Auditing the Immunization Data Quality from Routine Reports in Shangyu District, East China
Abstract
:1. Introduction
2. Methods
2.1. Selection of Research Sites
2.2. Immunization Information System
2.3. Evaluation Process
2.4. Evaluation Measures and Data Analysis
3. Results
3.1. Reporting Consistency
3.2. Quality of Immunization Reporting System
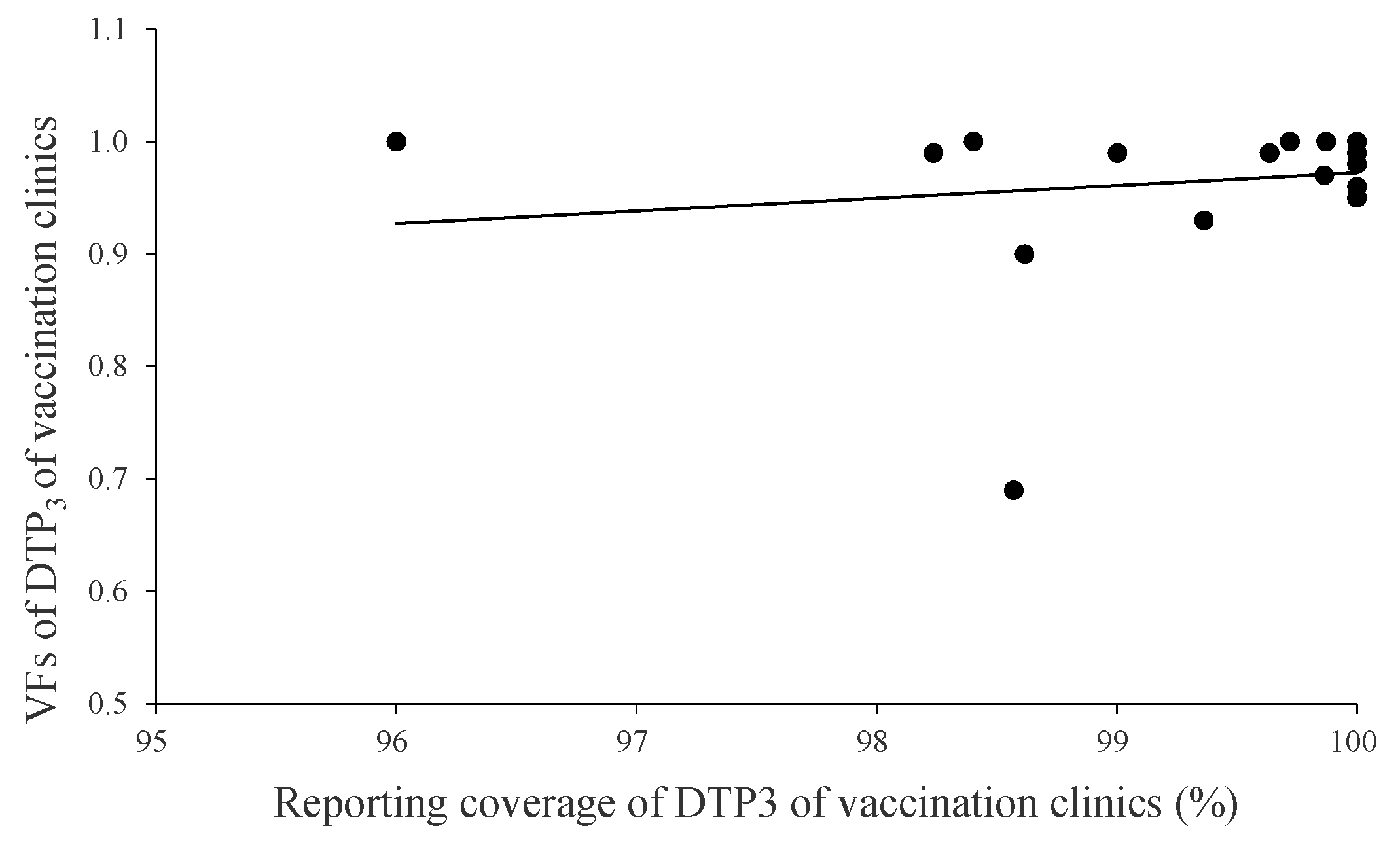
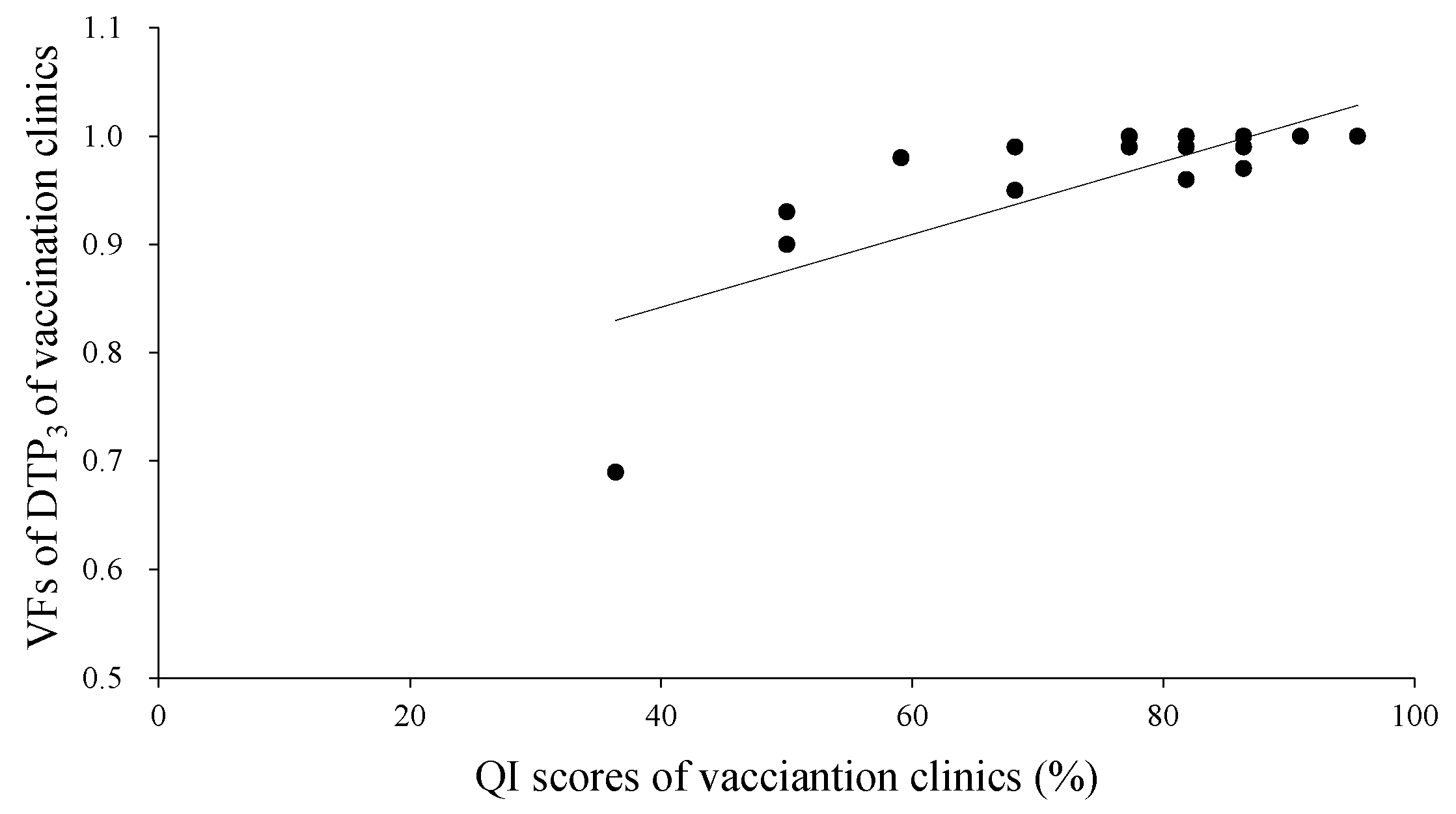
3.3. Correlation Analysis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hu, Y.; Luo, S.; Tang, X.; Lou, L.; Chen, Y.; Guo, J. Comparative assessment of immunization coverage of migrant children between national immunization program vaccines and non-national immunization program vaccines in East China. Hum. Vaccin. Immunother. 2015, 11, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Onta, S.R.; Sabroe, S.; Hansen, E.H. The quality of immunization data from routine primary health care reports: A case from Nepal. Health Policy Plan. 1998, 13, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Dietz, V.; Venczel, L.; Izurieta, H.; Stroh, G.; Zell, E.R.; Monterroso, E.; Tambini, G. Assessing and monitoring vaccination coverage levels: Lessons from the Americas. Rev. Panam. Salud Publica 2004, 16, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Freund, P.J.; Kalumba, K. Monitoring and evaluation of primary health care in rural Zambia. A comparative study. Scand. J. Soc. Med. 1985, 13, 137–146. [Google Scholar] [PubMed]
- Woodard, S.; Archer, L.; Zell, E.; Ronveaux, O.; Birmingham, M. Design and simulation study of the immunization Data Quality Audit (DQA). Ann. Epidemiol. 2007, 17, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Ronveaux, O.; Rickert, D.; Hadler, S.; Groom, H.; Lloyd, J.; Bchir, A.; Birmingham, M. The immunization data quality audit: Verifying the quality and consistency of immunization monitoring systems. Bull. World Health Organ. 2005, 83, 503–510. [Google Scholar] [PubMed]
- Hu, Y.; Chen, Y.; Zhang, B.; Li, Q. An Evaluation of Voluntary Varicella Vaccination Coverage in Zhejiang Province, East China. Int. J. Environ. Res. Public Health 2016, 13, 560. [Google Scholar] [CrossRef] [PubMed]
- Cutts, F.T.; Claquin, P.; Danovaro-Holliday, M.C.; Rhoda, D.A. Monitoring vaccination coverage: Defining the role of surveys. Vaccine 2016, 34, 4103–4109. [Google Scholar] [CrossRef] [PubMed]
- Danovaro-Holliday, M.C.; Ortiz, C.; Cochi, S.; Ruiz-Matus, C. Electronic immunization registries in Latin America: Progress and lessons learned. Rev. Panam. Salud Publica 2014, 35, 453–457. [Google Scholar] [PubMed]
- Lim, S.S.; Stein, D.B.; Charrow, A.; Murray, C.J. Tracking progress towards universal childhood immunisation and the impact of global initiatives: A systematic analysis of three-dose diphtheria, tetanus, and pertussis immunisation coverage. Lancet 2008, 372, 2031–2046. [Google Scholar] [CrossRef]
- Henderson, R.H.; Sundaresan, T. Cluster sampling to assess immunization coverage: A review of experience with a simplified sampling method. Bull. World Health Organ. 1982, 60, 253–260. [Google Scholar] [PubMed]
- Brogan, D.; Flagg, E.W.; Deming, M.; Waldman, R. Increasing the accuracy of the Expanded Programme on Immunization’s cluster survey design. Ann. Epidemiol. 1994, 4, 302–311. [Google Scholar] [CrossRef]
- Vandelaer, J.; Bilous, J.; Nshimirimana, D. Reaching Every District (RED) approach: A way to improve immunization performance. Bull. World Health Organ. 2008, 86, A–B. [Google Scholar] [CrossRef] [PubMed]
- Mavimbe, J.C.; Braa, J.; Bjune, G. Assessing immunization data quality from routine reports in Mozambique. BMC Public Health 2005, 5, 108. [Google Scholar] [CrossRef] [PubMed]
- Weeks, R.M.; Svetlana, F.; Noorgoul, S.; Valentina, G. Improving the monitoring of immunization services in Kyrgyzstan. Health Policy Plan. 2000, 15, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Williamson, L.; Stoops, N.; Heywood, A. Developing a District Health Information System in South Africa: A social process or technical solution? Stud. Health Technol. Inform. 2001, 84, 773–777. [Google Scholar] [PubMed]

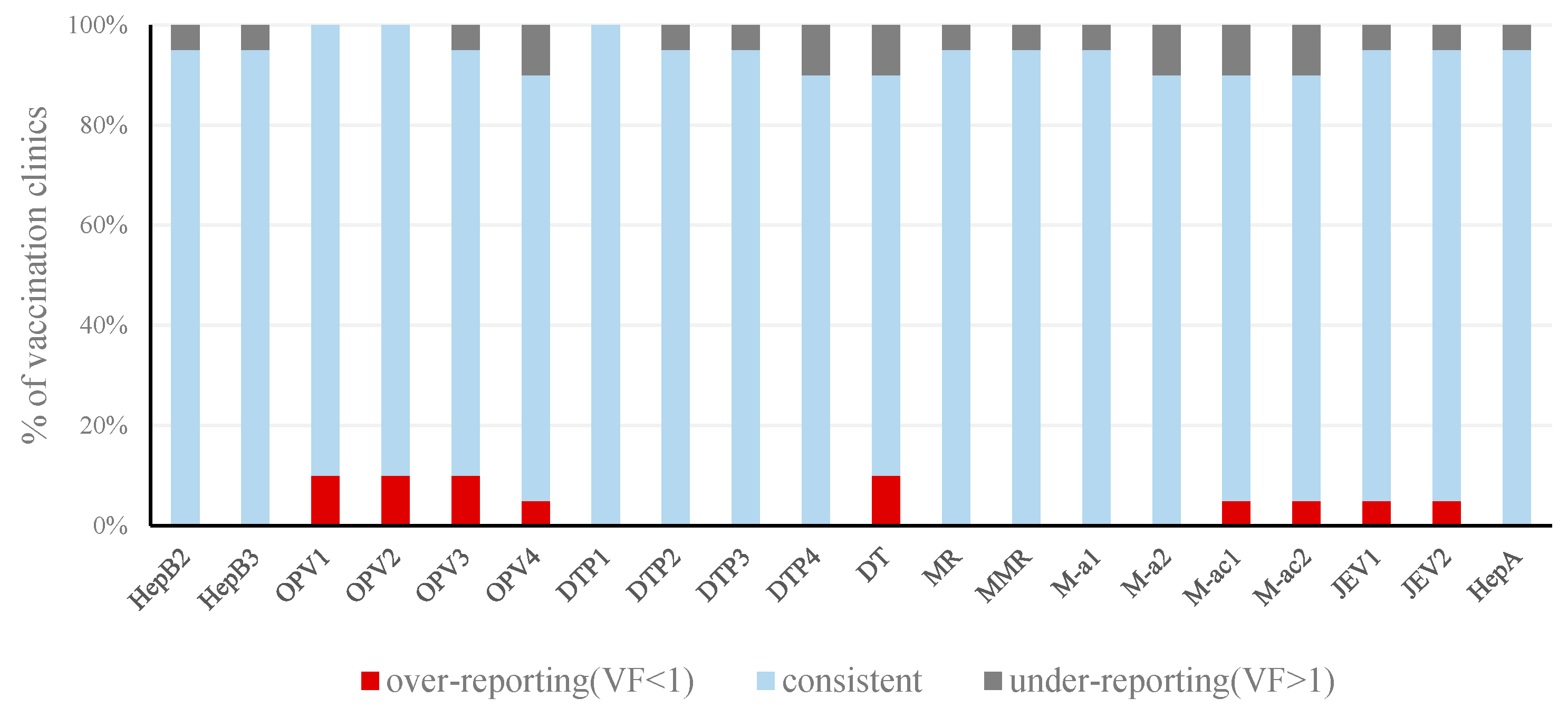
| Unit * | Weight | HepB2 | HepB3 | OPV1 | OPV2 | OPV3 | OPV4 | DTP1 | DTP2 | DTP3 | DTP4 | DT | MR | MMR | M-a1 | M-a2 | M-ac1 | M-ac2 | JEV1 | JEV2 | HepA | VF of Unit |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VC1 | 0.23 | 0.99 | 0.95 | 0.99 | 1.03 | 0.99 | 0.98 | 1.03 | 1.03 | 1.03 | 1.00 | 0.94 | 0.94 | 0.99 | 0.99 | 0.99 | 0.94 | 0.99 | 0.94 | 0.98 | 1.00 | 0.99 |
| VC2 | 0.12 | 0.97 | 0.97 | 1.01 | 1.01 | 1.01 | 0.99 | 1.01 | 1.00 | 1.00 | 1.00 | 0.94 | 0.92 | 0.94 | 1.00 | 0.96 | 1.00 | 0.91 | 0.97 | 0.96 | 0.87 | 0.97 |
| VC3 | 0.04 | 1.02 | 1.00 | 1.44 | 1.35 | 1.31 | 1.00 | 1.12 | 1.12 | 1.10 | 1.00 | 0.96 | 1.01 | 0.99 | 1.01 | 0.97 | 1.02 | 0.99 | 0.95 | 0.94 | 1.01 | 1.07 |
| VC4 | 0.04 | 0.99 | 0.93 | 0.98 | 1.01 | 0.94 | 0.98 | 1.00 | 0.95 | 1.01 | 0.98 | 0.99 | 1.02 | 0.93 | 0.99 | 1.00 | 0.99 | 1.00 | 0.87 | 0.94 | 0.98 | 0.97 |
| VC5 | 0.05 | 1.01 | 1.00 | 1.28 | 1.23 | 1.16 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.97 | 1.21 | 1.00 | 1.04 |
| VC6 | 0.02 | 1.13 | 0.69 | 0.93 | 0.91 | 0.70 | 0.24 | 0.92 | 0.77 | 0.69 | 0.39 | 0.20 | 0.52 | 0.32 | 0.59 | 0.36 | 0.55 | 0.14 | 0.50 | 0.47 | 0.38 | 0.57 |
| VC7 | 0.01 | 0.96 | 0.98 | 0.96 | 0.94 | 0.94 | 1.09 | 0.98 | 0.96 | 0.96 | 0.98 | 3.25 | 0.89 | 0.96 | 1.15 | 1.00 | 0.91 | 2.69 | 1.04 | 1.03 | 0.97 | 1.18 |
| VC8 | 0.01 | 1.02 | 1.00 | 1.02 | 1.00 | 1.00 | 1.03 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.02 | 1.02 | 0.98 | 1.00 | 1.05 | 0.83 | 1.00 | 0.94 | 0.98 | 0.99 |
| VC9 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| VC10 | 0.00 | 0.89 | 1.05 | 1.10 | 0.96 | 0.93 | 1.00 | 1.04 | 0.96 | 1.04 | 0.97 | 1.05 | 1.04 | 0.93 | 1.00 | 0.97 | 0.96 | 0.98 | 0.97 | 1.00 | 0.98 | 0.99 |
| VC11 | 0.06 | 1.02 | 0.99 | 1.07 | 1.05 | 1.03 | 1.00 | 1.01 | 1.01 | 1.00 | 1.01 | 0.95 | 1.09 | 1.00 | 1.00 | 1.01 | 1.00 | 0.96 | 1.28 | 1.29 | 1.01 | 1.04 |
| VC12 | 0.01 | 0.99 | 1.01 | 1.01 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.01 | 1.00 | 1.00 | 0.99 | 1.01 | 1.01 | 1.01 | 0.94 | 1.00 | 0.99 | 1.03 | 1.00 |
| VC13 | 0.03 | 1.10 | 0.99 | 1.01 | 1.01 | 1.01 | 0.99 | 1.00 | 1.01 | 1.00 | 1.01 | 1.03 | 1.00 | 1.01 | 1.00 | 1.01 | 1.00 | 1.07 | 1.01 | 1.01 | 1.01 | 1.01 |
| VC14 | 0.02 | 0.79 | 0.85 | 0.93 | 0.91 | 0.92 | 0.54 | 0.92 | 0.85 | 0.93 | 0.91 | 0.50 | 0.94 | 0.94 | 0.91 | 0.82 | 0.07 | 0.97 | 0.85 | 0.85 | 1.05 | 0.82 |
| VC15 | 0.03 | 0.99 | 1.00 | 1.00 | 0.98 | 1.00 | 0.98 | 0.98 | 1.01 | 0.98 | 1.00 | 1.01 | 1.06 | 1.00 | 0.99 | 1.00 | 1.01 | 0.97 | 0.99 | 0.99 | 0.99 | 1.00 |
| VC16 | 0.02 | 0.95 | 1.00 | 1.00 | 1.01 | 1.01 | 0.96 | 1.00 | 1.01 | 1.01 | 1.00 | 0.98 | 1.01 | 0.99 | 1.01 | 0.99 | 1.01 | 1.00 | 1.01 | 1.00 | 0.99 | 1.00 |
| VC17 | 0.05 | 1.02 | 1.03 | 1.01 | 1.01 | 1.02 | 1.29 | 1.00 | 1.00 | 1.01 | 0.78 | 1.20 | 1.02 | 1.06 | 1.00 | 1.01 | 1.19 | 1.10 | 1.04 | 1.19 | 1.10 | 1.05 |
| VC18 | 0.03 | 0.96 | 0.94 | 0.97 | 0.94 | 0.96 | 0.96 | 0.95 | 0.98 | 0.95 | 0.98 | 0.99 | 0.96 | 0.99 | 0.94 | 1.00 | 0.97 | 0.98 | 0.98 | 0.99 | 0.98 | 0.97 |
| VC19 | 0.06 | 1.00 | 1.00 | 1.00 | 1.00 | 1.01 | 1.00 | 1.01 | 1.01 | 0.99 | 1.00 | 1.00 | 1.01 | 0.99 | 1.00 | 1.00 | 1.02 | 1.00 | 1.04 | 1.00 | 1.00 | 1.00 |
| VC20 | 0.16 | 1.00 | 1.00 | 1.08 | 1.04 | 1.01 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.01 | 1.00 | 1.10 | 1.05 | 1.00 | 1.00 | 1.00 | 0.99 | 1.00 | 1.01 |
| District | - | 0.99 | 0.95 | 1.04 | 1.03 | 1.02 | 0.96 | 1.00 | 1.00 | 0.99 | 0.97 | 0.94 | 0.96 | 0.96 | 0.99 | 0.98 | 0.96 | 0.95 | 0.97 | 1.00 | 0.98 | 0.98 |
| Unit * | QI Score (%) | ||||
|---|---|---|---|---|---|
| Total | Recording Practices | Storage and Reporting Practices | Monitoring and Evaluation | Denominators | |
| VC1 | 86.36 | 85.71 | 75.00 | 100.00 | 100.00 |
| VC2 | 81.82 | 78.57 | 75.00 | 100.00 | 100.00 |
| VC3 | 50.00 | 57.14 | 25.00 | 50.00 | 50.00 |
| VC4 | 77.27 | 78.57 | 75.00 | 100.00 | 50.00 |
| VC5 | 86.36 | 85.71 | 75.00 | 100.00 | 100.00 |
| VC6 | 36.36 | 50.00 | 0.00 | 50.00 | 0.00 |
| VC7 | 81.82 | 85.71 | 50.00 | 100.00 | 100.00 |
| VC8 | 90.91 | 92.86 | 75.00 | 100.00 | 100.00 |
| VC9 | 77.27 | 85.71 | 25.00 | 100.00 | 100.00 |
| VC10 | 81.82 | 85.71 | 75.00 | 100.00 | 50.00 |
| VC11 | 95.45 | 100.00 | 75.00 | 100.00 | 100.00 |
| VC12 | 90.91 | 92.86 | 75.00 | 100.00 | 100.00 |
| VC13 | 90.91 | 92.86 | 75.00 | 100.00 | 100.00 |
| VC14 | 50.00 | 50.00 | 25.00 | 100.00 | 50.00 |
| VC15 | 59.09 | 71.43 | 25.00 | 50.00 | 50.00 |
| VC16 | 81.82 | 78.57 | 75.00 | 100.00 | 100.00 |
| VC17 | 86.36 | 85.71 | 75.00 | 100.00 | 100.00 |
| VC18 | 68.18 | 85.71 | 25.00 | 50.00 | 50.00 |
| VC19 | 68.18 | 71.43 | 50.00 | 100.00 | 50.00 |
| VC20 | 90.91 | 92.86 | 75.00 | 100.00 | 100.00 |
| Mean | 80.32 | 80.17 | 65.00 | 93.00 | 86.00 |
| Component (Number of Questions or Observations) | Number | Question or Observations | Correct Response Rate (%) |
|---|---|---|---|
| Recording Practices (14) | 1 | Are registers used for recording individual information about child immunization? | 100.00 |
| 2 | Can a child’s vaccination history be easily and rapidly retrieved in the registry? | 85.00 | |
| 3 | Did every person taking the child health card exercise get a perfect score for DTP1 < 1? | 80.00 | |
| 4 | Did every person taking the child health card exercise get a perfect score for DTP3 < 1? | 90.00 | |
| 5 | Did every person taking the child health card exercise get a perfect score for measles containing vaccine < 1? | 90.00 | |
| 6 | Was the correct vaccination given for every vaccination observed? | 60.00 | |
| 7 | Was the correct date to return given for every vaccination observed? | 55.00 | |
| 8 | Are the individual recording forms available for the entire audit year? | 100.00 | |
| 9 | Are vaccination staff aware of standard operating procedure and there forms to complete if there is a report of AEFI? | 60.00 | |
| 10 | Does the vaccination clinic use/maintain a ledger/stock control for vaccines? | 100.00 | |
| 11 | Is the ledger up-to-date in entries for DTP? | 50.00 | |
| 12 | Is the receipt of DTP in the ledger complete for the entries audit year? | 95.00 | |
| 13 | Is there a log of the receipt/issuing of syringes supplies? | 60.00 | |
| 14 | Does the vaccination clinic record vaccine batch number and expiry date? | 100.00 | |
| Monitoring and Evaluation (4) | 15 | Does the vaccination clinic monitor vaccine wastage? | 75.00 |
| 16 | Is there interaction with the community regarding immunization? | 0.00 | |
| 17 | Is there a mechanism in place to track vaccine doses that are due or track defaulters? | 70.00 | |
| 18 | Does the vaccination clinic monitor dropout rate? | 80.00 | |
| Storage and Reporting Practices (2) | 19 | Are all the vaccination reports available for the entire audit year? | 100.00 |
| 20 | Is there one location where reports and records before 2000 year concerning immunization data are stored appropriately? | 80.00 | |
| Denominators (2) | 21 | Does the vaccination clinic have a number of infants that they strive to vaccinate against DTP during a calendar year/reporting period/vaccination session? | 75.00 |
| 22 | Is the vaccination clinic aware of new births in the catchment area and attempts to follow up to ensure all newborns are immunized? | 80.00 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Zhang, X.; Li, Q.; Chen, Y. Auditing the Immunization Data Quality from Routine Reports in Shangyu District, East China. Int. J. Environ. Res. Public Health 2016, 13, 1158. https://doi.org/10.3390/ijerph13111158
Hu Y, Zhang X, Li Q, Chen Y. Auditing the Immunization Data Quality from Routine Reports in Shangyu District, East China. International Journal of Environmental Research and Public Health. 2016; 13(11):1158. https://doi.org/10.3390/ijerph13111158
Chicago/Turabian StyleHu, Yu, Xinpei Zhang, Qian Li, and Yaping Chen. 2016. "Auditing the Immunization Data Quality from Routine Reports in Shangyu District, East China" International Journal of Environmental Research and Public Health 13, no. 11: 1158. https://doi.org/10.3390/ijerph13111158





