholar] [CrossRef]
- Portela, R.; Tessinari, R.F.; Suarez, S.; Rasmussen, S.B.; Alonso, M.D.H.; Canela, M.C.; Avila, P.; Sanchez, B. Photocatalysis for continuous air purification in wastewater treatment plants: From lab to reality. Environ. Sci. Technol. 2012, 46, 5040–5048. [Google Scholar] [CrossRef]
- Hu, X.; Li, G.; Yu, J.C. Design, fabrication and modification of nanostructured semiconductor materials for environmental and energy applications. Langmuir 2010, 26, 3031–3039. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photoch. Photobio. 2008, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Han, F.; Kambala, V.S.R.; Srinivasan, M.; Rajarathnam, D.; Naidu, R. Tailored titanium dioxide photocatalysts for the degradation of organic dyes in wastewater treatment: A review. Appl. Catal. AGen. 2009, 359, 25–40. [Google Scholar] [CrossRef]
- Matsunaga, T.; Tomoda, R.; Nakajima, H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef]
- Ibanez, J.A.; Litter, M.I.; Pizarro, R.A. Photocatalytic bactericidal effect of TiO2 on Enterobacter cloacae: Comparative study with other Gram (−) bacteria. J. Photochem. Photobiol. AChem. 2003, 157, 81–85. [Google Scholar] [CrossRef]
- Madrid, P.A.; Moorillon, G.V.N.; Borunda, E.O.; Yoshida, M.M. Photoinduced bactericidal activity against Pseudomonas aeruginosa by TiO2 based thin films. FEMS Microbiol. Lett. 2002, 211, 183–188. [Google Scholar] [CrossRef]
- Melian, J.A.H.; Rodriguez, J.M.D.; Suarez, A.V.; Rendon, E.T.; Campo, C.V.D.; Arana, J.; Pena, J.P. The photocatalytic disinfection of urban waste waters. Chemosphere 2000, 41, 323–327. [Google Scholar] [CrossRef]
- Saito, T.; Iwase, T.; Horie, J.; Morioka, T. Mode of photocatalytic bactericidal action of powdered semiconductor TiO2 on mutans streptococci. J. Photochem. Photobio. B 1992, 14, 369–379. [Google Scholar] [CrossRef]
- Kim, B.; Kim, D.; Cho, D.; Cho, S. Bactericidal effect of TiO2 photocatalyst on selected food-borne pathogenic bacteria. Chemosphere 2003, 52, 277–281. [Google Scholar] [CrossRef]
- Watts, R.J.; Kong, S.; Orr, M.P.; Miller, G.C.; Henry, B.E. Photocatalytic inactivation of coliform bacteria and viruses in secondary waste-water effluent. Water Res. 1995, 29, 95–100. [Google Scholar] [CrossRef]
- Cornish, B.J.P.A.; Lawton, L.A.; Robertson, P.K.J. Hydrogen peroxide enhanced photocatalytic oxidation of microcystin-LR using titanium dioxide. Appl. Catal. B Environ. 2000, 25, 59–67. [Google Scholar] [CrossRef]
- Kuhn, K.P.; Chaberny, I.F.; Massholder, K.; Stickler, M.; Benz, V.W.; Sonntag, H.G.; Erdinger, L. Disinfection of surfaces by photocatalytic oxidation with titanium dioxide and UVA light. Chemosphere 2003, 53, 71–77. [Google Scholar] [CrossRef]
- Wamer, W.G.; Yin, J.J.; Wei, R.R. Oxidative damage to nucleic acids photosensitized by titanium dioxide. Free Rad. Biol. Med. 1997, 23, 851–858. [Google Scholar] [CrossRef]
- Yu, K.P.; Lee, G.W.M.; Lin, S.; Huang, C.P. Aerosol removal by unipolar ionization in indoor environments. J. Aerosol. Sci. 2004, 35, 923–941. [Google Scholar] [CrossRef]
- Chen, F.; Yang, X.; Mak, H.K.C.; Chan, Q.W.T. Photocatalytic oxidation for antimicrobial control in built environment: A brief literature overview. Build. Environ. 2010, 45, 1747–1754. [Google Scholar] [CrossRef]
- Estivill, S.; Hargreaves, D.M.; Puma, G.L. Evaluation of the intrinsic photocatalytic oxidation kinetics of indoor air pollutants. Environ. Sci. Technol. 2007, 41, 2028–2035. [Google Scholar] [CrossRef]
- Yamashita, H.; Anpo, M. Application of an ion beam technique for the design of visible light-sensitive, highly efficient and highly selective photocatalysts: Ion-implantation and ionized cluster beam methods. Catal. Surveys. Asia 2004, 8, 35–45. [Google Scholar]
- Bae, E.; Choi, W.; Park, J.; Shin, H.S.; Kim, S.B.; Lee, J.S. Effects of surface anchoring groups (carboxylate vs. phosphonate) in ruthenium-complex sensitized TiO2 on visible light reactivity in aqueous suspension. J. Phys. Chem. B 2004, 108, 14093–14101. [Google Scholar] [CrossRef]
- Karunakaran, C.; Abiramasundari, G.; Gomathisankar, P.; Manikandan, G.; Anandi, V. Cu-doped TiO2 nanoparticles for photocatalytic disinfection of bacteria under visible light. J. Colloid Interf. Sci. 2010, 352, 68–74. [Google Scholar] [CrossRef]
- Young, C.; Lim, T.M.; Chiang, K.; Scott, J.; Amal, R. Photocatalytic oxidation of toluene and trichloroethylene in the gas-phase by metallised (Pt, Ag) titanium dioxide. Appl. Catal. B Environ. 2008, 78, 1–10. [Google Scholar] [CrossRef]
- Paramasivam, I.; Macak, J.M.; Schmuki, P. Photocatalytic activity of TiO2 nanotube layers loaded with Ag and Au nanoparticles. Electrochem. Commun. 2008, 10, 71–75. [Google Scholar] [CrossRef]
- Sheel, D.W.; Brook, L.A.; Ditta, I.B.; Evans, P.; Foster, H.A.; Steele, A.; Yates, H.M. Biocidal silver and silver/titania composite films grown by chemical vapour deposition. J. Photochem. Photobio. A Chem. 2007, 187, 53–63. [Google Scholar] [CrossRef]
- Herrmann, J.M.; Tahiri, H.; Ait-Ichou, Y.; Lassaletta, G.; Gonzalez-Elipe, A.R.; Fernandez, A. Characterization and photocatalytic activity in aqueous medium of TiO2 and Ag-TiO2 coatings on quartz. Appl. Catal. B Environ. 1997, 13, 219–228. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, H.; Huo, K.; Cui, L.; Zhang, W.; Ni, H.; Zhang, Y.; Wu, Z.; Chu, P.K. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials 2011, 32, 5706–5716. [Google Scholar] [CrossRef]
- Zheng, S.K.; Wang, T.M.; Xiang, G.; Wang, C. Photocatalytic activity of nanostructured TiO2 thin films prepared by dc magnetron sputtering method. Vacuum 2001, 62, 361–366. [Google Scholar] [CrossRef]
- Kawakami, H.; Ilol, R.; Straka, L.; Papul, S.; Romu, J.; Hanninen, H.; Mahlberg, R.; Heikkila, M. Photocatalytic activity of atomic layer deposited TiO2 Coatings on austenitic stainless steels and copper alloys. J. Electrochem. Soc. 2008, 155, 62–68. [Google Scholar]
- Gamage, J.; Zhang, Z. Applications of photocatalytic disinfection. Int. J. Photoenergy 2010, 2010. [Google Scholar]
- Pham, T.D.; Lee, B.K.; Nguyen, M.V.; Lee, C.H. Germicide feasibility of TiO2/glass fiber and Ag-TiO2/glass fiber photocatalysts. Adv. Mat. Res. 2012, 518-523, 864–868. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, L.C.; Hui, R.Q.; Zhang, Z.; Yi, W.C. Effects of silver ion doping on the surface defect characteristics of TiO2. Res. Chem. Intermediat. 2004, 30, 569–577. [Google Scholar] [CrossRef]
- Cui, X.S. The Basic Theory of Solid Chemistry; Beijing Institute of Technology Printing: Beijing, China, 1991. [Google Scholar]
- Gao, X.; Zhao, M.; Zhang, Z.; Chen, C.; Ma, J.; Lu, J. Effects of hydrogen annealing on the microstructure and optical properties of single-phased Ag2O film deposited using direct-current reactive magnetron sputtering. Thin Solid Films 2011, 519, 6620–6623. [Google Scholar] [CrossRef]
- He, C.; Yu, Y. Influence of silver doping on the photocatalytic activity of titania films. Appl. Surf. Sci. 2002, 200, 239–247. [Google Scholar] [CrossRef]
- Seery, M.K.; George, R.; Floris, P.; Pillai, S.C. Silver doped titanium dioxide nanomaterials for enhanced visible light photocatalysis. J. Photochem. Photobiol. A Chem. 2007, 189, 258–263. [Google Scholar] [CrossRef]
- Vohra, A.; Goswami, D.Y.; Deshpande, D.A.; Block, S.S. Enhanced photocatalytic disinfection of indoor air. Appl. Catal. BEnviron. 2006, 65, 57–65. [Google Scholar]
- Behnajady, M.A.; Modirshahla, N.; Shokri, M.; Rad, B. Enhancement of photocatalytic activity of TiO2 nanoparticles by silver doping: Photodeposition versus liquid impregnation methods. Global NEST J. 2008, 10, 1–7. [Google Scholar]
- Zhang, H.; Wang, G.; Chen, D.; Li, X.; Li, J. Tuning photoelectrochemical performances of Ag-TiO2 nanocomposites via reduction/oxidation of Ag. Chem. Mater. 2008, 20, 6543–6549. [Google Scholar] [CrossRef]
- Fu, Y.; Du, H.; Zhang, S.; Huang, W. XPS characterization of surface and interfacial structure of sputtered TiNi films on Si substrate. Mater. Sci. Eng. A 2005, 403, 25–31. [Google Scholar] [CrossRef]
- Rengaraj, S.; Li, X.Z. Enhanced photocatalytic activity of TiO2 by doping with Ag for degradation of 2,4,6-trichlorophenol in aqueous suspension. J. Mol. Catal. A Chem. 2006, 243, 60–67. [Google Scholar] [CrossRef]
- Meng, F.; Sun, Z. Enhanced photocatalytic activity of silver nanoparticles modified TiO2 thin films prepared by RF magnetron sputtering. Mater. Chem. Phys. 2009, 118, 349–353. [Google Scholar] [CrossRef]
- Chao, H.E.; Yun, Y.U.; Xingfang, H.U.; Larbot, A. Effect of silver doping on the phase transformation and grain growth of sol-gel titania powder. J. Eur. Ceram. Soc. 2003, 23, 1457–1464. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Sunada, K.; Watanabe, T.; Hashimoto, K. Studies on photokilling of bacteria on TiO2 thin film. J. Photochem. Photobiol. A Chem. 2003, 156, 227–233. [Google Scholar] [CrossRef]
- Yu, J.; Xiong, J.; Cheng, B.; Liu, S. Fabrication and characterization of Ag-TiO2 multiphase nanocomposite thin films with enhanced photocatalytic activity. Appl. Catal. B Environ. 2005, 60, 211–221. [Google Scholar] [CrossRef]
- Li, Y.; Wu, C.Y. Role of moisture in adsorption, photocatalytic oxidation, and reemission of elemental mercury on a SiO2-TiO2 nanocomposite. Environ. Sci. Technol. 2006, 40, 6444–6448. [Google Scholar] [CrossRef]
- Peccia, J.; Werth, H.M.; Miller, S.; Hernandez, M. Effects of relative humidity on the ultraviolet induced inactivation of airborne bacteria. Aerosol. Sci. Technol. 2001, 35, 728–740. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pham, T.-D.; Lee, B.-K. Feasibility of Silver Doped TiO2/Glass Fiber Photocatalyst under Visible Irradiation as an Indoor Air Germicide. Int. J. Environ. Res. Public Health 2014, 11, 3271-3288. https://doi.org/10.3390/ijerph110303271
Pham T-D, Lee B-K. Feasibility of Silver Doped TiO2/Glass Fiber Photocatalyst under Visible Irradiation as an Indoor Air Germicide. International Journal of Environmental Research and Public Health. 2014; 11(3):3271-3288. https://doi.org/10.3390/ijerph110303271
Chicago/Turabian StylePham, Thanh-Dong, and Byeong-Kyu Lee. 2014. "Feasibility of Silver Doped TiO2/Glass Fiber Photocatalyst under Visible Irradiation as an Indoor Air Germicide" International Journal of Environmental Research and Public Health 11, no. 3: 3271-3288. https://doi.org/10.3390/ijerph110303271




