Essential Oil of Japanese Cedar (Cryptomeria japonica) Wood Increases Salivary Dehydroepiandrosterone Sulfate Levels after Monotonous Work
Abstract
:1. Introduction
2. Methods
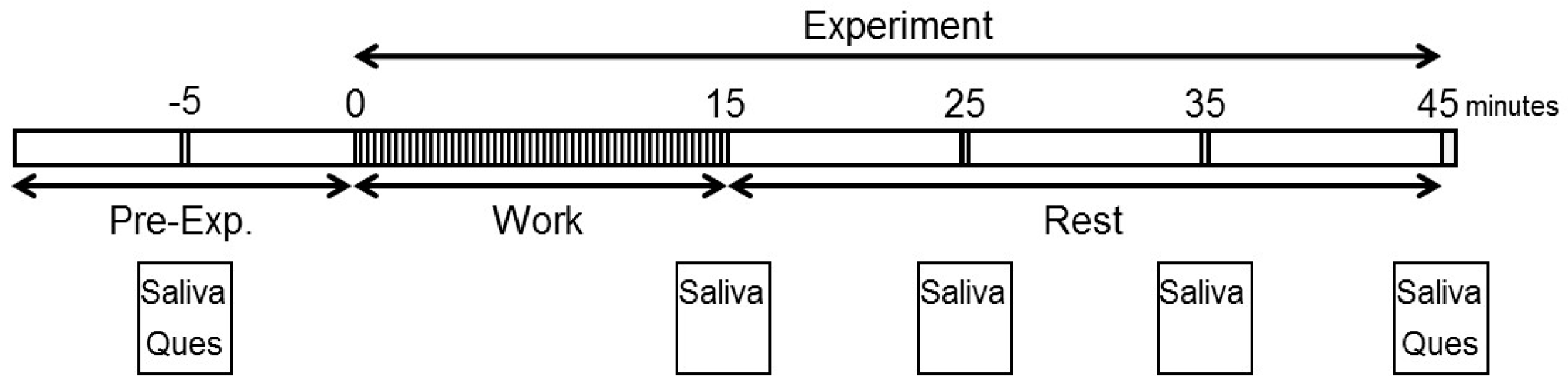
2.1. Participants and Experimental Design
2.2. Preparation of the Essential Oil and Sample for Use with a Diffuser
2.3. Arithmetic Work as Monotonous Work
2.4. Subjective Assessment
2.5. Salivary Stress Marker Assay
2.6. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.7. Statistical Analysis
3. Results
3.1. Constituent Analysis of Japanese Cedar Wood Essential Oil in the Experiment Room
3.2. Analysis of Subjective Assessments
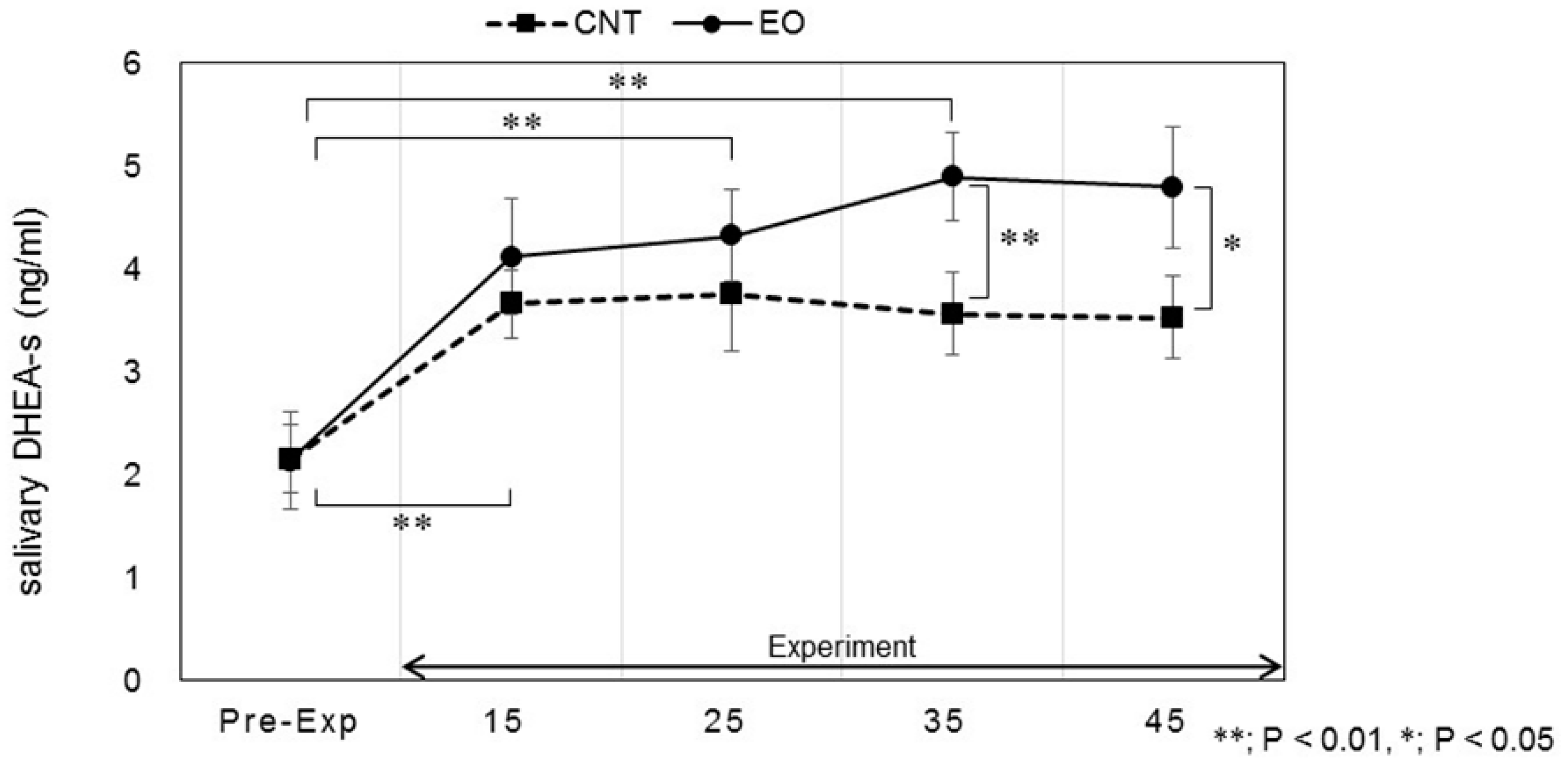
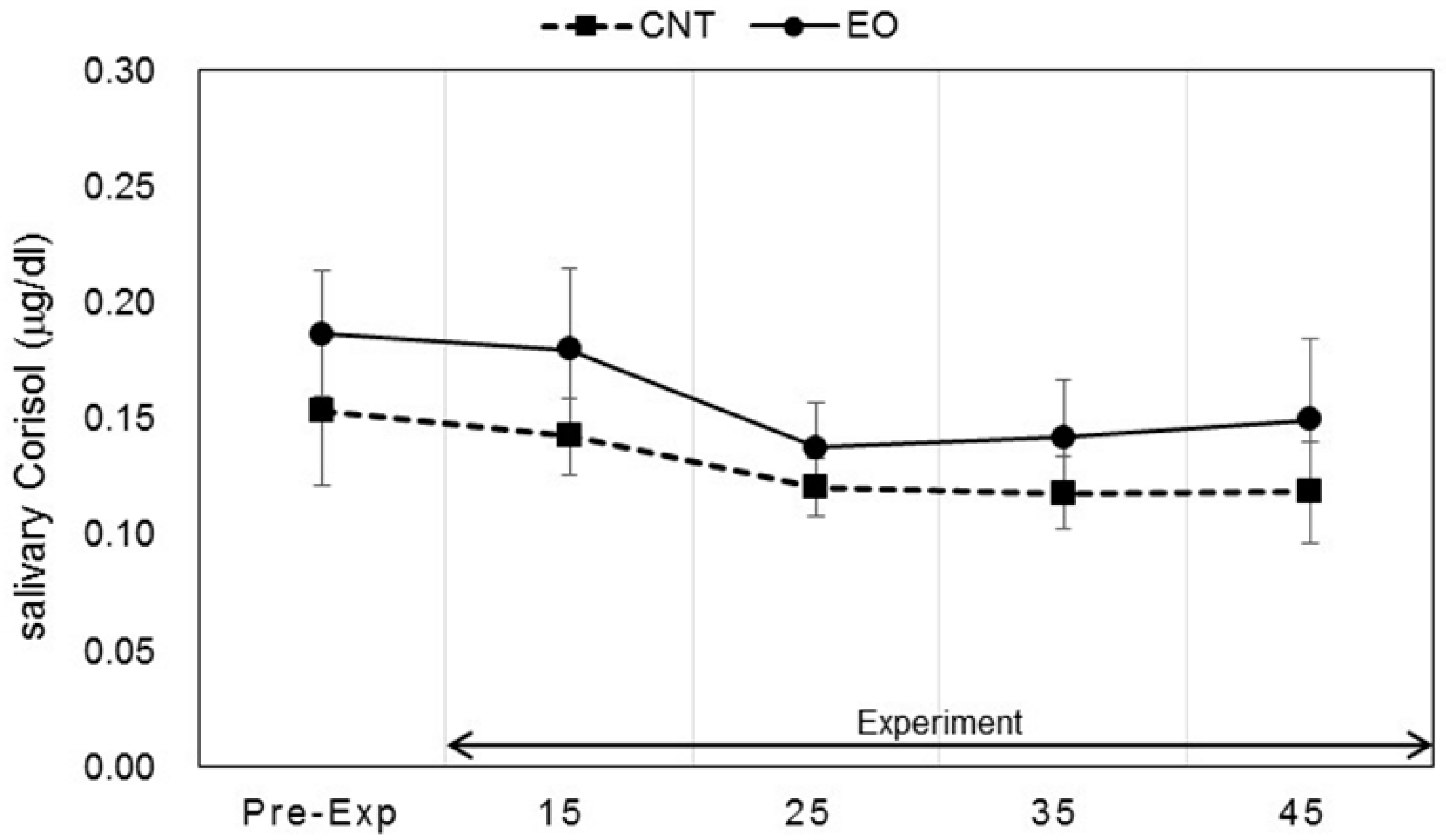
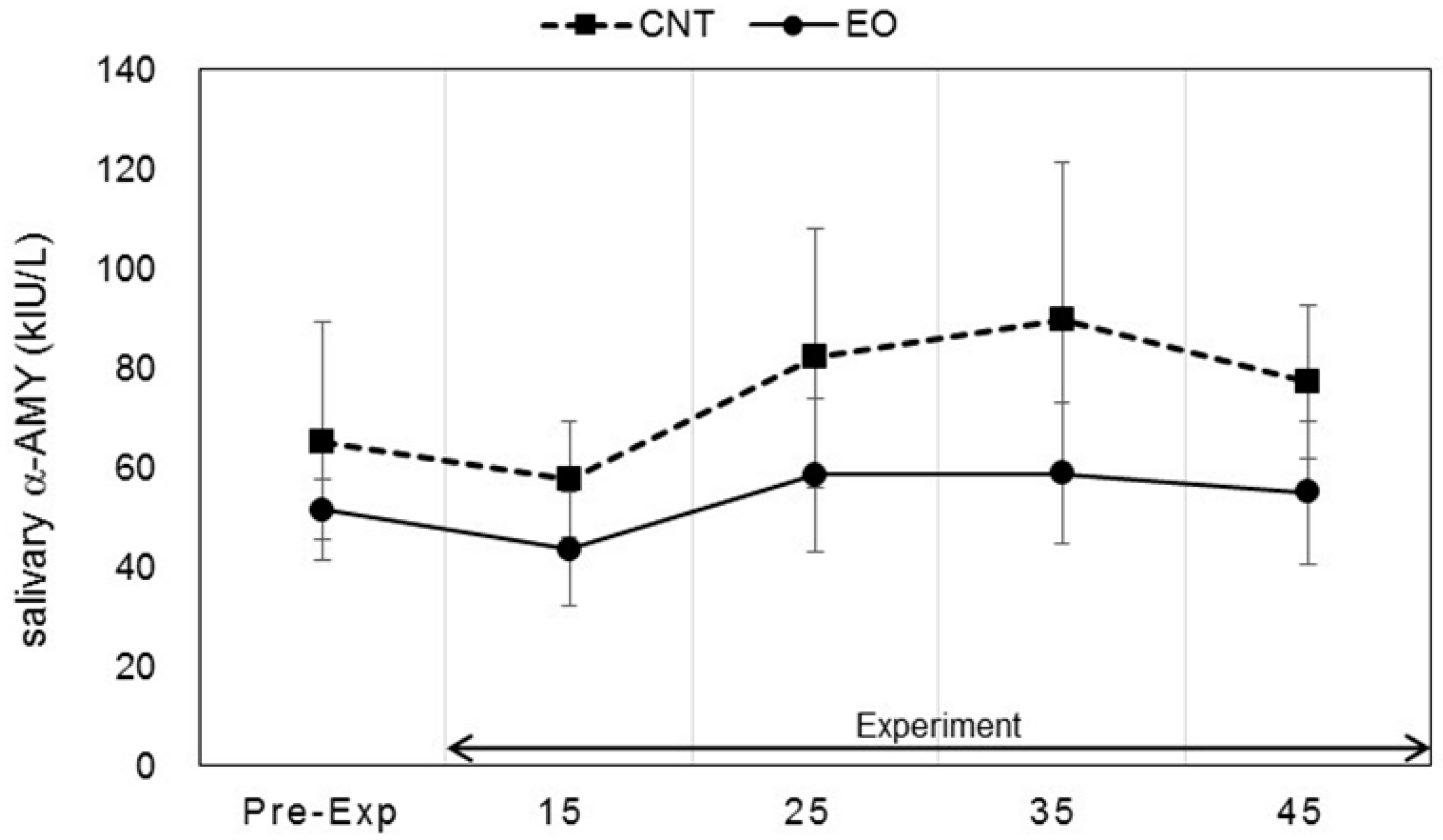
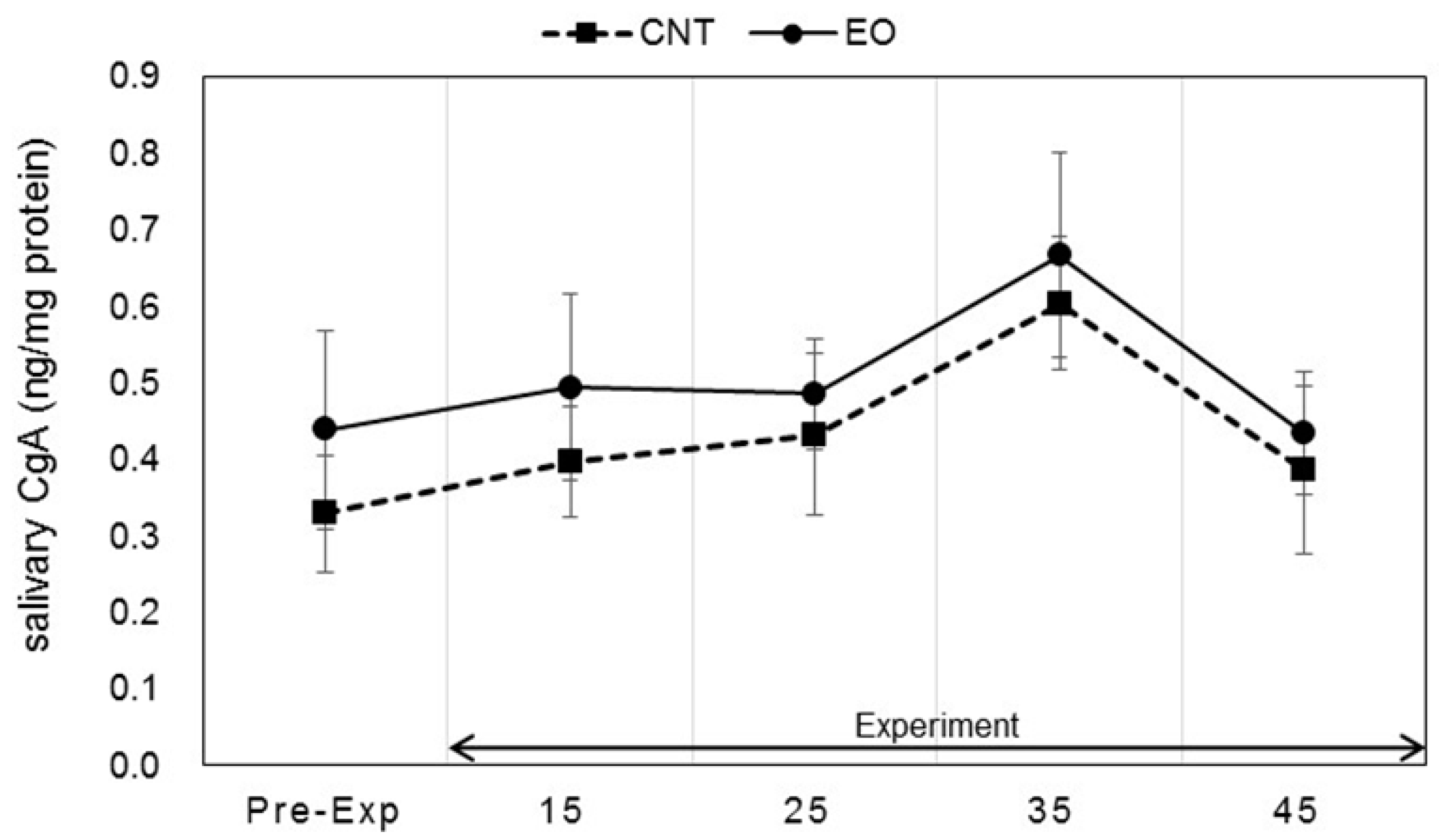
3.3. Analysis of Salivary Stress Markers
3.4. Arithmetic Work as a Monotonous Task
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yamamoto, K.; Okazaki, A.; Ohmori, S. The relationship between psychosocial stress, age, BMI, CRP, lifestyle, and the metabolic syndrome in apparently healthy subjects. J. Physiol. Anthropol. 2011, 3, 15–22. [Google Scholar] [CrossRef]
- Eriksson, A.K.; van den Donk, M.; Hilding, A.; Östenson, C.G. Work stress, sense of coherence, and risk of type 2 diabetes in a prospective study of middle-aged Swedish men and women. Diabetes Care 2013, 36, 2683–2689. [Google Scholar] [CrossRef] [PubMed]
- Luckhaupt, S.E.; Cohen, M.A.; Calvert, G.M. Prevalence of obesity among U.S. workers and associations with occupational factors. Am. J. Prev. Med. 2014, 46, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, K.; Cleal, B.; Clausen, T.; Anderson, L.L. Work, diabetes and obesity: A seven year follow-up study among Danish health care workers. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Kivimäki, M.; Kawachi, I. Work stress as a risk factor for cardiovascular disease. Curr. Cardiol. Rep. 2015, 17. [Google Scholar] [CrossRef] [PubMed]
- Halsam, C.; Atkinson, S.; Brown, S.S.; Halsam, R.A. Anxiety and depression in the workplace: Effects on the individual and organization (a focus group investigation). J. Affect. Disord. 2005, 88, 209–215. [Google Scholar]
- Ngoundo-Mbongue, T.B.; Niezborala, M.; Sulem, P.; Briant-Vincens, D.; Bancarel, Y.; Jansou, P.; Chastan, E.; Montastruc, J.L.; Lapeyre-Mestre, M. Psychoactive drug consumption: Performance-enhancing behavior and pharmacodependence in workers. Pharmacoepidemiol. Drug Saf. 2005, 14, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, H.G.; Kessler, R.C.; Kelley, D.; Ben-Hamadi, R.; Joish, V.N.; Greenberg, P.E. Employer burden of mild, moderate, and severe major depressive disorder: Mental health services utilization and costs, and work performance. Depress. Anxiety 2010, 27, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Dobetsberger, C.; Buchbauer, G. Actions of essential oils on the central nervous system: An updated review. Flavour Frag. J. 2011, 26, 300–316. [Google Scholar] [CrossRef]
- Angelucci, F.L.; Silva, V.V.; Pizzol, C.D.; Spir, L.G.; Praes, C.E.O.; Maibach, H. Physiological effects of olfactory stimuli inhalation in humans: An overview. Int. J. Cosmet. Sci. 2014, 36, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.M.; Shen, C.W. Aromatherapy benefits autonomic nervous system regulation for elementary school faculty in Taiwan. Evid. Base Complement. Altern. Med. 2011. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Fang, S.H.; Fang, L. The effects of aromatherapy in relieving symptoms related to job stress among nurses. Int. J. Nurs. Pract. 2015, 21, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Warm, J.S.; Dember, W.N.; Parasuraman, R. Effects of olfactory stimulation on performance and stress in a visual sustained attention task. J. Soc. Cosmet. Chem. 1991, 42, 199–210. [Google Scholar]
- Gould, A.; Martin, G.N. “A good odour to breathe”? The effect of pleasant ambient odour on human visual vigilance. Appl. Cognit. Psychol. 2001, 15, 225–232. [Google Scholar] [CrossRef]
- Shimizu, K.; Gyokusen, M.; Kitamura, S.; Kawabe, T.; Kozaki, T.; Ishibashi, K.; Izumi, R.; Mizunoya, W.; Ohnuki, K.; Kondo, R. Essential oil of lavender inhibited the decreased attention during a long-term task in humans. Biosci. Biotechnol. Biochem. 2008, 72, 1944–1947. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, E.; Fukagawa, M.; Okamoto, T.; Fukuda, A.; Hayashi, C.; Ohnuki, K.; Shimizu, K.; Kondo, R. Volatiles emitted from the leaves of Laurus nobilis L. improve vigilance performance in visual discrimination task. Biomed. Res. 2011, 32, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Motohashi, Y.; Kobayashi, S. Changes in mood by inhalation of essential oils in humans 2: Effect of essential oils on blood pressure, heart rate, R-R intervals, performance, sensory evaluation and POMS. Mokuzai Gakkaishi 1992, 38, 909–913. [Google Scholar]
- Dayawansa, S.; Umeno, K.; Takakura, H.; Hori, E.; Tabuchi, E.; Nagashima, Y.; Oosu, H.; Yada, Y.; Suzuki, T.; Ono, T.; et al. Autonomic responses during inhalation of natural fragrance of cedrol in humans. Auton. Neurosci.—Basic Clin. 2003, 108, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Tsunetsugu, Y.; Morikawa, T.; Miyazaki, Y. The relaxing effect of the smell of wood. Wood Ind. 2005, 60, 598–602. [Google Scholar]
- Kimura, A.; Sasaki, S.; Shibutani, S.; Kobayashi, D.; Iijima, Y.; Yatagai, M. Effects of inhalation of Akita Sugi (Cryptomeria japonica) wood oil on psychological and physiological responses. Aroma Res. 2009, 10, 162–169. [Google Scholar]
- Ikei, H.; Song, C.; Lee, J.; Miyazaki, Y. Comparison of the effects of olfactory stimulation by air-dried and high-temperature-dried wood chips of hinoki cypress (Chamaecyparis obutusa) on prefrontal cortex activity. J. Wood Sci. 2015. [Google Scholar] [CrossRef]
- Matsubara, E.; Kawai, S. VOCs emitted from Japanese cedar (Cryptomeria japonica) interior walls induce physiological relaxation. Build. Environ. 2014, 72, 125–130. [Google Scholar] [CrossRef]
- Howard, S.; Hughes, B.M. Expectancies, not aroma, explain impact of lavender aromatherapy on psychophysiological indices of relaxation in young healthy women. Br. J. Health Psychol. 2008, 13, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Chamine, I.; Oken, B.S. Aroma effects on physiologic and cognitive function following acute stress: A mechanism investigation. J. Altern. Complement. Med. 2016, 22, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, S.; Yanai, H.; Aoki, T.; Tamai, H.; Tanaka, Y.; Hokugoh, K. A factor analytic study of the items for the personality description based on the principle of the three traits theory for the work curve of addition of the Uchida-Kraepelin psychodiagnostic test. Shinrigaku Kenkyu 1985, 56, 79–182. [Google Scholar] [CrossRef]
- Yamaguchi, M. Stress evaluation using a biomarker in Saliva. Folia Pharmacol. Jpn. 2007, 129, 80–84. [Google Scholar] [CrossRef]
- Danhof-Pont, M.B.; Veen, T.; Zitman, F.G. Biomarkers in burnout: A systematic review. J. Psychosom. Res. 2011, 70, 505–524. [Google Scholar] [CrossRef] [PubMed]
- Sandone, C.; Dinallo, V.; Paci, M.; D’Ottavio, S.; Barbato, G.; Bernardini, S. Saliva metabolomics by NMR for the evaluation of sport performance. J. Pharm. Biomed. Anal. 2014, 88, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Morimoto, K. Effect of lavender aroma on salivary endocrinological stress markers. Arch. Oral Biol. 2008, 3, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Morimoto, K. Evaluation of effects of lavender and peppermint aromatherapy using sensitive salivary endocrinological stress markers. Stress Health 2011, 27, 430–435. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Tahara, Y.; Kosaka, S. Influence of concentration of fragrances on salivary α-amylase. Int. J. Cosmet. Sci. 2009, 31, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, T.; Tani, Y.; Yamaguchi, T.; Sawa, M.; Kobayashi, H. Effects of inhaled the Cyperi rhizoma and perillae herba essential oil on emotional states, autonomix nervous system and salivary biomarker. Health 2015, 7, 533–541. [Google Scholar] [CrossRef]
- Morgan, C.A.; Southwick, S.; Hazlett, G.; Rasmusson, A.; Hoyt, G.; Zimolo, Z.; Charney, D. Relationships among plasma dehydroepiandrosterone sulfate and cortisol levels, symptoms of dissociation, anf objective performance in humans exposed to acute stress. Arch. Gen. Psychiatry 2004, 61, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Izawa, S.; Sugaya, N.; Shirotsuki, K.; Yamada, K.C.; Ogawa, N.; Ouchi, Y.; Nagano, Y.; Suzuki, K.; Nomura, S. Salivary dehydroepiandrosterone secretion in response to acute psychosocial stress and its correlations with biological and psychological changes. Biol. Psychol. 2008, 79, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Lennartsson, A.K.; Kushnir, M.M.; Bergquist, J.; Jonsdottir, I.H. DHEA and DHEA-S response to acute psychosocial stress in healthy men and women. Biol. Psychol. 2012, 90, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Otsuka, T.; Kobayashi, M.; Wakayama, Y.; Igarashi, H.; Katsumata, M.; Hirata, Y.; Li, Y.J.; Hirata, K.; Shimizu, T.; et al. Acute effects of walking in forest environments on cardiovascular and metabolic parameters. Eur. J. Appl. Physiol. 2011, 111, 2845–2853. [Google Scholar] [CrossRef] [PubMed]
- Hosoi, J.; Haze, S.; Gozu, Y.; Sakai, K.; Moriyama, M.; Tsuchiya, T. Stimulation of DHEA secretion and regulation of skin functions by odorants or skin care. Auton. Neurosci.-Basic Clin. 2007, 137, 103–104. [Google Scholar] [CrossRef]
- Bjørnerem, A.; Straume, B.; Midtby, M.; Fønnebø, V.; Sundsfjord, J.; Svartberg, J.; Acharya, G.; Oian, P.; Berntsen, G.K. Endogenous sex hormones in relation to age, sex, lifestyle factors, and chronic diseases in a general population: The Tromso study. J. Clin. Endocrinol. Metab. 2004, 89, 6039–6047. [Google Scholar] [CrossRef] [PubMed]
- Maninger, N.; Wolkowitz, O.M.; Reus, V.I.; Epel, E.S.; Mellon, S.H. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front. Neuroendocrinol. 2009, 30, 65–91. [Google Scholar] [CrossRef] [PubMed]
- Khorram, O.; Vu, L.; Yen, S.S.C. Activation of immune function by dehydroepiandrosterone (DHEA) in age-advanced men. Biol. Sci. Med. Sci. 1997, 52, M1–M7. [Google Scholar] [CrossRef]
- Shirotsuki, K.; Izawa, S.; Sugaya, N.; Yamada, K.C.; Ogawa, N.; Ouchi, Y.; Nagano, Y.; Nomura, S. Salivary cortisol and DHEA reactivity to psychosocial stress in socially anzious males. Int. J. Psychophysiol. 2009, 72, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Speirs, R.L.; Herring, J.; Cooper, W.D.; Hardy, C.C.; Hind, C.R. The influence of sympathetic activity and isoprenaline on the secretion of amylase from the human parotid gland. Arch. Oral Biol. 1974, 19, 747–752. [Google Scholar] [CrossRef]
- Winkler, H.; Fischer-Colbrie, R. The Chromogranins A and B: The first 25 years and future perspectives. Neuroscience 1992, 49, 497–528. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Morikawa, T.; Yamamoto, N. Effect of wooden odoriferous substances on humans. Jpn. J. Physiol. Anthropol. 1999, 4, 49–50. [Google Scholar]
- Cheng, S.S.; Lin, H.Y.; Chang, S.T. Chemical composition and antifungal activity of essential oils from different tissues of Japanese cedar (Cryptomeria japonica). J. Agric. Food Chem. 2005, 53, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Ohira, T.; Park, B.J.; Kurosumi, Y.; Miyazaki, Y. Evaluation of dried-wood odors: Comparison between analytical and sensory data on odors from dried Sugi (Cryptomeria japonica) wood. J. Wood Sci. 2009, 55, 144–148. [Google Scholar] [CrossRef]
- Su, H.J.; Chao, C.J.; Chang, H.Y.; Wu, P.C. The effects of evaporating essential oils on indoor air quality. Atmos. Environ. 2007, 41, 1230–1236. [Google Scholar] [CrossRef]
- Huang, H.L.; Tsai, T.J.; Hsu, N.Y.; Lee, C.C.; Wu, P.C.; Su, H.J. Effects of essential oils on the formation of formaldehyde and secondary organic aerosols in an aromatherapy environment. Build. Environ. 2012, 57, 120–125. [Google Scholar] [CrossRef]




| RT (min) | Components | Composition (%) |
|---|---|---|
| 26.9 | a-Cubebene | 0.8 |
| 28.2 | Copaene | 0.3 |
| 28.8 | b-Cubebene | 0.9 |
| 30.1 | b-Caryophyllene | 1.1 |
| 31.4 | Muurora-3,5-diene | 1.2 |
| 31.6 | a-Humulene | 1.0 |
| 32.4 | Cadina-1(6),4-diene | 2.8 |
| 32.5 | g-Muurolene | 0.3 |
| 32.7 | Germacrene D | 0.2 |
| 33.2 | cis-Muurola-4(15),5-diene | 4.5 |
| 33.4 | Cubebol | 16.4 |
| 33.5 | a-Muurolene | 6.5 |
| 34.0 | b-Bisabolene | 0.3 |
| 34.2 | 4-epi-Cubebol | 18.0 |
| 34.4 | d-Cadinene | 21.4 |
| 34.5 | (-)-Calamenene | 1.3 |
| 34.5 | b-Cadinene | 2.1 |
| 34.9 | Cubenene | 1.4 |
| 35.6 | a-Elemol | 1.5 |
| 37.2 | Gleenol | 1.5 |
| 38.7 | 1,10-di-epi-Cubenol | 5.8 |
| 38.9 | g-Eudesmol | 0.5 |
| 39.3 | Epicubenol | 3.8 |
| 39.5 | a-Muurolol | 1.1 |
| 39.7 | b-Eudesmol | 3.2 |
| 40.1 | Dihydroeudesmol | 0.3 |
| 42.5 | Cryptomerione | 0.7 |
| 51.1 | Sandaracopimaridiene | 0.2 |
| 55.2 | Abietadiene | 1.0 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsubara, E.; Tsunetsugu, Y.; Ohira, T.; Sugiyama, M. Essential Oil of Japanese Cedar (Cryptomeria japonica) Wood Increases Salivary Dehydroepiandrosterone Sulfate Levels after Monotonous Work. Int. J. Environ. Res. Public Health 2017, 14, 97. https://doi.org/10.3390/ijerph14010097
Matsubara E, Tsunetsugu Y, Ohira T, Sugiyama M. Essential Oil of Japanese Cedar (Cryptomeria japonica) Wood Increases Salivary Dehydroepiandrosterone Sulfate Levels after Monotonous Work. International Journal of Environmental Research and Public Health. 2017; 14(1):97. https://doi.org/10.3390/ijerph14010097
Chicago/Turabian StyleMatsubara, Eri, Yuko Tsunetsugu, Tatsuro Ohira, and Masaki Sugiyama. 2017. "Essential Oil of Japanese Cedar (Cryptomeria japonica) Wood Increases Salivary Dehydroepiandrosterone Sulfate Levels after Monotonous Work" International Journal of Environmental Research and Public Health 14, no. 1: 97. https://doi.org/10.3390/ijerph14010097





