1. Introduction
Di-(2-ethylhexyl) phthalate (DEHP), an environmental endocrine disruptor, is one of the organic chemicals that is used in a large variety of industrial and consumer applications. It is the most commonly used plasticizer worldwide [
1,
2,
3]. An endocrine disruptor alters the function of the endocrine systems and causes adverse effects in organisms [
4].
DEHP has been approved for use and has also been detected in a wide range of consumer products [
5,
6], including personal care products [
7], infant toys [
8], food wraps [
9], cosmetics, medical consumables, nutritional supplements, cleaning materials, lubricants, insecticides, solvents, adhesives, paints, lacquers, etc. Unfortunately, DEHP may leach slowly from these plastic products into foods, beverages, and even directly into body fluids [
10,
11]. It is also detected in indoor air [
12,
13,
14], indoor dust [
15,
16,
17,
18], and air inside vehicles [
19]. The general population may be exposed since DEHP is ubiquitous environmental contaminants [
20,
21,
22]. Epidemiological investigation showed that the exposure dose of DEHP for the population was 5 mg/kg/d [
23].
Studies have shown that correlating DEHP levels with negative endocrine effects in females are increasing [
24]. DEHP can cause the abortion of female animals, increase ovarian weight and the follicle developmental disorders, and gonadal synthesis of estradiol and gonadotropin receptor gene expression decreased pituitary gonadotropin upregulated genes [
25,
26,
27]. DEHP can enter the fetus through the placental barrier and gather in the fetus. This may have an impact on the fetus directly [
28,
29].
Until now, most of the research has focused on reproductive toxicity of adult females’ exposure to DEHP, and little is known regarding the mechanism of DEHP in pubertal females. With the development of society and economy, precocious puberty has been getting more and more attention. The rate of precocious puberty is increasing. Meanwhile, the incidents of reproductive system diseases are also rising. The average age of menarche of girls in Australia and Europe are three years earlier than before [
30,
31,
32]. Epidemiology found that exposure to phthalates are associated with endometritis, earlier breast development, sexual precocity and earlier onset of puberty [
33,
34,
35,
36]. However, the effect of DEHP on pubertal female reproductive system is still not well studied.
Animal reproduction is regulated by a variety of factors both inside and outside the body, and the hypothalamic-pituitary-gonadal axis (HPGA) plays a major regulatory role in it. The central precocious puberty (CPP) is due to the hypothalamic-pituitary-gonadal axis function starting early, and because the level of gonadotropin-releasing hormone (GnRH) is premature increased [
37]. GnRH is a hypothalamic neuronal secretory decapitate that plays an important regulatory role in the mammalian reproductive system. The present study supports the notion that GnRH-(1-5) is functionally capable of regulating the reproductive neuroendocrine system [
38]. GnRH influences the reproductive processes mainly by regulating pituitary gonadotropin synthesis and release, which, in turn, modulate steroid genesis and gametogenesis [
39,
40]. Even if the hypothalamus is the principal source of GnRH, and the pituitary is the target site for it, several studies have reported that GnRH receptor (GnRHR) will be in many other tissues including reproductive organs, such as testes, prostates, ovaries, and uteruses [
41,
42,
43,
44].
Precocious puberty may cause polycystic ovary syndrome, and thus will cause heart disease, diabetes, endometrial cancer, infertility and other diseases [
45,
46]. This study investigated the effect of pubertal female rats’ exposure to DEHP on the expression of GnRH in hypothalamus and GnRHR in the uterus and to explore the role of DEHP in reproductive endocrine disruption. The DEHP doses were set from the human exposure dose (5 mg/kg/d) [
23] and the LD
50 (30 g/kg) in rats [
47].
2. Materials and Methods
2.1. Animals and Treatment
Female Wistar rats (15 days old, 60 ± 10 g) were purchased from the Experimental Animal Center of Jilin University. They were allowed at least a 7-day acclimatization-period and observed for signs of illness before starting experimental procedures, and their body weight was measured every day. The 48 rats were randomly apportioned into 4 groups (n = 12, each). The rats were housed in polypropylene cages (6 rats per cage) with sterilized bedding, and maintained under controlled conditions of temperature (23 ± 1 °C), humidity (55% ± 5%), and a 12:12 h light-dark cycle. The experiment lasted approximately 4 weeks including the period of adaptation to the feeding regime. Rats were administered via oral gavage 0, 250 mg/kg/d DEHP (1/120 LD50), 500 mg/kg/d DEHP (1/60 LD50), 1000 mg/kg/d DEHP (1/30 LD50) (Sinopharm Chemical Reagent, purity >99%, Shanghai, China) in 0.1 mL corn oil/20 g body weight for up to 4 weeks. The experimental protocol was subject to approval by the Animal Use and Care Committee of Jilin University, and the ethical approval code of the animal experiment is 2015-03-14.
2.2. Tissue Collection
After 4 weeks, the rats were weighed and killed by decapitation. The hypothalamus and uterus of each rat were also dissected, weighed, and collected immediately after decapitation.
2.3. Quantification of GnRH
GnRH in the hypothalamus was quantified using enzyme-linked immunosorbent assay kits (R&D systems, Minneapolis, MN, USA). The total protein from the hypothalamus sample was extracted, and 0.1 mL diluted sample from each group was mixed with 10 µL GnRH antibody and 50 µL horseradish peroxidase-labeled streptavidin. This was incubated at 37 °C for 60 min. The wells were rinsed five times with distilled water and incubated with chromogen solution from the kit (50 µL A and 50 µL B) for 10 min at 37 °C in dark. The reaction was then stopped with 50 µL of stop solution, and the absorbance was read at 450 nm within 10 min.
2.4. Immunohistochemistry
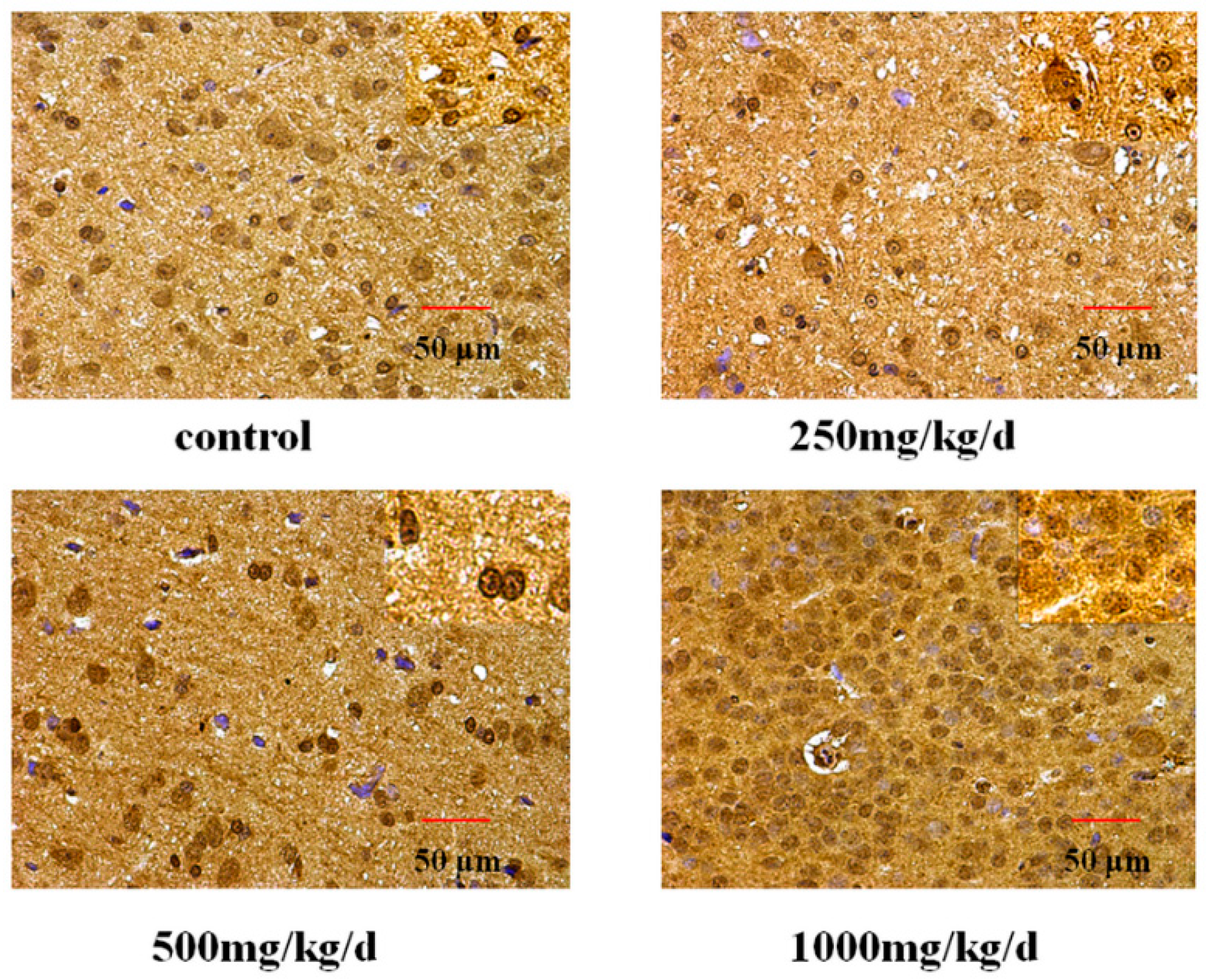
Tissue sections of hypothalamus and uterus were deparaffinized in xylene, rehydrated through graded ethanols, and finally rinsed in distilled water. The specimens were treated with antigen retrieval in 0.1 M citric acid solution by boiling for 15 min and cooling to room temperature. They were then incubated with 3% H
2O
2 (v/v) in methanol for 10 min to quench the endogenous peroxidase activity. The sections were blotted with normal rat serum (1:10 dilution) for 30 min at room temperature, and incubated with rabbit polyclonal primary antibody (GnRH and GnRHR, Proteintech, Rosemont, IL, USA) (1:1000 in phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin) overnight at 4 °C in a moist chamber. The slides were incubated with peroxidase-conjugated anti-rabbit secondary antibody (Bioss Biotechnology Company, Shanghai, China) (1:200 dilution in PBS) for 30 min at room temperature after three washes in PBS. The staining was visualized using a 3,3′-diaminobenzidine (DAB) (Bioss Biotechnology Company, Shanghai, China) kit. The specimens were counterstained with hematoxylin (Bogoo Biotechnology Company, Shanghai, China), mounted, observed under a light microscope, and photographed [
48].
2.5. RNA Extraction
Total RNA was isolated using Trizol reagent (Invitrogen, Waltham, MA, USA). The quantity and the integrity of total RNA were determined by a UV spectrophotometer and evaluated by formaldehyde denaturing gel electrophoresis.
2.6. Real-Time Reverse Transcription-PCR
Real-time reverse transcription-PCR was used to verify gene expression using Stratagene MX3000p (TaKaRa, Shiga, Japan). Reverse transcription was performed with 500 ng total RNA in a 10-ìL reaction, and 1 ìL of cDNA was then used for a 25-ìL PCR reaction mixture containing an optimal concentration of primers and SYBR-Green Supermix (SYBR premix Ex Tap II, TaKaRa, Shiga, Japan). The PCR reaction was carried out in 45 cycles of 95 °C for 20 s and 60 °C for 20 s. â-actin was used as an internal control [
49]. The primers are listed in
Table 1.
2.7. Western Blotting
Isolated tissues were immediately put on ice and homogenized in lysis buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 1% deoxycholate, 1 mM sodium fluoride, 2 mM sodium orthovanadate, and complete protease inhibitor tablets). Homogenates were centrifuged at 12,000
g for 5 min at 4 °C. Protein in the supernatant was quantified using the BCA protein assay (Beyotime, Shanghai, China). Equal amounts of protein from each sample were mixed with sodium dodecyl sulfate (SDS) sample buffer (Beyotime, Shanghai, China). Samples were separated using pre-cast 10% Bis-Tris gel (Beyotime, Shanghai, China) in Tris-Glycine-SDS running buffer and transferred to nitrocellulose filter membrane (NC) (Phamacia, Goteborg, Sweden) in transfer buffer. Prior to immunoblotting, NC membranes were blocked with 5% nonfat milk in tris buffered saline tween (TBST) buffer (20 mmol/L Tris-HCl, 140 mmol/L NaCl, and 0.05% Tween 20, pH 7.5). Following incubation with the primary antibodies specific for each protein for 24 h at 4 °C—mouse anti-GnRH antibody (1:1000, 34 KDa, Abcam, Cambridge, UK), rabbit anti-GnRHR antibody (1:500, 36 KDa, Abcam, Cambridge, UK), and rabbit anti-beta Actin antibody (1:1000, 42 KDa, Abcam, Cambridge, UK)—the blots were washed with TBST and then incubated with the horseradish peroxidase-conjugated secondary antibodies (1:2000, Proteintech, Rosemont, IL, USA) for 1 h at room temperature. Immunolabeling was detected by enhanced chemiluminescence (Proteintech, Rosemont, IL, USA) according to the recommended conditions [
50]. Western blot analysis was performed in duplicates for each sample and its average protein level was calculated for comparison. Digital images of the blots were created by scanning the blots on a scanner in transparency mode and optical density measurements of the bands were taken with Image-Pro software (Media Cybernetics Company, Bethesda, MD, USA). Each protein level was normalized to control samples from the same membrane and presented in percentage.
2.8. Statistical Analysis
Statistical evaluations were calculated using SPSS 22.0 statistical software (SPSS Inc., Chicago, IL, USA). All data were tested for normal distribution and independence using the Normal Plots in SPSS. Shapiro–Wilk significance was over 0.05, indicating that the assumptions were valid. Differences between the treatment and control groups were analyzed by analysis of Bonferroni’s test. Data are presented as mean ± standard error of the mean. p < 0.05 was considered significantly significant.
4. Discussion
The puberty of female rats is one of the key stages of the development of their reproductive system and sexual differentiation. During puberty, female rats are extremely sensitive to both endogenous and exogenous hormone changes, and any small change in their sex hormone levels could exert a persistent effect on the development of the reproductive system. Pubertal development is complicated and orderly, which is affected by heredity, nutrition, systemic diseases, endocrine hormone, psycho-mental factors, etc. It begins with the releasing of GnRH by the hypothalamus. By acting on the GnRH receptors on the luteinizing hormone (LH)- and follicle-stimulating hormone (FSH) secreting cells in the anterior pituitary, GnRH promotes the secretion of LH and FSH in the pituitary, which then act on the gonad and promotes the development of it, the generation of mature gametes and the secretion of sex hormones. GnRH plays a pivotal role in regulating hypothalamic-pituitary-gonadal axis function. HPG axis is temporarily activated in the late fetal period and early infancy, suppressed during childhood, and then activated again when puberty begins. During puberty, the secretion of GnRH in the hypothalamus increases, which enters the adenohypophysis through the portal system of the hypothalamus and pituitary. With its sensitivity to GnRH gradually increasing, the adenohypophysis secretes LH and FSH when stimulated by GnRH.
DEHP may have negative impacts on the development and function of female reproductive system. According to previous studies, exposure to steroid hormones or environmental endocrine disruptors (EEDs) during pregnancy or neonatal period could affect the starting time of puberty. This proves that exposure to exogenous estrogenic chemicals in perinatal period could affect the development of the whole endocrine axis [
51,
52]. Exogenous estrogens, like estradiol estradiol benzoate (EB), can decrease the vaginal opening time of pubertal female rats, increase the level of GnRH in hypothalamus of rats, start the hypothalamus-pituitary-gonadal axis, and then lead to the onset of puberty [
53]. Liu et al.’s study also said, compared with control rats, the DEHP-treated adult female rats showed higher GnRH levels in the hypothalamus [
54].
In this study, DEHP is applied on female rats in puberty. We observed that DEHP treatment resulted in higher food consumption and body weight, and they were the same as previous results. Some studies have reported that the body weight, food intake and visceral fat content of female mice exposed to DEHP were significantly increased [
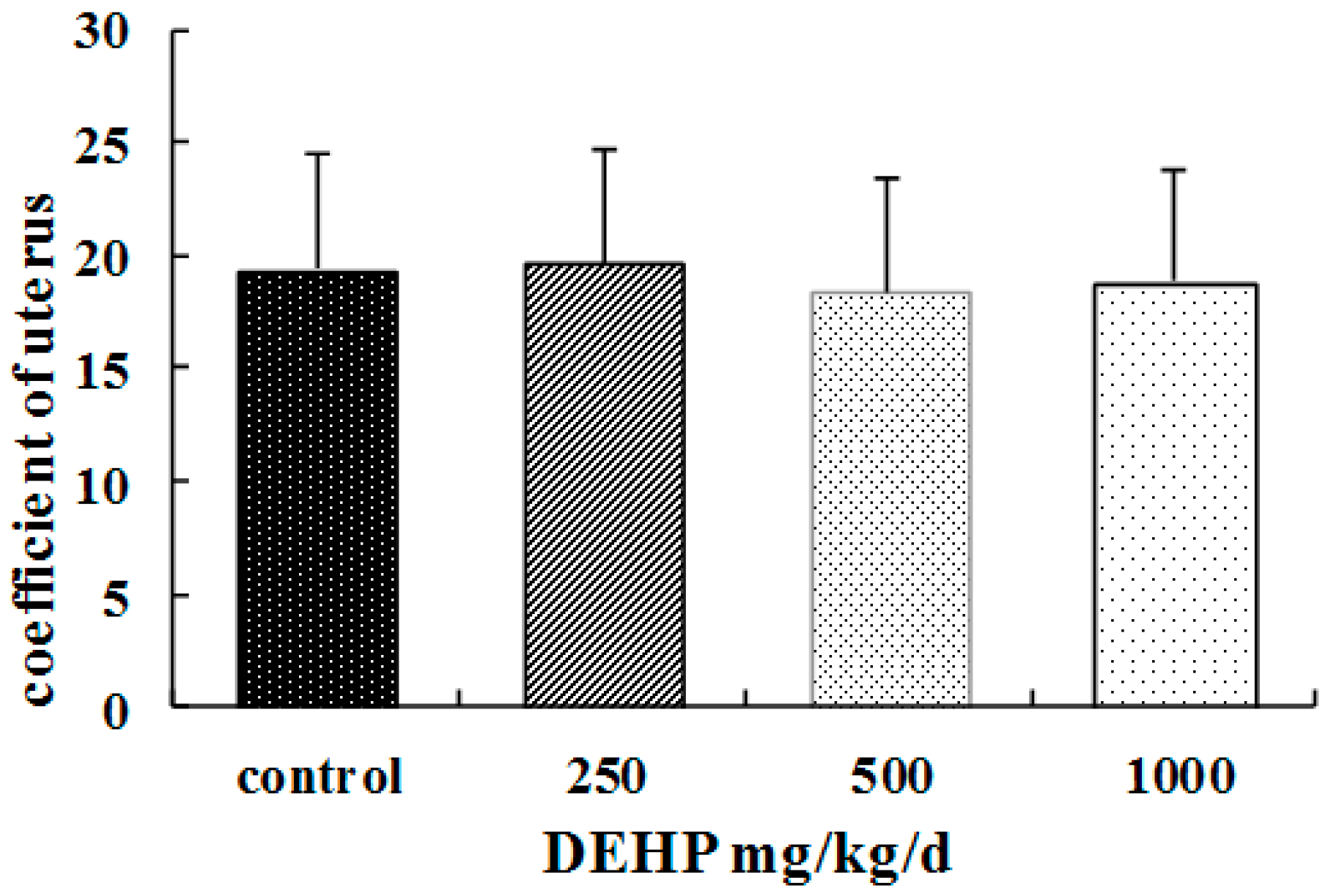
26]. The association between DEHP and higher body weight suggests its negative effects on the growth and development of pubertal female rats. In our study, we also find that the ovaries of the rats treated with DEHP were congestive and swelling, and the volume of the ovaries and uterus became bigger. It suggests ovary and uterus might be the target organ of DEHP.
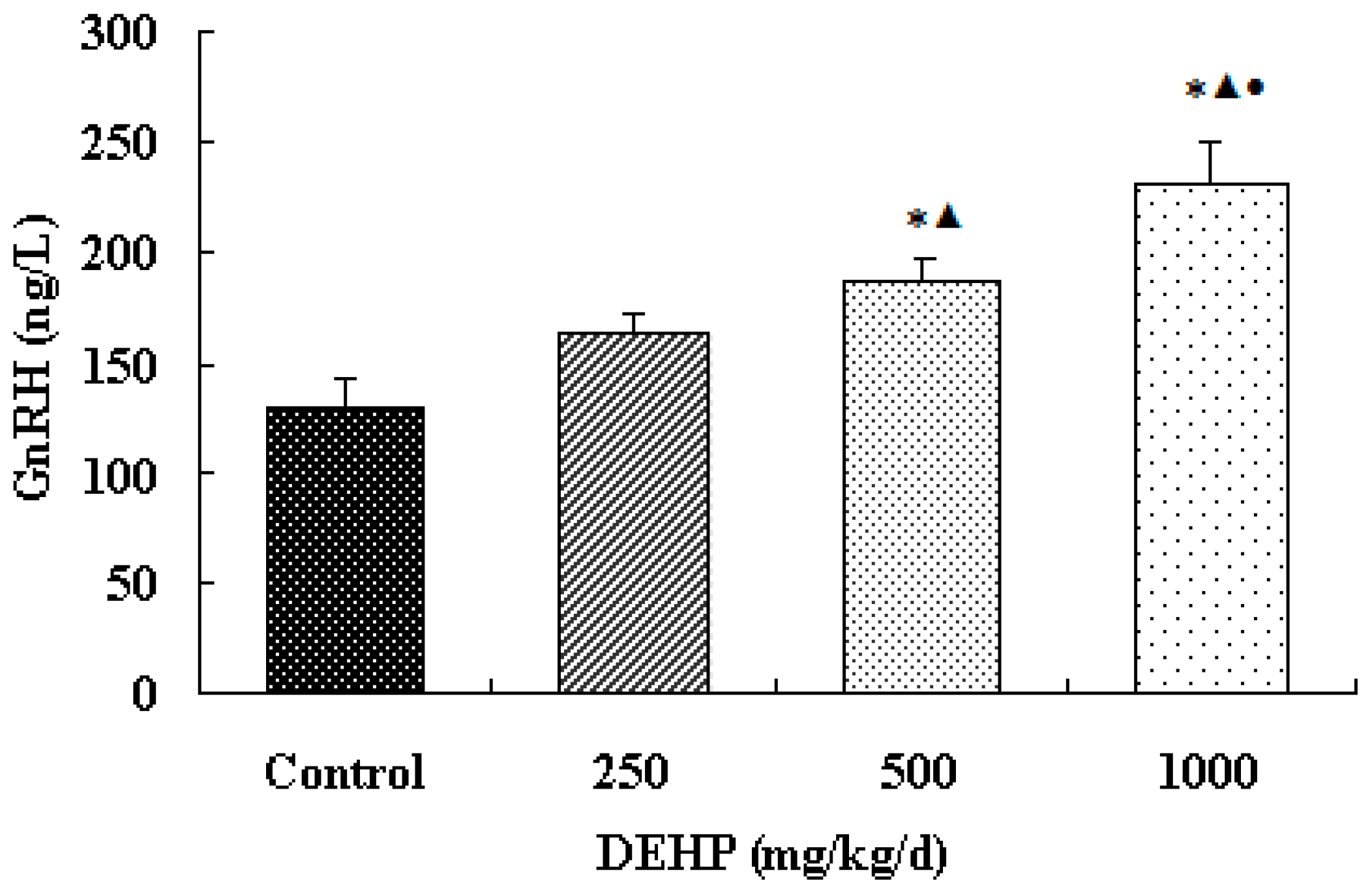
The result shows that the secretion of GnRH in the hypothalamus of the exposure group significantly increased. It indicates that the effect of DEHP is similar to that of GnRH agonists and estrogen. Sustained DEHP exposure might increase the sensitivity of GnRH in the hypothalamus and then raise the secretion of GnRH.
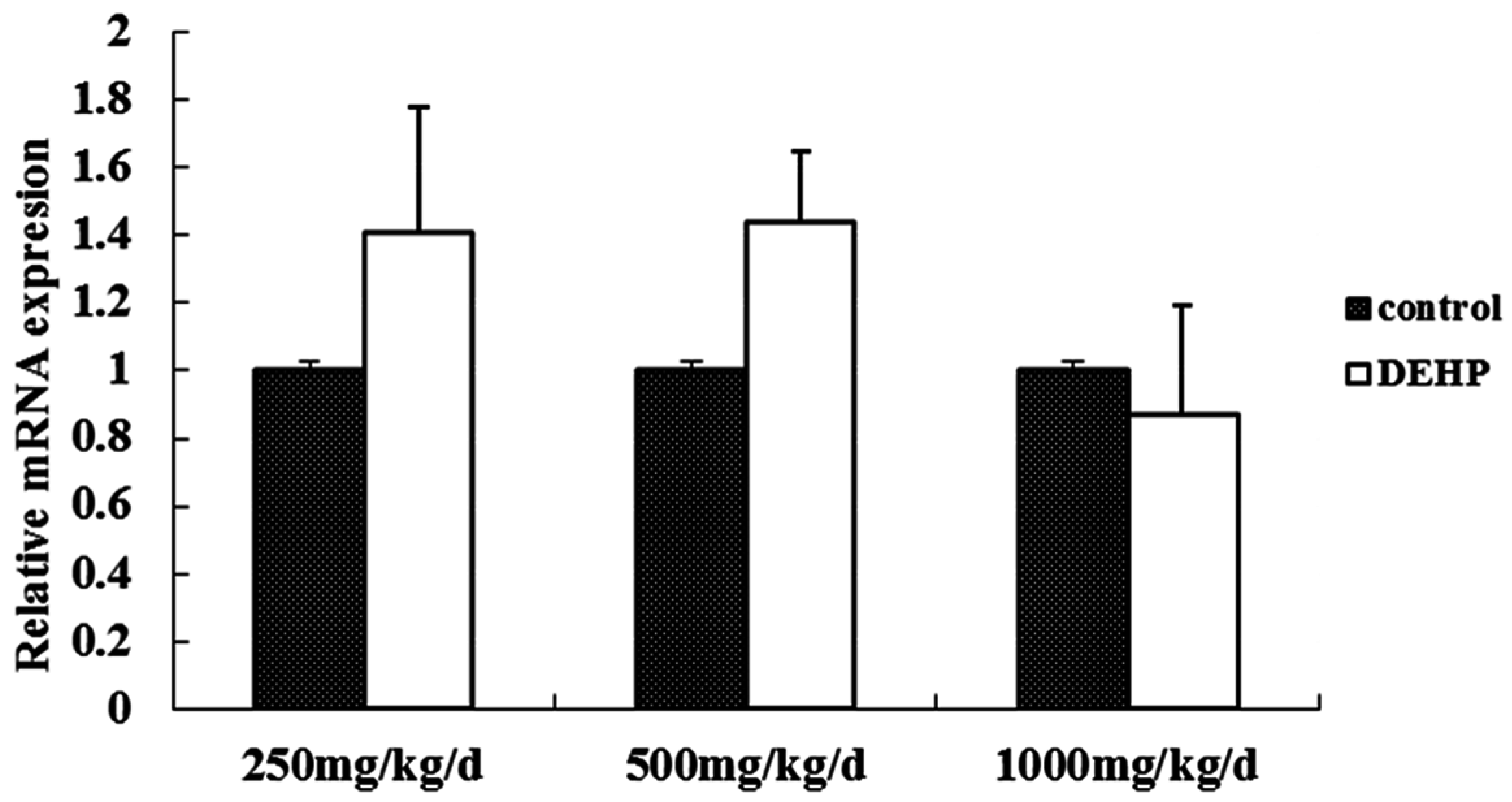
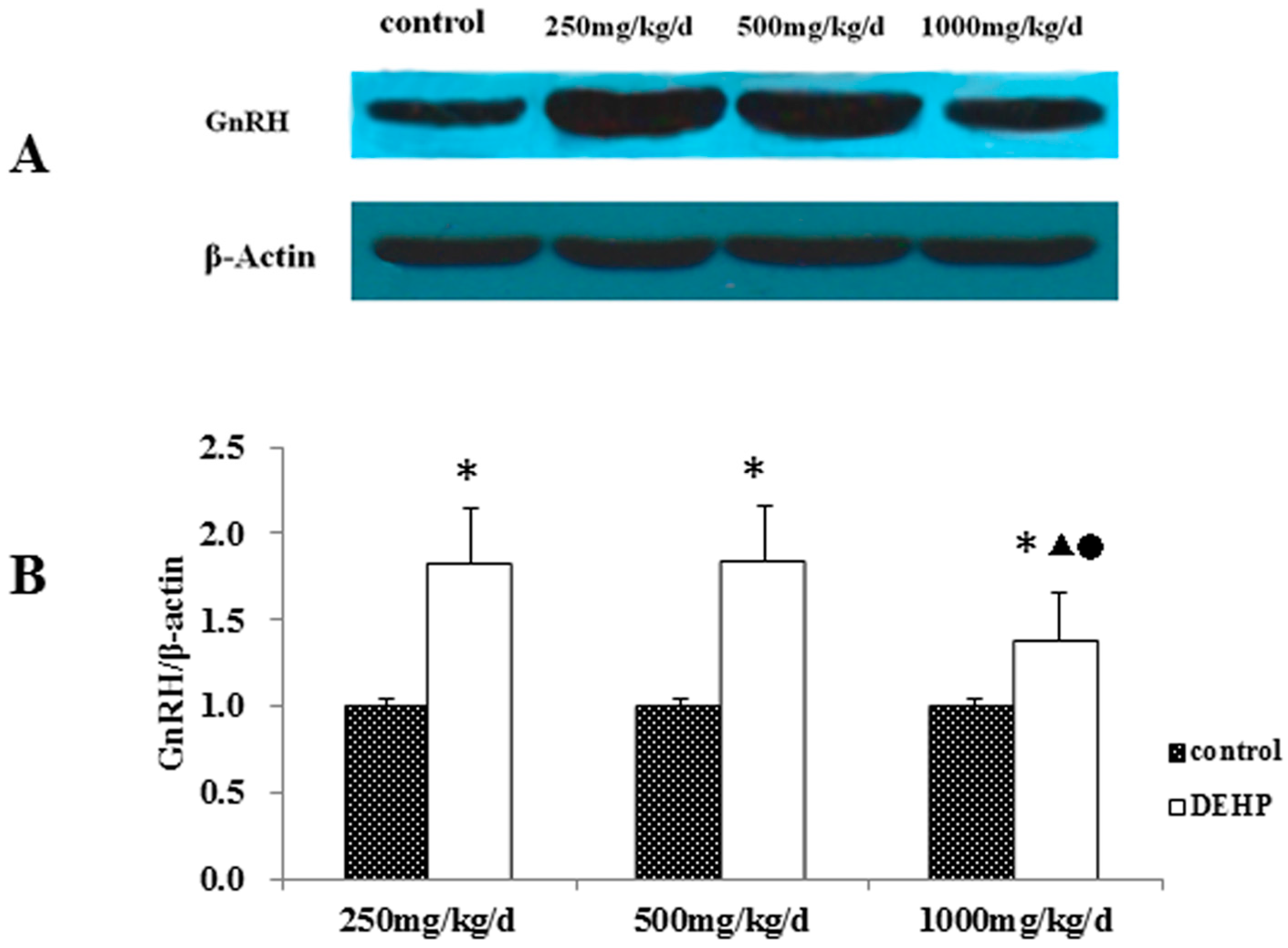
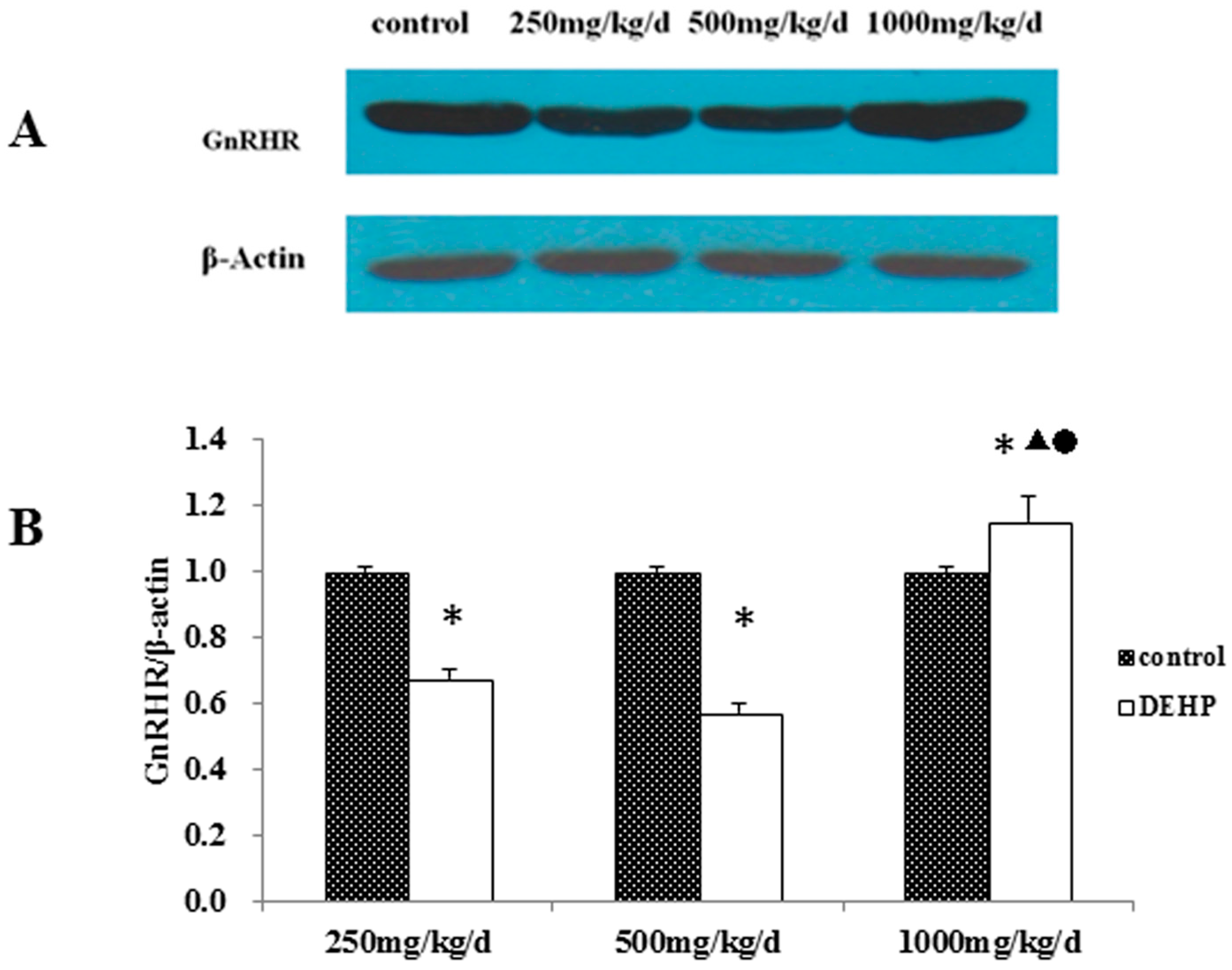
Meanwhile, however, there is no significant difference in the mRNA level of GnRH in the hypothalamus of the rats treated with DEHP. The protein level in the hypothalamus is significantly higher in the rats treated with DEHP compared with the rats in control group, and the level was significantly lower in the rats treated with 1000 mg·kg−1·d−1 DEHP compared with the rats in 250 and 500 mg/kg/d DEHP. This means that DEHP could affect the regulation of GnRH after translation, and the protein expression of GnRH is much more stable. However, 1000 mg/kg/d DEHP may inhibit the protein level of GnRH in the hypothalamus. This suggests that GnRH of hypothalamus might be an important target of DEHP.
Studies have found that EEDs, like bisphenol A and 4-nonylphenol, can have significant adverse effects on the gene regulatory network related to the function of the pituitary-ovary axis. GnRHR mRNA, FSH β-subunit, mRNA of LH β-subunit, FSH-R, mRNA of LH-R, the receptor of E
2α and mRNA of the receptor of E
2α all increased significantly. Those that also increased significantly included the expression levels of the mRNA of GnRH-R pathway (phosphatidylinositol pathway), E
2-R receptor pathway (including E
2-R receptor signal transduction pathway and crosstalk pathway between IGF, EGF and TGF) and the estrogen-metabolizing enzymes [
55]. Liu et al.’s study said, compared with control rats, the DEHP-treated adult female rats showed higher mRNA and protein levels of GnRHR in the pituitary [
54].
Under the influence of GnRH in the hypothalamus, the pituitary releases FSH and LH that decide the secretion of sex hormones in the ovary. The cyclic variation of the endometrium is regulated by sex hormones. Previous research suggested that GnRHR only exists in the pituitary. However, basic research on GnRH and GnRHR in recent years shows that GnRHR also exists in other organs, including the ovaries, testes, uteruses, etc. [
41,
42,
43,
44]. In addition, tissues of malignant tumors, such as breast cancer, ovarian cancer and endometrial cancer, experience high affinity of GnRHR expression as well [
56,
57,
58].
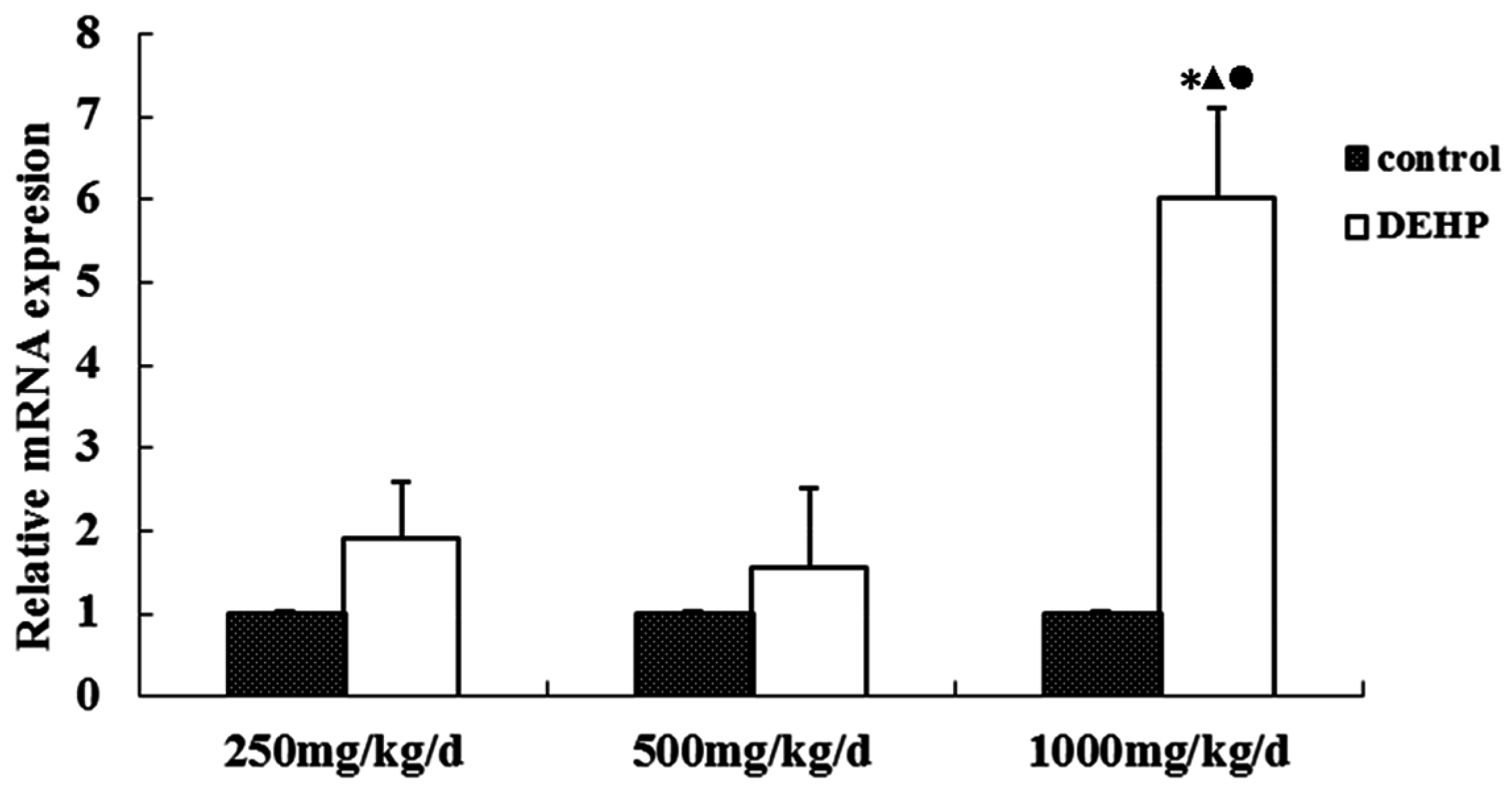
DEHP is an environmental endocrine disruptor, and it has estrogen-like activity. Estrogen plays a role in both normal uterine physiology and uterine pathologies, such as dysfunctional uterine bleeding or breakthrough bleeding, endometriosis, infertility, leiomyoma, and endometrial cancer [
59]. Previous study investigated the in vitro and in vivo effects of DEHP and also the comparison of the urinary levels of several phthalate metabolites between women with and without endometriosis. These findings suggest that exposure to phthalate may lead to establishment of endometriosis by enhancing invasive and proliferative activities of endometrial cells [
60]. A report showed a 31-year old woman in Italy who had a small uterus, sterility and secondary amenorrhea. Cytogenetic analysis demonstrated the complete loss of a copy of the GnRHR gene. GnRHR may associate with gonadal function [
61]. The result of this study shows that the cytomembrane, cytoplasm and nucleus of uterus epithelial cells and glandular epithelial cells are GnRHR-positive. The immunoreactive substance is yellow or yellow brown. In addition, 250 and 500 mg/kg/d DEHP can decrease the protein expression level of GnRHR in uteruses, while high doses of DEHP may increase the GnRHR mRNA and protein expression levels in uteruses of pubertal female rats. This proves that DEHP may, through the toxic effect of GnRH in the hypothalamus and its receptor on the uterus, do damage to the regulatory function of autocrine or paracrine of the uterus and result in uterus related diseases.