Infection Rates by Dengue Virus in Mosquitoes and the Influence of Temperature May Be Related to Different Endemicity Patterns in Three Colombian Cities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Field Work
2.2. Experimental Infection of Mosquitoes
2.3. RNA Extraction
2.4. Molecular Detection of Virus in Mosquitoes
2.5. Detection of Dengue Virus and Serotype Identification in Field-Captured Mosquitoes
2.6. Epidemiological Data
2.7. Climate Data
2.8. Statistical Analyses
3. Results
3.1. Field-Captured Mosquitoes Support the Breteau Indices
3.2. Molecular Detection of DENV in Experimentally Infected Mosquitoes
3.3. Dengue Infection in Mosquitoes Is Not Related to Mosquito Density
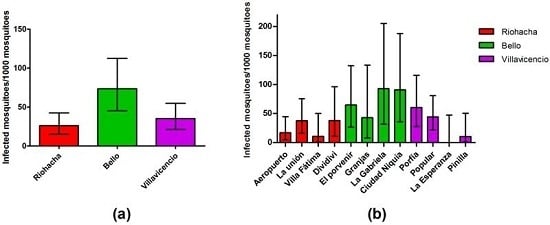
3.4. Infection Rate (IR) of Field-Captured Mosquitoes, Vector Index (VI) and Its Relation to Dengue Cases
3.5. Dengue Serotypes Circulating in Field Mosquitoes
3.6. Mosquitoes Infection Rate by Serotypes and Human Cases
3.7. Mosquitoes Infection Rate and Climatic Variables
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DENV | Dengue virus |
| WHO | World Health Organization |
| GLM | Generalized linear model |
| LRM | Logistic regression model |
| IR | Infection rates |
| VI | Vector index |
References
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- World Health Organization. Dengue Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition; World Health Organization: Geneva, Switzerland, 2009; p. 147. [Google Scholar]
- Arboleda, S.; Jaramillo-O, N.; Peterson, A.T. Spatial and temporal dynamics of Aedes aegypti larval sites in Bello, Colombia. J. Vector Ecol. 2012, 37, 37–48. [Google Scholar] [CrossRef] [PubMed]
- De Melo, D.P.; Scherrer, L.R.; Eiras, Á. Dengue fever occurrence and vector detection by larval survey, ovitrap and mosquitrap: A space-time clusters analysis. PLoS ONE 2012, 7, e42125. [Google Scholar] [CrossRef] [PubMed]
- Bowman, L.R.; Runge-Ranzinger, S.; McCall, P.J. Assessing the relationship between vector indices and dengue transmission: A systematic review of the evidence. PLoS Negl. Trop. Dis. 2014, 8, e2848. [Google Scholar] [CrossRef] [PubMed]
- Tun-Lin, W.; Kay, B.H.; Barnes, A.; Forsyth, S. Critical examination of Aedes aegypti indices: Correlations with abundance. Am. J. Trop. Med. Hyg. 1996, 54, 543–547. [Google Scholar] [PubMed]
- Focks, D. A Review of Entomological Samplings Methods and Indicators for Dengue Vectors; World Health Organization: Geneva, Switzerland, 2004; p. 38. [Google Scholar]
- Focks, D.; Barrera, R. Dengue Transmission Dynamics: Assessment and Implications for Control; World Health Organization: Geneva, Switzerland, 2007; p. 26. [Google Scholar]
- Guedes, D.R.; Cordeiro, M.T.; Melo-Santos, M.A.; Magalhaes, T.; Marques, E.; Regis, L.; Furtado, A.F.; Ayres, C.F. Patient-based dengue virus surveillance in Aedes aegypti from Recife, Brazil. J. Vector Borne Dis. 2010, 47, 67–75. [Google Scholar] [PubMed]
- Castro, M.G.; Nogueira, R.M.; Filippis, A.M.; Ferreira, A.A.; Lima, M.a.R.; Faria, N.R.; Nogueira, F.E.B.; Simões, J.B.; Nunes, P.C.; Sampaio, S.A.; et al. Dengue virus type 4 in Niterói, Rio de Janeiro: The role of molecular techniques in laboratory diagnosis and entomological surveillance. Mem. Inst. Oswaldo Cruz 2012, 107, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Victor, T.J. Detection of dengue viral infections in Aedes mosquitoes: An essential tool for epidemiological surveillance. Indian J. Med. Res. 2009, 129, 634–636. [Google Scholar] [PubMed]
- Chen, C.F.; Shu, P.Y.; Teng, H.J.; Su, C.L.; Wu, J.W.; Wang, J.H.; Lin, T.H.; Huang, J.H.; Wu, H.S. Screening of dengue virus in field-caught Aedes aegypti and Aedes albopictus (Diptera: Culicidae) by one-step SYBR green-based reverse transcriptase-polymerase chain reaction assay during 2004–2007 in Southern Taiwan. Vector Borne Zoonotic Dis. 2010, 10, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Liu-Helmersson, J.; Stenlund, H.; Wilder-Smith, A.; Rocklöv, J. Vectorial capacity of Aedes aegypti: Effects of temperature and implications for global dengue epidemic potential. PLoS ONE 2014, 9, e89783. [Google Scholar] [CrossRef] [PubMed]
- Liotta, D.J.; Cabanne, G.; Campos, R.; Tonon, S.A. Molecular detection of dengue viruses in field caught Aedes aegypti mosquitoes from northeastern Argentina. Rev. Latinoam. Microbiol. 2005, 47, 82–87. [Google Scholar] [PubMed]
- Pessanha, J.E.; Caiaffa, W.T.; Cecilio, A.B.; Iani, F.C.; Araujo, S.C.; Nascimento, J.C.; Kroon, E.G.; Proietti, F.A.; Arias, J.R. Cocirculation of two dengue virus serotypes in individual and pooled samples of Aedes aegypti and Aedes albopictus larvae. Rev. Soc. Bras. Med. Trop. 2011, 44, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.S.; Calisher, C.H.; Gubler, D.J.; Chang, G.J.; Vorndam, A.V. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J. Clin. Microbiol. 1992, 30, 545–551. [Google Scholar] [PubMed]
- Seah, C.L.; Chow, V.T.; Chan, Y.C. Semi-nested PCR using NS3 primers for the detection and typing of dengue viruses in clinical serum specimens. Clin. Diagn. Virol. 1995, 4, 113–120. [Google Scholar] [CrossRef]
- Shu, P.Y.; Chang, S.F.; Kuo, Y.C.; Yueh, Y.Y.; Chien, L.J.; Sue, C.L.; Lin, T.H.; Huang, J.H. Development of group- and serotype-specific one-step SYBR green I-based real-time reverse transcription-PCR assay for dengue virus. J. Clin. Microbiol. 2003, 41, 2408–2416. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.W.; Russell, B.J.; Lanciotti, R.S. Serotype-specific detection of dengue viruses in a fourplex real-time reverse transcriptase PCR assay. J. Clin. Microbiol. 2005, 43, 4977–4983. [Google Scholar] [CrossRef] [PubMed]
- Chien, L.J.; Liao, T.L.; Shu, P.Y.; Huang, J.H.; Gubler, D.J.; Chang, G.J. Development of real-time reverse transcriptase PCR assays to detect and serotype dengue viruses. J. Clin. Microbiol. 2006, 44, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Gurukumar, K.R.; Priyadarshini, D.; Patil, J.A.; Bhagat, A.; Singh, A.; Shah, P.S.; Cecilia, D. Development of real time PCR for detection and quantitation of dengue viruses. Virol. J. 2009, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Leparc-Goffart, I.; Baragatti, M.; Temmam, S.; Tuiskunen, A.; Moureau, G.; Charrel, R.; de Lamballerie, X. Development and validation of real-time one-step reverse transcription-PCR for the detection and typing of dengue viruses. J. Clin. Virol. 2009, 45, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Waggoner, J.J.; Abeynayake, J.; Sahoo, M.K.; Gresh, L.; Tellez, Y.; Gonzalez, K.; Ballesteros, G.; Pierro, A.M.; Gaibani, P.; Guo, F.P.; et al. Single-reaction, multiplex, real-time RT-PCR for the detection, quantitation, and serotyping of dengue viruses. PLoS Negl. Trop Dis. 2013, 7, e2116. [Google Scholar] [CrossRef] [PubMed]
- Pankhong, P.; Siriprasertkul, W.; Patpoparn, S.; Srisuphanunt, M.; Rojanapremsuk, J.; Sithiprasasna, R.; Coleman, R.E.; Nisalak, A.; Endy, T.P.; Attatippaholkun, M.K.; et al. Molecular serotyping of dengue viruses in field-caught Aedes mosquitos by in-house RNA extraction/RT-PCR reagent kits. Southeast Asian J. Trop Med. Public Health 2002, 33 (Suppl. 3), 139–144. [Google Scholar] [PubMed]
- Morin, C.W.; Comrie, A.C.; Ernst, K. Climate and dengue transmission: Evidence and implications. Environ Health Perspect 2013, 121, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Macoris, M.L.; Galvani, K.C.; Andrighetti, M.T.; Wanderley, D.M. Assessing the effects of temperature on the population of Aedes aegypti, the vector of dengue. Epidemiol. Infect. 2009, 137, 1188–1202. [Google Scholar] [CrossRef] [PubMed]
- Rueda, L.M.; Patel, K.J.; Axtell, R.C.; Stinner, R.E. Temperature-dependent development and survival rates of Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 1990, 27, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Barrera, R.; Amador, M.; MacKay, A.J. Population dynamics of Aedes aegypti and dengue as influenced by weather and human behavior in San Juan, Puerto Rico. PLoS Negl. Trop Dis. 2011, 5, e1378. [Google Scholar] [CrossRef] [PubMed]
- Christophers, S.R. Aedes aegypti (L.), the Yellow Fever Mosquito; Its Life History, Bionomics, and Structure; Cambridge University Press: New York, NY, USA, 1960; p. 738. [Google Scholar]
- Parker, A.H. The effect of a difference in temperature and humidity on certain reactions of female Aedes aegypti. Bull. Entomol. Res. 1952, 43, 221–229. [Google Scholar] [CrossRef]
- Jetten, T.H.; Focks, D.A. Potential changes in the distribution of dengue transmission under climate warming. Am. J. Trop. Med. Hyg. 1997, 57, 285–297. [Google Scholar] [PubMed]
- Reiter, P. Climate change and mosquito-borne disease. Environ. Health Perspect. 2001, 109 (Suppl. 1), 141–161. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.; Johansson, M.A. The incubation periods of dengue viruses. PLoS ONE 2012, 7, e50972. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, L.; Paaijmans, K.P.; Fansiri, T.; Carrington, L.B.; Kramer, L.D.; Thomas, M.B.; Scott, T.W. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc. Natl. Acad. Sci. USA 2011, 108, 7460–7465. [Google Scholar] [CrossRef] [PubMed]
- Azil, A.H.; Long, S.A.; Ritchie, S.A.; Williams, C.R. The development of predictive tools for pre-emptive dengue vector control: A study of Aedes aegypti abundance and meteorological variables in North Queensland, Australia. Trop. Med. Int. Health 2010, 15, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Usme-Ciro, J.A.; Mendez, J.A.; Tenorio, A.; Rey, G.J.; Domingo, C.; Gallego-Gomez, J.C. Simultaneous circulation of genotypes I and III of dengue virus 3 in Colombia. Virol. J. 2008, 5, 101. [Google Scholar] [CrossRef] [PubMed]
- Vega, L.R. Informe Final del Evento Dengue, año 2012; Instituto Nacional de Salud (INS): Bogotá, Colombia, 2013. [Google Scholar]
- Mercado-Reyes, M. Informe del Evento Dengue, año 2013; Instituto Nacional de Salud (INS): Bogotá, Colombia, 2014. [Google Scholar]
- MinSalud. Situación Actual de Dengue a Semana 12 de 2013. Periodo de Análisis: 2008–2013; Ministerio de Salud y Protección Social: Bogotá, Colombia, 2013. [Google Scholar]
- DANE. Censo General 2005; Departamento Administrativo Nacional de Estadística: Bogotá, Colombia, 2010. [Google Scholar]
- Rueda, L.M. Pictorial keys for the identification of mosquitoes (Diptera: Culicidae) associated with dengue virus transmission. Zootaxa 2004, 589, 1–60. [Google Scholar]
- Medina, F.; Medina, J.F.; Colón, C.; Vergne, E.; Santiago, G.A.; Muñoz-Jordán, J.L. Dengue virus: Isolation, propagation, quantification, and storage. Curr. Protoc. Microbiol. 2012. [Google Scholar] [CrossRef]
- Chutinimitkul, S.; Payungporn, S.; Theamboonlers, A.; Poovorawan, Y. Dengue typing assay based on real-time PCR using SYBR green I. J. Virol. Methods 2005, 129, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Seah, C.L.; Chow, V.T.; Tan, H.C.; Can, Y.C. Rapid, single-step RT-PCR typing of dengue viruses using five NS3 gene primers. J. Virol. Methods 1995, 51, 193–200. [Google Scholar] [CrossRef]
- INS. Available online: http://www.ins.gov.co/lineas-de-accion/Subdireccion-Vigilancia/sivigila/Paginas/vigilancia-rutinaria.aspx (accessed on 20 May 2014).
- Gu, W.; Lampman, R.; Novak, R.J. Assessment of arbovirus vector infection rates using variable size pooling. Med. Vet. Entomol. 2004, 18, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.G.; Barker, C.M.; Moore, C.G.; Pape, W.J.; Eisen, L. Seasonal patterns for entomological measures of risk for exposure to Culex vectors and West Nile virus in relation to human disease cases in Northeastern Colorado. J. Med. Entomol. 2009, 46, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- Gujral, I.B.; Zielinski-Gutierrez, E.C.; LeBailly, A.; Nasci, R. Behavioral risks for West Nile virus disease, Northern Colorado, 2003. Emerg. Infect. Dis. 2007, 13, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. West Nile Virus in the United States: Guidelines for Surveillance, Prevention, and Control; CDC: Fort Collins, CO, USA, 2013.
- Scott, T.W.; Morrison, A.C. Aedes aegypti density and the risk of dengue virus transmission. In Ecological Aspects for Application of Genetically Modified Mosquitoes; Takken, W., Scott, T.W., Eds.; FRONTIS: Dordrecht, The Netherlands, 2003; pp. 187–206. [Google Scholar]
- Scott, T.W.; Morrison, A.C. Vector dynamics and transmission of dengue virus: Implications for dengue surveillance and prevention strategies: Vector dynamics and dengue prevention. Curr. Top. Microbiol. Immunol. 2010, 338, 115–128. [Google Scholar] [PubMed]
- Tun-Lin, W.; Burkot, T.R.; Kay, B.H. Effects of temperature and larval diet on development rates and survival of the dengue vector Aedes aegypti in North Queensland, Australia. Med. Vet. Entomol. 2000, 14, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Delatte, H.; Gimonneau, G.; Triboire, A.; Fontenille, D. Influence of temperature on immature development, survival, longevity, fecundity, and gonotrophic cycles of Aedes albopictus, vector of chikungunya and dengue in the Indian Ocean. J. Med. Entomol. 2009, 46, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Farjana, T.; Tuno, N.; Higa, Y. Effects of temperature and diet on development and interspecies competition in Aedes aegypti and Aedes albopictus. Med. Vet. Entomol. 2012, 26, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Couret, J.; Dotson, E.; Benedict, M.Q. Temperature, larval diet, and density effects on development rate and survival of Aedes aegypti (Diptera: Culicidae). PLoS ONE 2014, 9, e87468. [Google Scholar] [CrossRef] [PubMed]
- Méndez, F.; Barreto, M.; Arias, J.F.; Rengifo, G.; Muñoz, J.; Burbano, M.E.; Parra, B. Human and mosquito infections by dengue viruses during and after epidemics in a dengue-endemic region of Colombia. Am. J. Trop. Med. Hyg. 2006, 74, 678–683. [Google Scholar] [PubMed]
- Chow, V.T.; Chan, Y.C.; Yong, R.; Lee, K.M.; Lim, L.K.; Chung, Y.K.; Lam-Phua, S.G.; Tan, B.T. Monitoring of dengue viruses in field-caught Aedes aegypti and Aedes albopictus mosquitoes by a type-specific polymerase chain reaction and cycle sequencing. Am. J. Trop. Med. Hyg. 1998, 58, 578–586. [Google Scholar] [PubMed]
- Grange, L.; Simon-Loriere, E.; Sakuntabhai, A.; Gresh, L.; Paul, R.; Harris, E. Epidemiological risk factors associated with high global frequency of inapparent dengue virus infections. Front. Immunol. 2014, 5, 280. [Google Scholar] [CrossRef] [PubMed]
- Herrero, L.J.; Zakhary, A.; Gahan, M.E.; Nelson, M.A.; Herring, B.L.; Hapel, A.J.; Keller, P.A.; Obeysekera, M.; Chen, W.; Sheng, K.C.; et al. Dengue virus therapeutic intervention strategies based on viral, vector and host factors involved in disease pathogenesis. Pharmacol. Ther. 2013, 137, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yang, S.; Yen, H.; Christakos, G. A spatio-temporal climate-based model of early dengue fever warning in Southern Taiwan. Stoch. Environ. Res. Risk Assess. 2011, 25, 485–494. [Google Scholar] [CrossRef]
- Kramer, L.D.; Hardy, J.L.; Presser, S.B. Effect of temperature of extrinsic incubation on the vector competence of Culex tarsalis for western equine encephalomyelitis virus. Am. J. Trop. Med. Hyg. 1983, 32, 1130–1139. [Google Scholar] [PubMed]
- Turell, M.J. Effect of environmental temperature on the vector competence of Aedes taeniorhynchus for RIFT valley fever and venezuelan equine encephalitis viruses. Am. J. Trop. Med. Hyg. 1993, 49, 672–676. [Google Scholar] [PubMed]
- Kay, B.H.; Jennings, C.D. Enhancement or modulation of the vector competence of Ochlerotatus vigilax (Diptera: Culicidae) for ross river virus by temperature. J. Med. Entomol. 2002, 39, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, C.J.; Reiskind, M.H.; Pesko, K.N.; Greene, K.E.; Lounibos, L.P. Larval environmental temperature and the susceptibility of Aedes albopictus skuse (Diptera: Culicidae) to chikungunya virus. Vector Borne Zoonotic Dis. 2010, 10, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sheng, J.; Plevka, P.; Kuhn, R.J.; Diamond, M.S.; Rossmann, M.G. Dengue structure differs at the temperatures of its human and mosquito hosts. Proc. Natl. Acad. Sci. USA 2013, 110, 6795–6799. [Google Scholar] [CrossRef] [PubMed]
- Rey, F.A. Dengue virus: Two hosts, two structures. Nature 2013, 497, 443–444. [Google Scholar] [CrossRef] [PubMed]
- Adelman, Z.N.; Anderson, M.A.; Wiley, M.R.; Murreddu, M.G.; Samuel, G.H.; Morazzani, E.M.; Myles, K.M. Cooler temperatures destabilize RNA interference and increase susceptibility of disease vector mosquitoes to viral infection. PLoS Negl. Trop. Dis. 2013, 7, e2239. [Google Scholar] [CrossRef] [PubMed]
- Goindin, D.; Delannay, C.; Ramdini, C.; Gustave, J.; Fouque, F. Parity and longevity of Aedes aegypti according to temperatures in controlled conditions and consequences on dengue transmission risks. PLoS ONE 2015, 10, e0135489. [Google Scholar] [CrossRef] [PubMed]
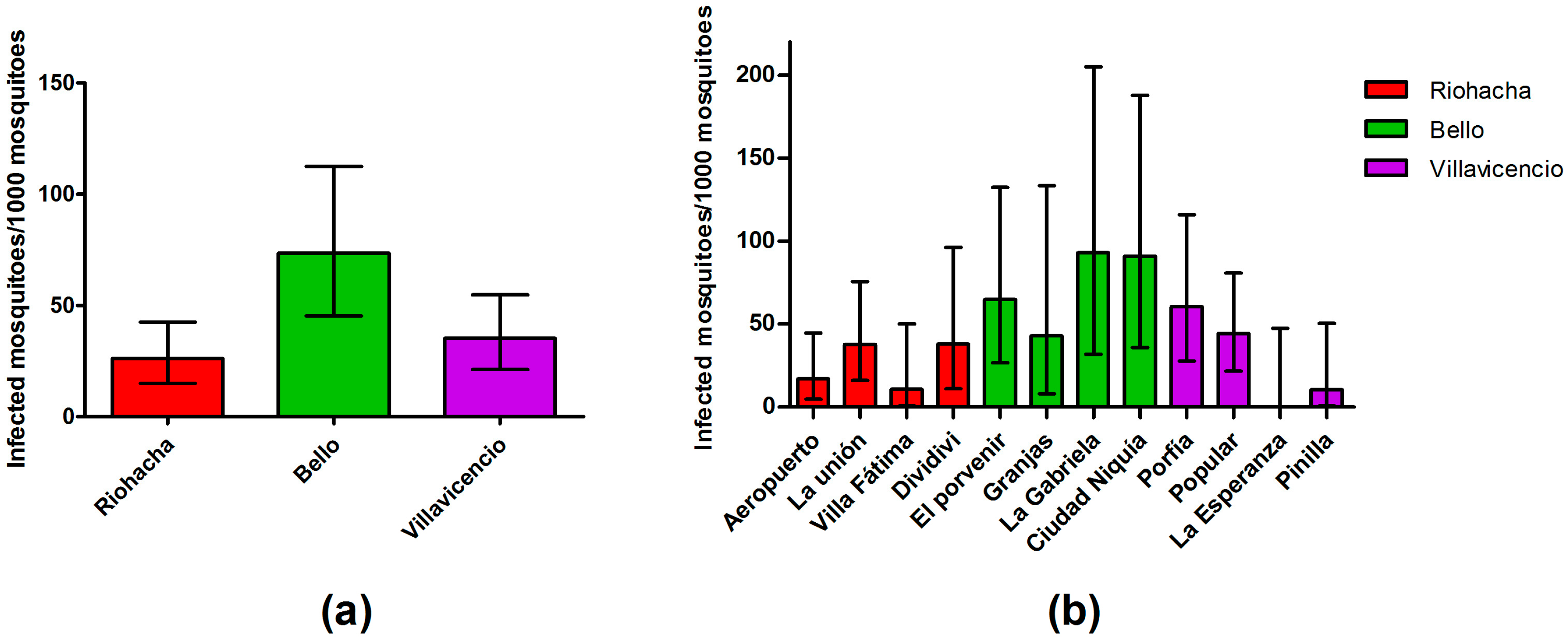


| City | Neighborhood | Females Captured | Number of Pools | Positive Pools (%) |
|---|---|---|---|---|
| Riohacha | Aeropuerto | 175 | 44 | 3 (6.82) |
| La Unión | 167 | 46 | 6 (13.04) | |
| Villa Fátima | 90 | 23 | 1 (4.35) | |
| Dividivi | 77 | 22 | 3 (13.64) | |
| Bello | El Porvenir | 77 | 24 | 5 (20.83) |
| Granjas | 47 | 20 | 2 (10) | |
| La Gabriela | 43 | 27 | 4 (14.81) | |
| Ciudad Niquía | 57 | 24 | 5 (20.83) | |
| Villavicencio | Porfía | 128 | 40 | 7 (17.5) |
| Popular | 185 | 58 | 8 (13.79) | |
| La Esperanza | 70 | 21 | 0 (0) | |
| Pinilla | 94 | 24 | 1 (4.17) | |
| Total | 1210 | 373 | 45 (12.06) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña-García, V.H.; Triana-Chávez, O.; Mejía-Jaramillo, A.M.; Díaz, F.J.; Gómez-Palacio, A.; Arboleda-Sánchez, S. Infection Rates by Dengue Virus in Mosquitoes and the Influence of Temperature May Be Related to Different Endemicity Patterns in Three Colombian Cities. Int. J. Environ. Res. Public Health 2016, 13, 734. https://doi.org/10.3390/ijerph13070734
Peña-García VH, Triana-Chávez O, Mejía-Jaramillo AM, Díaz FJ, Gómez-Palacio A, Arboleda-Sánchez S. Infection Rates by Dengue Virus in Mosquitoes and the Influence of Temperature May Be Related to Different Endemicity Patterns in Three Colombian Cities. International Journal of Environmental Research and Public Health. 2016; 13(7):734. https://doi.org/10.3390/ijerph13070734
Chicago/Turabian StylePeña-García, Víctor Hugo, Omar Triana-Chávez, Ana María Mejía-Jaramillo, Francisco J. Díaz, Andrés Gómez-Palacio, and Sair Arboleda-Sánchez. 2016. "Infection Rates by Dengue Virus in Mosquitoes and the Influence of Temperature May Be Related to Different Endemicity Patterns in Three Colombian Cities" International Journal of Environmental Research and Public Health 13, no. 7: 734. https://doi.org/10.3390/ijerph13070734