Antenatal Dexamethasone Exposure in Preterm Infants Is Associated with Allergic Diseases and the Mental Development Index in Children
Abstract
:1. Introduction
2. Material and Methods
2.1. Measurement of Plasma Cytokine Levels Associated with Th Cell Subset Immunity Using the Magpix® My-System
2.2. Real-Time Quantitative RT-PCR Analysis of Th Cell-Related mRNA Expression
2.3. Statistical Analysis
3. Results
3.1. Dexamethasone Exposure Group Had a Higher Incidence of Allergic Diseases
3.2. Higher Plasma Levels of IL-5 and IL-10 Were Found in the Asthma and Allergic Rhinitis Group
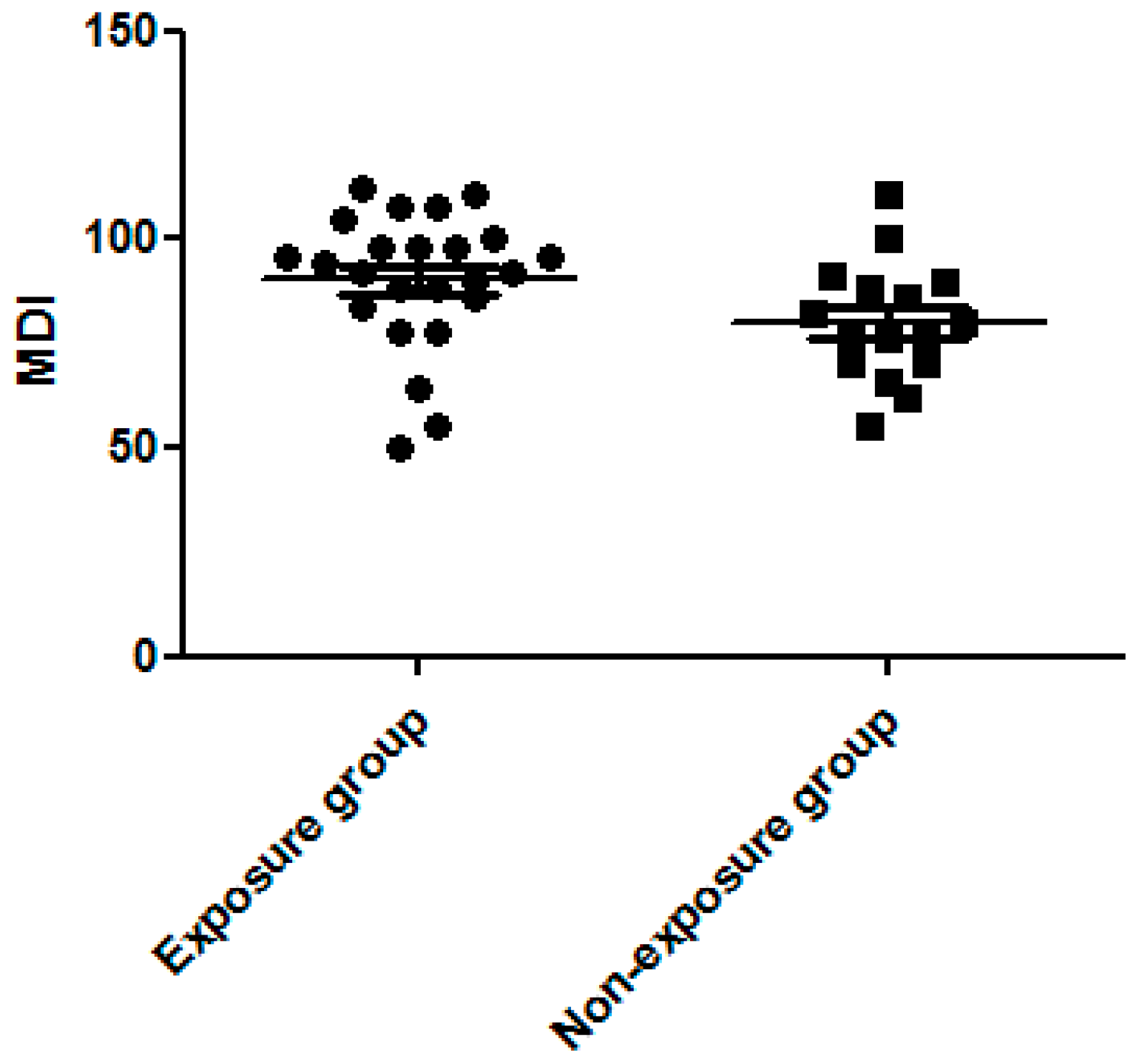
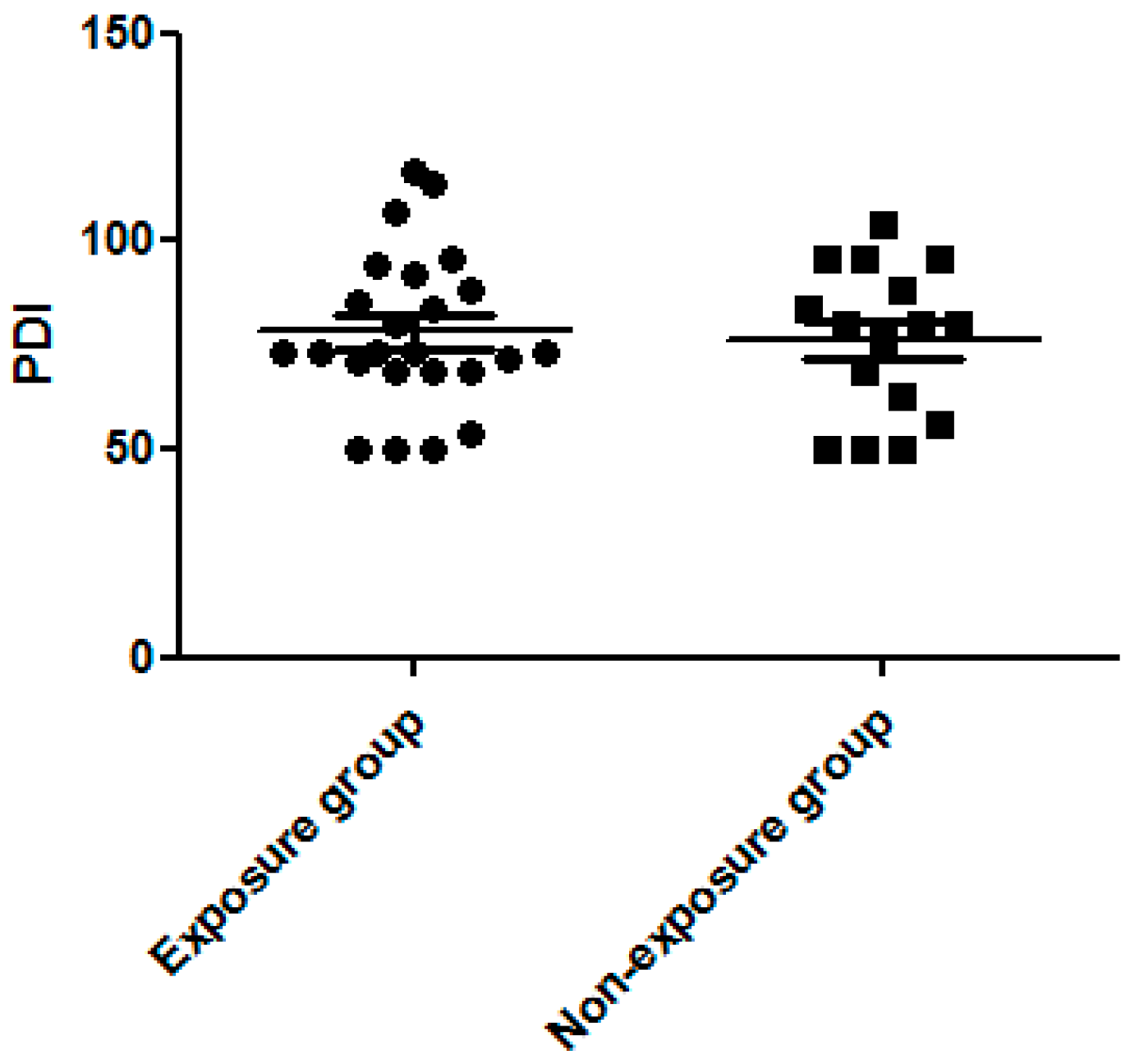
3.3. Dexamethasone Exposure Group Had a Higher MDI Score
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, C.A.; Wang, C.L.; Chuang, H.; Ou, C.Y.; Hsu, T.Y.; Yang, K.D. Prenatal prediction of infant atopy by maternal but not paternal total IgE levels. J. Allergy Clin. Immunol. 2003, 112, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Eder, W.; Ege, M.J.; von Mutius, E. The asthma epidemic. N. Engl. J. Med. 2006, 355, 2226–2235. [Google Scholar] [CrossRef] [PubMed]
- Ronmark, E.; Bjerg, A.; Perzanowski, M.; Platts-Mills, T.; Lundback, B. Major increase in allergic sensitization in schoolchildren from 1996 to 2006 in northern Sweden. J. Allergy Clin. Immunol. 2009, 124, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, H.; Simpson, A.; Palmer, C.N.; Bonnelykke, K.; McLean, I.; Mukhopadhyay, S.; Pipper, C.B.; Halkjaer, L.B.; Lipworth, B.; Hankinson, J.; et al. Gene-environment interaction in the onset of eczema in infancy: Filaggrin loss-of-function mutations enhanced by neonatal cat exposure. PLoS Med. 2008, 5, e131. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.W.; Pan, A.Y. Cumulative environmental changes, skewed antigen exposure, and the increase of allergy. Adv. Immunol. 2008, 98, 39–83. [Google Scholar] [PubMed]
- Putney, J.W. Capacitative calcium entry: From concept to molecules. Immunol. Rev. 2009, 231, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Matsubara, T.; Motohashi, T.; Sasai, K.; Nakachi, S.; Umezawa, Y.; Yabuta, K. Increased expression of Fc epsilon R2/CD23 on peripheral blood B lymphocytes and serum IgE levels in Kawasaki disease. Int. Arch. Allergy Appl. Immunol. 1991, 95, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.J.; Watson, L. Asthma and the hygiene hypothesis. N. Engl. J. Med. 2001, 344, 1643–1644. [Google Scholar] [PubMed]
- Riedler, J.; Braun-Fahrlander, C.; Eder, W.; Schreuer, M.; Waser, M.; Maisch, S.; Carr, D.; Schierl, R.; Nowak, D.; von Mutius, E.; et al. Exposure to farming in early life and development of asthma and allergy: A cross-sectional survey. Lancet 2001, 358, 1129–1133. [Google Scholar] [CrossRef]
- Cullinan, P.; Harris, J.M.; Newman Taylor, A.J.; Jones, M.; Taylor, P.; Dave, J.R.; Mills, P.; Moffat, S.A.; White, C.W.; Figg, J.K.; et al. Can early infection explain the sibling effect in adult atopy? Eur. Respir. J. 2003, 22, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Bach, J.F. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 2002, 347, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.D.; Ou, C.Y.; Hsu, T.Y.; Chang, J.C.; Chuang, H.; Liu, C.A.; Liang, H.M.; Kuo, H.C.; Chen, R.F.; Huang, E.Y. Interaction of maternal atopy, CTLA-4 gene polymorphism and gender on antenatal immunoglobulin E production. Clin. Exp. Allergy 2007, 37, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.T. The link between perinatal glucocorticoids exposure and psychiatric disorders. Pediatr. Res. 2011, 69, 19R–25R. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.T. Perinatal programming of neuropsychiatric disorders. J. Formos Med. Assoc. 2011, 110, 347–349. [Google Scholar] [CrossRef]
- Pole, J.D.; Mustard, C.A.; To, T.; Beyene, J.; Allen, A.C. Antenatal steroid therapy for fetal lung maturation: Is there an association with childhood asthma? J. Asthma 2009, 46, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Dietert, R.R.; Lee, J.E.; Olsen, J.; Fitch, K.; Marsh, J.A. Developmental immunotoxicity of dexamethasone: Comparison of fetal versus adult exposures. Toxicology 2003, 194, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.J.; Fanaroff, A.A. The preterm lung and airway: Past, present, and future. Pediatr. Neonatol. 2013, 54, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Abdel-Latif, M.; Kent, A.; NICUS Network. Antenatal steroid exposure and outcomes of very premature infants: A regional cohort study. Arch. Dis. Child. Fetal Neonatal Ed. 2014, 99, F14–F20. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.; Haglund, B.; Ewald, U.; Odlind, V.; Kieler, H. Short and long-term effects of antenatal corticosteroids assessed in a cohort of 7827 children born preterm. Acta Obstet. Gynecol. Scand. 2009, 88, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Husar, A.; Challis, J.R.; Dudenhausen, J.W.; Henrich, W.; Plagemann, A.; Sloboda, D.M. Growth restricting effects of a single course of antenatal betamethasone treatment and the role of human placental lactogen. Placenta 2013, 34, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.E.; Willan, A.R.; Hannah, M.E.; Ohlsson, A.; Kelly, E.N.; Matthews, S.G.; Saigal, S.; Asztalos, E.; Ross, S.; Delisle, M.F.; et al. Effect of antenatal corticosteroids on fetal growth and gestational age at birth. Obstet. Gynecol. 2012, 119, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Pole, J.D.; Mustard, C.A.; To, T.; Beyene, J.; Allen, A.C. Antenatal steroid therapy and childhood asthma: Is there a possible link? Med. Hypotheses 2008, 70, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Watterberg, K.L.; Shaffer, M.L.; Mishefske, M.J.; Leach, C.L.; Mammel, M.C.; Couser, R.J.; Abbasi, S.; Cole, C.H.; Aucott, S.W.; Thilo, E.H.; et al. Growth and neurodevelopmental outcomes after early low-dose hydrocortisone treatment in extremely low birth weight infants. Pediatrics 2007, 120, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Wilson-Costello, D.; Walsh, M.C.; Langer, J.C.; Guillet, R.; Laptook, A.R.; Stoll, B.J.; Shankaran, S.; Finer, N.N.; van Meurs, K.P.; Engle, W.A.; et al. Impact of postnatal corticosteroid use on neurodevelopment at 18 to 22 months’ adjusted age: Effects of dose, timing, and risk of bronchopulmonary dysplasia in extremely low birth weight infants. Pediatrics 2009, 123, e430–e437. [Google Scholar] [CrossRef] [PubMed]
- Tsakok, T.; Weinmayr, G.; Jaensch, A.; Strachan, D.P.; Williams, H.C.; Flohr, C.; ISAAC Phase 2 Study Group. Eczema and indoor environment: Lessons from the International Study of Asthma and Allergies in Childhood (ISAAC) Phase 2. Lancet 2015, 385 (Suppl. 1), S99. [Google Scholar] [CrossRef]
- Kuo, H.C.; Wang, C.L.; Liang, C.D.; Yu, H.R.; Huang, C.F.; Wang, L.; Hwang, K.P.; Yang, K.D. Association of lower eosinophil-related T helper 2 (Th2) cytokines with coronary artery lesions in Kawasaki disease. Pediatr. Allergy Immunol. 2009, 20, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shan, J.; Zhang, C.; Zhang, J.; Feng, L.; Li, S.; Li, Y. Peripheral blood T regulatory cell counts may not predict transplant rejection. BMC Immunol. 2010, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Cattarelli, D.; Chirico, G.; Simeoni, U. Renal effects of antenatally or postnatally administered steroids. Pediatr. Med. Chir. 2002, 24, 157–162. [Google Scholar] [PubMed]
- Morrison, J.L.; Botting, K.J.; Soo, P.S.; McGillick, E.V.; Hiscock, J.; Zhang, S.; McMillen, I.C.; Orgeig, S. Antenatal steroids and the IUGR fetus: Are exposure and physiological effects on the lung and cardiovascular system the same as in normally grown fetuses? J. Pregnancy 2012, 2012, 839656. [Google Scholar] [CrossRef] [PubMed]
- Annesi-Maesano, I.; Moreau, D.; Strachan, D. In utero and perinatal complications preceding asthma. Allergy 2001, 56, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Committee on Obstetric Practice. ACOG committee opnion: Antenatal corticosteroid therapy for fetal maturation. Obstet. Gynecol. 2002, 99, 871–873. [Google Scholar]
- Alexander, N.; Rosenlocher, F.; Stalder, T.; Linke, J.; Distler, W.; Morgner, J.; Kirschbaum, C. Impact of antenatal synthetic glucocorticoid exposure on endocrine stress reactivity in term-born children. J. Clin. Endocrinol. Metab. 2012, 97, 3538–3544. [Google Scholar] [CrossRef] [PubMed]
- Asztalos, E.; Willan, A.; Murphy, K.; Matthews, S.; Ohlsson, A.; Saigal, S.; Armson, A.; Kelly, E.; Delisle, M.F.; Gafni, A.; et al. Association between gestational age at birth, antenatal corticosteroids, and outcomes at 5 years: Multiple courses of antenatal corticosteroids for preterm birth study at 5 years of age (MACS-5). BMC Pregnancy Childbirth 2014, 14, 272. [Google Scholar] [CrossRef] [PubMed]
- Miracle, X.; Di Renzo, G.C.; Stark, A.; Fanaroff, A.; Carbonell-Estrany, X.; Saling, E.; Coordinators of World Associatin of Perinatal Medicine Prematurity Working Group. Guideline for the use of antenatal corticosteroids for fetal maturation. J. Perinat. Med. 2008, 36, 191–196. [Google Scholar] [CrossRef] [PubMed]
| Demographic Data | Exposure Group (n = 24) | Non-Exposure Group (n = 16) | p-Value |
|---|---|---|---|
| Male gender | 13 (54.2%) | 8 (50.0%) | 0.796 |
| Gestational age (week) | 29.63 ± 0.50 | 28.06 ± 0.73 | 0.074 |
| Birth body weight (gm) | 1210.83 ± 43.06 | 1010.00 ± 44.13 | 0.003 * |
| Age (years) | 3.89 ± 0.14 | 4.17 ± 0.20 | 0.242 |
| Allergic disease | 18 (75.0%) | 3 (18.8%) | <0.0001 * |
| Asthma | 10 (41.7%) | 0 (0.0%) | 0.003 * |
| Allergic rhinitis | 14 (58.3%) | 3 (18.8%) | 0.013 * |
| Atopic dermatitis | 6 (25.0%) | 1 (6.3%) | 0.126 |
| Cytokines | Exposure Group (n = 24) | Non-Exposure Group (n = 16) | p-Value |
|---|---|---|---|
| Interferon-gamma (pg/mL) | 3.20 ± 0.75 | 3.39 ± 0.65 | 0.856 |
| IL-2 (pg/mL) | 1.16 ± 0.22 | 0.68 ± 0.09 | 0.093 |
| IL-5 (pg/mL) | 0.67 ± 0.12 | 0.42 ± 0.06 | 0.137 |
| IL-10 (pg/mL) | 2.44 ± 0.67 | 1.19 ± 0.27 | 0.148 |
| IL-13 (pg/mL) | 0.34 ± 0.24 | 0.20 ± 0.13 | 0.647 |
| IL-17A (pg/mL) | 2.91 ± 1.08 | 2.01 ± 0.34 | 0.508 |
| IP-10 (pg/mL) | 240.46 ± 21.66 | 216.41 ± 26.19 | 0.485 |
| mRNA | Exposure Group (n = 24) | Non-Exposure Group (n = 16) | p-Value |
|---|---|---|---|
| T-bet | 0.74 ± 0.18 | 0.81 ± 0.36 | 0.857 |
| GATA-3 | 0.74 ± 0.13 | 0.97 ± 0.22 | 0.322 |
| Foxp-3 | 0.86 ± 0.12 | 0.96 ± 0.16 | 0.600 |
| RORγt | 0.83 ± 0.17 | 1.01 ± 0.25 | 0.537 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, W.-N.; Chen, C.-C.; Yu, H.-R.; Huang, L.-T.; Kuo, H.-C. Antenatal Dexamethasone Exposure in Preterm Infants Is Associated with Allergic Diseases and the Mental Development Index in Children. Int. J. Environ. Res. Public Health 2016, 13, 1206. https://doi.org/10.3390/ijerph13121206
Tseng W-N, Chen C-C, Yu H-R, Huang L-T, Kuo H-C. Antenatal Dexamethasone Exposure in Preterm Infants Is Associated with Allergic Diseases and the Mental Development Index in Children. International Journal of Environmental Research and Public Health. 2016; 13(12):1206. https://doi.org/10.3390/ijerph13121206
Chicago/Turabian StyleTseng, Wan-Ning, Chih-Cheng Chen, Hong-Ren Yu, Li-Tung Huang, and Ho-Chang Kuo. 2016. "Antenatal Dexamethasone Exposure in Preterm Infants Is Associated with Allergic Diseases and the Mental Development Index in Children" International Journal of Environmental Research and Public Health 13, no. 12: 1206. https://doi.org/10.3390/ijerph13121206







