Effects of Gender on the Association of Urinary Phthalate Metabolites with Thyroid Hormones in Children: A Prospective Cohort Study in Taiwan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants and Design
2.2. Measurement of Phthalate Metabolite Concentrations
2.3. Thyroid Hormone Measurement
2.4. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Association between Concentrations of Urine Phthalate Metabolites and Thyroid Hormones
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BZBP | Benzylbutyl phthalate metabolite |
| DBP | Dibutyl phthalate |
| DCHP | Dicyclohexyl phthalate metabolite |
| DEP | Diethyl phthalate |
| DEHP | Di-2-ethylhexyl phthalate |
| DiNP | Diisononyl phthalate |
| DMP | Dimethyl phthalate |
| DOP | Di-n-octyl phthalate |
| LOQ | Limit of quantification |
| MBzP | Mono-benzyl phthalate |
| MCPP | Mono-3-carboxypropyl phthalate |
| MCHP | Monocyclohexyl phthalate |
| MEHHP | Mono-2-ethyl-5-hydroxyhexyl phthalate |
| MEHP | Mono-(2-ethylhexyl) phthalate |
| MEOHP | Mono-2-ethyl-5-oxohexyl phthalate |
| MEP | Monoethyl phthalate |
| MiBP | Mono-isobutyl phthalate |
| MnBP | Mono-n-butyl phthalate |
| MiNP | Mono-isononyl phthalate |
| MMP | Monomethyl phthalate |
| MOP | Mono-n-octyl phthalate |
| TSH | Thyroid-stimulating hormone |
| T3 | Triiodothyronine |
| T4 | Thyroxine |
| UPLC –MS/MS | Ultrahigh-performance liquid chromatography (UPLC)–tandem mass spectrometry |
References
- Oehlmann, J.; Schulte-Oehlmann, U.; Kloas, W.; Jagnytsch, O.; Lutz, I.; Kusk, K.O.; Wollenberger, L.; Santos, E.M.; Paull, G.C.; Van Look, K.J.; Tyler, C.R. A critical analysis of the biological impacts of plasticizers on wildlife. Philos. Trans. R Soc. Lond. B Biol. Sci. 2009, 364, 2047–2062. [Google Scholar] [CrossRef] [PubMed]
- Hubinger, J.C. A survey of phthalate esters in consumer cosmetic products. J. Cosmet. Sci. 2010, 61, 457–465. [Google Scholar] [PubMed]
- Huang, P.C.; Kuo, P.L.; Chou, Y.Y.; Lin, S.J.; Lee, C.C. Association between prenatal exposure to phthalates and the health of newborns. Environ. Int. 2009, 35, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Jurewicz, J.; Hanke, W. Exposure to phthalates: Reproductive outcome and children health. A review of epidemiological studies. Int. J. Occup. Med. Environ. Health 2011, 24, 115–141. [Google Scholar] [CrossRef] [PubMed]
- Lyche, J.L.; Gutleb, A.C.; Bergman, A.; Eriksen, G.S.; Murk, A.J.; Ropstad, E.; Saunders, M.; Skaare, J.U. Reproductive and developmental toxicity of phthalates. J. Toxicol. Environ. Health B Crit. Rev. 2009, 12, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Kay, V.R.; Bloom, M.S.; Foster, W.G. Reproductive and developmental effects of phthalate diesters in males. Crit. Rev. Toxicol. 2014, 44, 467–498. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. Executive Summary to EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Breous, E.; Wenzel, A.; Loos, U. The promoter of the human sodium/iodide symporter responds to certain phthalate plasticisers. Mol. Cell. Endocrinol. 2005, 244, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Ghisari, M.; Bonefeld-Jorgensen, E.C. Effects of plasticizers and their mixtures on estrogen receptor and thyroid hormone functions. Toxicol. Lett. 2009, 189, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Jugan, M.L.; Levi, Y.; Blondeau, J.P. Endocrine disruptors and thyroid hormone physiology. Biochem. Pharmacol. 2010, 79, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Mathieu-Denoncourt, J.; Wallace, S.J.; de Solla, S.R.; Langlois, V.S. Plasticizer endocrine disruption: Highlighting developmental and reproductive effects in mammals and non-mammalian aquatic species. Gen. Comp. Endocrinol. 2015, 219, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Shen, O.; Wu, W.; Du, G.; Liu, R.; Yu, L.; Sun, H.; Han, X.; Jiang, Y.; Shi, W.; Hu, W.; et al. Thyroid disruption by Di-n-butyl phthalate (DBP) and mono-n-butyl phthalate (MBP) in Xenopus laevis. PLoS ONE 2011, 6, e19159. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, A.; Sawatsubashi, S.; Yamauchi, K. Endocrine disrupting chemicals: Interference of thyroid hormone binding to transthyretins and to thyroid hormone receptors. Mol. Cell. Endocrinol. 2003, 199, 105–117. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, L.; Wei, L.; Li, L. DEHP reduces thyroid hormones via interacting with hormone synthesis-related proteins, deiodinases, transthyretin, receptors, and hepatic enzymes in rats. Environ. Sci. Pollut. Res. Int. 2015, 22, 12711–12719. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Calafat, A.M.; Hauser, R. Di(2-ethylhexyl) phthalate metabolites may alter thyroid hormone levels in men. Environ. Health Perspect. 2007, 115, 1029–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, P.C.; Kuo, P.L.; Guo, Y.L.; Liao, P.C.; Lee, C.C. Associations between urinary phthalate monoesters and thyroid hormones in pregnant women. Hum. Reprod. 2007, 22, 2715–2722. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.M.; Becker, K.; Wittassek, M.; Seiwert, M.; Angerer, J.; Kolossa-Gehring, M. Di-n-butylphthalate and butylbenzylphthalate - urinary metabolite levels and estimated daily intakes: Pilot study for the German Environmental Survey on children. J. Expo. Sci. Environ. Epidemiol. 2007, 17, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.T.; Wu, C.F.; Chen, B.H.; Chen, E.K.; Chen, Y.L.; Shiea, J.; Lee, W.T.; Chao, M.C.; Wu, J.R. Intake of phthalate-tainted foods alters thyroid functions in Taiwanese children. PLoS ONE 2013, 8, e55005. [Google Scholar] [CrossRef] [PubMed]
- Lien, G.W.; Huang, C.C.; Shiu, J.S.; Chen, M.H.; Hsieh, W.S.; Guo, Y.L.; Chen, P.C. Perfluoroalkyl substances in cord blood and attention deficit/hyperactivity disorder symptoms in seven-year-old children. Chemosphere 2016, 156, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.J.; Lin, C.C.; Lin, Y.J.; Hsieh, W.S.; Chen, P.C. Early life phthalate exposure and atopic disorders in children: A prospective birth cohort study. Environ. Int. 2014, 62, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Goen, T.; Seiwert, M.; Conrad, A.; Pick-Fuss, H.; Muller, J.; Wittassek, M.; Schulz, C.; Kolossa-Gehring, M. GerES IV: Phthalate metabolites and bisphenol A in urine of German children. Int. J. Hyg. Environ. Health 2009, 212, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Wallner, P.; Kundi, M.; Hohenblum, P.; Scharf, S.; Hutter, H.P. Phthalate Metabolites, Consumer Habits and Health Effects. Int. J. Environ. Res. Public Health 2016, 13, 717. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Carrillo, L.; Hernandez-Ramirez, R.U.; Calafat, A.M.; Torres-Sanchez, L.; Galvan-Portillo, M.; Needham, L.L.; Ruiz-Ramos, R.; Cebrian, M.E. Exposure to phthalates and breast cancer risk in northern Mexico. Environ. Health Perspect. 2010, 118, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Ferguson, K.K. Relationship between urinary phthalate and bisphenol A concentrations and serum thyroid measures in U.S. adults and adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007–2008. Environ. Health Perspect. 2011, 119, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Gomez Ramos, M.J.; Heffernan, A.L.; Toms, L.M.; Calafat, A.M.; Ye, X.; Hobson, P.; Broomhall, S.; Mueller, J.F. Concentrations of phthalates and DINCH metabolites in pooled urine from Queensland, Australia. Environ. Int. 2016, 88, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Myridakis, A.; Chalkiadaki, G.; Fotou, M.; Kogevinas, M.; Chatzi, L.; Stephanou, E.G. Exposure of Preschool-Age Greek Children (RHEA Cohort) to Bisphenol A, Parabens, Phthalates, and Organophosphates. Environ. Sci. Technol. 2016, 50, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Boas, M.; Frederiksen, H.; Feldt-Rasmussen, U.; Skakkebaek, N.E.; Hegedus, L.; Hilsted, L.; Juul, A.; Main, K.M. Childhood exposure to phthalates: Associations with thyroid function, insulin-like growth factor I, and growth. Environ. Health Perspect. 2010, 118, 1458–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ait Bamai, Y.; Araki, A.; Kawai, T.; Tsuboi, T.; Yoshioka, E.; Kanazawa, A.; Cong, S.; Kishi, R. Comparisons of urinary phthalate metabolites and daily phthalate intakes among Japanese families. Int. J. Hyg. Environ. Health 2015, 218, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.J.; Wu, C.F.; Tsai, Y.C.; Huang, P.C.; Chen, M.L.; Wang, S.L.; Chen, B.H.; Chen, C.C.; Wu, W.C.; Hsu, P.S.; et al. Intake of Phthalate-tainted Foods and Serum Thyroid Hormones in Taiwanese Children and Adolescents. Sci. Rep. 2016, 6, 30589. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Cao, Y.; Shen, Q.; Zhao, Y.; Zhang, Z.; Zhang, Y. Association Between Urinary Phthalates and Pubertal Timing in Chinese Adolescents. J. Epidemiol. 2015, 25, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, N.S.; Dhanya, C.R.; Indu, A.R.; Kurup, P.A. Changes in some hormones by low doses of di(2-ethyl hexyl) phthalate (DEHP), a commonly used plasticizer in PVC blood storage bags & medical tubing. Indian J. Med. Res. 2004, 119, 139–144. [Google Scholar] [PubMed]
- Hashimoto, K.; Yamakita, N.; Ikeda, T.; Matsuhisa, T.; Kuwayama, A.; Sano, T.; Hashimoto, K.; Yasuda, K. Longitudinal study of patients with idiopathic isolated TSH deficiency: Possible progression of pituitary dysfunction in lymphocytic adenohypophysitis. Endocr. J. 2006, 53, 593–601. [Google Scholar] [CrossRef] [PubMed]
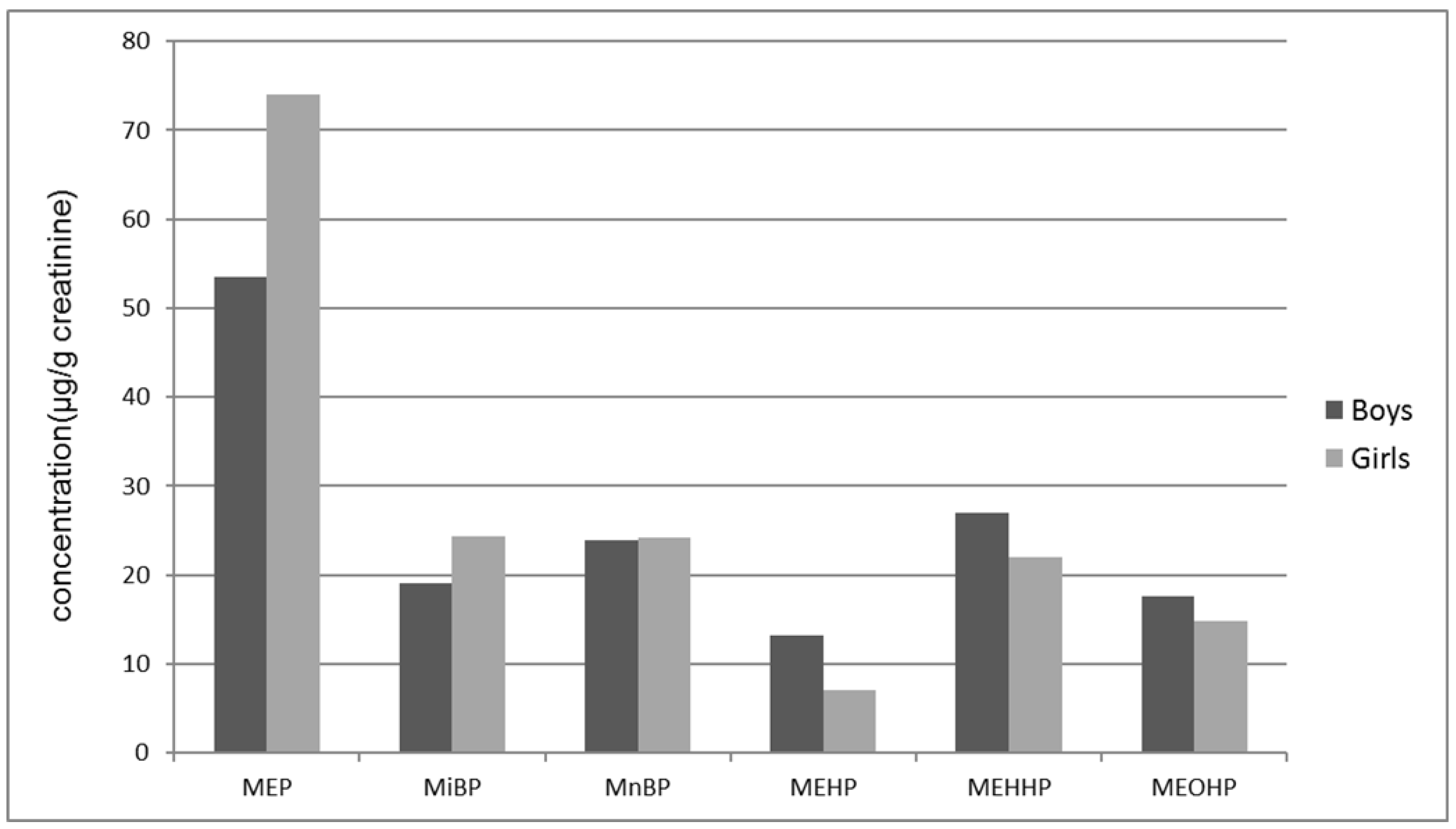
| Boys (92) | Girls (97) | Total (189) | |
|---|---|---|---|
| Mother | |||
| Maternal age while delivery (≥35 years old) (%) | 24.30 | 31.70 | 27.90 |
| Maternal education ≥college (%) | 47.70 | 55.40 | 51.40 |
| Occupation (non-housewife) (%) | 86.00 | 82.20 | 84.10 |
| Children | |||
| Birth weight <2500 g (%) | 2.56 | 5.00 | 3.80 |
| Gestational age (<37 weeks) (%) | 3.70 | 5.00 | 4.30 |
| Parity <2 (%) | 37.40 | 42.60 | 39.90 |
| Height (cm) (8 years old) (mean ± SD) | 135.75 ± 5.77 | 135.62 ± 6.09 | 135.69 ± 5.91 |
| Weight (kg) (mean ± SD) | 33.17 ± 7.43 | 31.35 ± 6.68 | 32.29 ± 7.12 |
| BMI (kg/m2) (mean ± SD) | 17.89 ± 3.22 | 16.93 ± 2.74 | 17.42 ± 3.02 |
| Environmental factors | |||
| ETS exposures (%) | 30.20 | 18.60 | 24.40 |
| Family income per year >1,500,000 (NT dollars) (%) | 15.90 | 17.80 | 16.80 |
| Phthalate Metabolites | LOQ (μg/L) | Percent (%) > LOQ | 25th Percentile | Median | 75th Percentile | 95th Percentile |
|---|---|---|---|---|---|---|
| Without creatinine correction (μg/L) | ||||||
| MMP | 0.10 | 29.20 | <LOQ | <LOQ | 1.72 | 12.06 |
| MEP | 0.50 | 96.30 | 16.83 | 30.49 | 69.60 | 156.90 |
| MiBP | 0.50 | 92.70 | 5.96 | 15.46 | 31.21 | 78.36 |
| MnBP | 0.50 | 90.40 | 5.64 | 15.60 | 35.72 | 96.84 |
| MBzP | 0.05 | 47.00 | <LOQ | <LOQ | 1.43 | 7.28 |
| MEHP | 0.50 | 86.30 | 1.31 | 4.44 | 9.38 | 30.95 |
| MEHHP | 0.05 | 100.00 | 8.03 | 17.21 | 33.37 | 69.97 |
| MEOHP | 0.05 | 100.00 | 5.36 | 11.06 | 21.93 | 49.18 |
| MCHP | 0.50 | 0.90 | <LOQ | <LOQ | <LOQ | <LOQ |
| MCPP | 0.50 | 1.80 | <LOQ | <LOQ | <LOQ | <LOQ |
| MOP | 0.50 | 0.00 | <LOQ | <LOQ | <LOQ | <LOQ |
| MiNP | 0.50 | 3.20 | <LOQ | <LOQ | <LOQ | <LOQ |
| Creatinine-corrected concentration (μg/g creatinine) a | ||||||
| MMP | <LOQ | <LOQ | 2.34 | 15.08 | ||
| MEP | 18.60 | 34.82 | 71.55 | 153.74 | ||
| MiBP | 7.07 | 16.57 | 28.01 | 62.06 | ||
| MnBP | 6.44 | 17.17 | 31.99 | 75.79 | ||
| MBzP | <LOQ | <LOQ | 1.53 | 5.04 | ||
| MEHP | 1.86 | 4.40 | 8.80 | 26.28 | ||
| MEHHP | 10.03 | 20.45 | 30.91 | 53.44 | ||
| MEOHP | 6.87 | 12.84 | 20.51 | 34.61 | ||
| MCHP | <LOQ | <LOQ | <LOQ | <LOQ | ||
| MCPP | <LOQ | <LOQ | <LOQ | <LOQ | ||
| MOP | <LOQ | <LOQ | <LOQ | <LOQ | ||
| MiNP | <LOQ | <LOQ | <LOQ | <LOQ | ||
| Samples | Free T4 (ng/dL) | Total T4 (μg/dL) | Free T3 (ng/dL) | Total T3 (μg/dL) | TSH (miu/mL) |
|---|---|---|---|---|---|
| all | |||||
| (n = 189) | |||||
| mean ± SD | 1.39 ± 0.14 | 8.72 ± 1.35 | 4.06 ± 0.52 | 151.83 ± 24.00 | 2.53 ± 1.23 |
| median | 1.38 | 8.60 | 4.09 | 151.65 | 2.33 |
| boys | |||||
| (n = 92) | |||||
| mean ± SD | 1.39 ± 0.14 | 8.85 ± 1.39 | 4.06 ± 0.46 | 151.17 ± 24.61 | 2.63 ± 1.26 |
| median | 1.40 | 8.96 | 4.13 | 150.30 | 2.42 |
| girls | |||||
| (n = 97) | |||||
| mean ± SD | 1.38 ± 0.14 | 8.57 ± 1.30 | 4.06 ± 0.59 | 152.55 ± 23.43 | 2.45 ± 1.20 |
| median | 1.36 | 8.43 | 4.05 | 153.4 | 2.22 |
| Crude Analysis | Creatinine-Corrected Analysis | |||||
|---|---|---|---|---|---|---|
| All | Boys | Girls | All | Boys | Girls | |
| Free T4 | ||||||
| MEP | 0.00001 | −0.00001 | 0.00001 | −0.00001 | −0.0001 | 0.00001 |
| (−0.0001, 0.0001) | (−0.0004, 0.0004) | (−0.0001, 0.0002) | (−0.0001, 0.0001) | (−0.0004, 0.0003) | (−0.0001, 0.0001) | |
| MiBP | 0.0004 | −0.0003 | 0.0009 * | 0.0004 | −0.0004 | 0.0007 |
| (−0.0002, 0.0009) | (−0.0011, 0.0006) | (0.0002, 0.0016) | (−0.0003, 0.0001) | (−0.0016, 0.0008) | (−0.0001, 0.0015) | |
| MnBP | 0.0003 | 0.0004 | 0.0003 | 0.0004 | 0.0004 | 0.0003 |
| (−0.00001, 0.0007) | (−0.0002, 0.0010) | (−0.0001, 0.0007) | (−0.0002, 0.0009) | (−0.0004, 0.0011) | (−0.0004, 0.0010) | |
| ΣDBP | 0.0002 | 0.0001 | 0.0003 | 0.0002 | 0.0001 | 0.0003 |
| (−0.00001, 0.0004) | (−0.0003, 0.0005) | (−0.00001, 0.0006) | (−0.0001, 0.0006) | (−0.0004, 0.0006) | (−0.0001, 0.0008) | |
| MEHP | −0.00001 | −0.0001 | 0.0018 * | −0.00001 | −0.0001 | 0.0026 * |
| (−0.0002, 0.0002) | (−0.0002, 0.0001) | (0.0004, 0.0031) | (−0.0004, 0.0003) | (−0.0005, 0.0002) | (0.0005, 0.0047) | |
| MEHHP | 0.0001 | −0.0001 | 0.0010 | 0.0001 | −0.0002 | 0.0015 * |
| (−0.0002, 0.0004) | (−0.0005, 0.0002) | (0.0002, 0.0018) | (−0.0004, 0.0006) | (−0.0008, 0.0001) | (0.0002, 0.0028) | |
| MEOHP | 0.0001 | −0.0002 | 0.0014 * | 0.0002 | −0.0004 | 0.0024 * |
| (−0.0003, 0.0006) | (−0.0007, 0.0003) | (0.0003, 0.0024) | (−0.0006, 0.0010) | (−0.0013, 0.0006) | (0.0005, 0.0043) | |
| ΣDEHP | 0.00001 | −0.00001 | 0.0005 * | 0.00001 | −0.0001 | 0.0008 * |
| (−0.0001, 0.0001) | (−0.0001, 0.0001) | (0.0001, 0.0008) | (−0.0002, 0.0002) | (−0.0003, 0.0001) | (0.0002, 0.0014) | |
| Total T4 | ||||||
| MEP | −0.00001 | −0.0001 | −0.00001 | −0.00001 | −0.0001 | 0.00001 |
| (−0.0002, 0.0002) | (−0.0006, 0.0005) | (−0.0002, 0.0002) | (−0.0001, 0.0001) | (−0.0006, 0.0004) | (−0.0001, 0.0001) | |
| MiBP | 0.0007 | 0.0007 | 0.0005 | 0.0010 * | 0.0012 | 0.0009 |
| (−0.0001, 0.0015) | (−0.0005, 0.0020) | (−0.0006, 0.0016) | (0.00001, 0.0019) | (−0.0007, 0.0030) | (−0.0003, 0.0020) | |
| MnBP | 0.0003 | 0.0005 | 0.0001 | 0.0004 | 0.0004 | 0.0002 |
| (−0.0002, 0.0008) | (−0.0004, 0.0014) | (−0.0006, 0.0007) | (−0.0004, 0.0012) | (−0.0007, 0.0016) | (−0.0009, 0.0012) | |
| ΣDBP | 0.0003 | 0.0003 | 0.0001 | 0.0004 | 0.0004 | 0.0003 |
| (−0.0001, 0.0006) | (−0.0002, 0.0009) | (−0.0003, 0.0006) | (−0.0001 (0.0009) | (−0.0004, 0.0011) | (−0.0003, 0.0010) | |
| MEHP | 0.00001 | 0.00001 | 0.0002 | 0.0001 | 0.00001 | 0.0006 |
| (−0.0002, 0.0003) | (−0.0003, 0.0003) | (−0.0020, 0.0024) | (−0.0004, 0.0006) | (−0.0005, 0.0005) | (−0.0027, 0.0039) | |
| MEHHP | 0.0001 | 0.00001 | −0.0002 | 0.0002 | 0.0001 | −0.0002 |
| (−0.0003, 0.0005) | (−0.0005, 0.0005) | (−0.0015, 0.0011) | (−0.0006, 0.0010) | (−0.0007, 0.0010) | (−0.0022, 0.0018) | |
| MEOHP | 0.0001 | 0.0001 | −0.0001 | 0.0003 | 0.0002 | −0.0002 |
| (−0.0005, 0.0008) | (−0.0007, 0.0008) | (−0.0018, 0.0016) | (−0.0008, 0.0015) | (−0.0011, 0.0015) | (−0.0032, 0.0027) | |
| ΣDEHP | 0.00001 | 0.00001 | −0.00001 | 0.0002 | 0.00001 | 0.00001 |
| (−0.0001, 0.0002) | (−0.0001, 0.0001) | (−0.0006, 0.0005) | (−0.0002, 0.0003) | (−0.0002, 0.0003) | (−0.0010, 0.0009) | |
| Free T3 | ||||||
| MEP | 0.00001 | 0.0002 | 0.00001 | 0.00001 | 0.0002 | 0.00001 |
| (−0.0001, 0.0002) | (−0.0002, 0.0006) | (−0.0002, 0.0002) | (−0.0001, 0.0001) | (−0.0002, 0.0006) | (−0.0001, 0.0001) | |
| MiBP | 0.0005 | 0.0009 * | 0.0003 | 0.0003 | 0.0013 * | 0.0001 |
| (−0.0002, 0.0011) | (−0.00001, 0.0017) | (−0.0008, 0.0014) | (−0.0005, 0.0012) | (0.00001, 0.0025) | (−0.0011, 0.0013) | |
| MnBP | 0.0003 | 0.0006 * | 0.0002 | 0.0005 | 0.0009 * | 0.0003 |
| (−0.0001, 0.0008) | (0.00001, 0.0013) | (−0.0005, 0.0009) | (0.0001, 0.0012) | (0.0002, 0.0017) | (−0.0008, 0.0014) | |
| ΣDBP | 0.0002 | 0.0004 * | 0.0002 | 0.0003 | 0.0006 | 0.0001 |
| (−0.0001, 0.0005) | (0.00001, 0.0008) | (−0.0003, 0.0006) | (−0.0001, 0.0007) | (0.0001, 0.0011) | (−0.0006, 0.0008) | |
| MEHP | 0.0001 | 0.0001 | 0.0004 | 0.00001 | 0.0001 | 0.0004 |
| (−0.0001, 0.0003) | (−0.0001, 0.0002) | (−0.0018, 0.0025) | (−0.0003, 0.0005) | (−0.0002, 0.0005) | (−0.0028, 0.0037) | |
| MEHHP | 0.0002 | 0.0002 | 0.0003 | 0.0004 | 0.0005 | 0.0001 |
| (−0.0001, 0.0006) | (−0.0001, 0.0005) | (−0.0010, 0.0015) | (−0.0002, 0.0010) | (−0.0001, 0.0010) | (−0.0019, 0.0020) | |
| MEOHP | 0.0003 | 0.0003 | 0.0004 | 0.0005 | 0.0006 | 0.0003 |
| (−0.0003, 0.0008) | (−0.0002, 0.0008) | (−0.0013, 0.0020) | (−0.0004, 0.0015) | (−0.0002, 0.0015) | (−0.0026, 0.0032) | |
| ΣDEHP | 0.00001 | 0.00001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| (−0.0001, 0.0002) | (−0.00001, 0.0001) | (−0.0004, 0.0007) | (−0.0001, 0.0003) | (−0.0001, 0.0003) | (−0.0009, 0.0010) | |
| Total T3 | ||||||
| MEP | 0.00001 | −0.00001 | 0.00001 | 0.00001 | 0.00001 | 0.00001 |
| (−0.0002, 0.0002) | (−0.0006, 0.0005) | (−0.0002, 0.0002) | (−0.0001, 0.0001) | (−0.0005, 0.0005) | (−0.0001, 0.0001) | |
| MiBP | −0.0002 | 0.0002 | −0.0005 | −0.0003 | 0.0002 | −0.0004 |
| (−0.0010, 0.0007) | (−0.0010, 0.0015) | (−0.0017, 0.0007) | (−0.0013, 0.0008) | (−0.0017, 0.0020) | (−0.0018, 0.0009) | |
| MnBP | −0.0001 | −0.0001 | −0.0002 | −0.001 | −0.0001 | −0.0002 |
| (−0.0007, 0.0005) | (−0.0010, 0.0009) | (−0.0009, 0.0006) | (−0.0010, 0.0007) | (−0.0012, 0.0011) | (−0.0014, 0.0011) | |
| ΣDBP | −0.0001 | 0.00001 | −0.0002 | −0.0001 | −0.00001 | −0.0002 |
| (−0.0005, 0.0003) | (−0.0006, 0.0006) | (−0.0007, 0.0004) | (−0.0006, 0.0004) | (−0.0008, 0.0007) | (−0.0010, 0.0006) | |
| MEHP | 0.00001 | 0.00001 | −0.0014 | 0.00001 | 0.0001 | −0.0023 |
| (−0.0002, 0.0003) | (−0.0002, 0.0003) | (−0.0039, 0.0010) | (−0.0005, 0.0005) | (−0.0004, 0.0006) | (−0.0059, 0.0014) | |
| MEHHP | −0.0001 | 0.0001 | −0.0012 | −0.0001 | 0.0002 | −0.0021 |
| (−0.0006, 0.0004) | (−0.0004, 0.0006) | (−0.0026, 0.0002) | (−0.0010, 0.0007) | (−0.0006, 0.0011) | (−0.0042, 0.0001) | |
| MEOHP | −0.0001 | 0.0002 | −0.0014 | −0.0002 | 0.0004 | −0.0027 |
| (−0.0009, 0.0006) | (−0.0006, 0.0009) | (−0.0033, 0.0004) | (−0.0014, 0.0011) | (−0.0009, 0.0016) | (−0.0059, 0.0004) | |
| ΣDEHP | −0.00001 | 0.00001 | −0.0005 | −0.00001 | 0.0001 | −0.0009 |
| (−0.0002, 0.0001) | (−0.0001, 0.0002) | (−0.0011, 0.0001) | (−0.0003, 0.0003) | (−0.0002, 0.0003) | (−0.0019, 0.0001) | |
| TSH | ||||||
| MEP | 0.0001 | −0.0008 | 0.0002 | 0.00001 | −0.0005 | 0.0001 |
| (−0.0004, 0.0006) | (−0.0029, 0.0013) | (−0.0003, 0.0007) | (−0.0003, 0.0004) | (−0.0023, 0.0014) | (−0.0003, 0.0004) | |
| MiBP | 0.0001 | −0.0007 | 0.0009 | −0.0019 | −0.0022 | −0.0014 |
| (−0.0027, 0.0029) | (−0.0056, 0.0041) | (−0.0026, 0.0043) | (−0.0056, 0.0017) | (−0.0092, 0.0048) | (−0.0058, 0.0029) | |
| MnBP | 0.0016 | −0.0013 | 0.0028 * | 0.0014 | −0.0019 | 0.0045 * |
| (0.0001, 0.0031) | (−0.0051, 0.0024) | (0.0013, 0.0044) | (−0.0011, 0.0039) | (−0.0063, 0.0024) | (0.0016, 0.0073) | |
| ΣDBP | 0.0008 | −0.0007 | 0.0018 * | 0.0001 | −0.0013 | 0.0013 |
| (−0.0004, 0.0020) | (−0.0029, 0.0016) | (0.0005, 0.0031) | (−0.0016, 0.0018) | (−0.0042, 0.0017) | (−0.0008, 0.0035) | |
| MEHP | 0.0004 | 0.0003 | 0.0021 | 0.0007 | 0.0007 | 0.0019 |
| (−0.0002, 0.0010) | (−0.0003, 0.0010) | (−0.0047, 0.0089) | (−0.0005, 0.0019) | (−0.0006, 0.0019) | (−0.0092, 0.0130) | |
| MEHHP | 0.0007 | 0.0006 | 0.0017 | 0.0011 | 0.0010 | 0.0007 |
| (−0.0005, 0.0019) | (−0.0008, 0.0019) | (−0.0022, 0.0057) | (−0.0013, 0.0034) | (−0.0017, 0.0036) | (−0.0061, 0.0075) | |
| MEOHP | 0.0012 | 0.0009 | 0.0028 | 0.0020 | 0.0017 | 0.0024 |
| (−0.0005, 0.0029) | (−0.0011, 0.0030) | (−0.0022, 0.0077) | (−0.0014, 0.0053) | (−0.0021, 0.0055) | (−0.0074, 0.0123) | |
| ΣDEHP | 0.0002 | 0.0002 | 0.0008 | 0.0004 | 0.0003 | 0.0006 |
| (−0.0001, 0.0005) | (−0.0002, 0.0005) | (−0.0009, 0.0025) | (−0.0003, 0.0010) | (−0.0004, 0.0010) | (−0.0026, 0.0038) | |
| Concentrations (μg/L) | Free T4 (ng/dL) | p | Total T4 (μg/dL) | p | Free T3 (ng/dL) | p | Total T3 (μg/dL) | p | TSH (miu/mL) | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| all | |||||||||||
| MEP | 0.533 | 0.825 | 0.166 | 0.492 | 0.810 | ||||||
| <20.07 | 1.37 | 8.69 | 3.98 | 153.47 | 2.58 | ||||||
| 20.07–51.41 | 1.39 | 8.62 | 4.15 | 150.72 | 2.49 | ||||||
| >51.41 | 1.39 | 8.77 | 4.10 | 150.98 | 2.53 | ||||||
| MiBP | 0.047 * | 0.167 | 0.236 | 0.178 | 0.260 | ||||||
| <7.96 | 1.36 | 8.58 | 4.07 | 156.74 | 2.77 | ||||||
| 7.96–23.12 | 1.38 | 8.64 | 3.99 | 148.14 | 2.29 | ||||||
| >23.12 | 1.40 | 8.86 | 4.16 | 150.22 | 2.53 | ||||||
| MnBP | 0.060 | 0.338 | 0.322 | 0.310 | 0.533 | ||||||
| <8.45 | 1.36 | 8.55 | 4.07 | 154.65 | 2.44 | ||||||
| 8.45–15.65 | 1.38 | 8.82 | 4.01 | 151.03 | 2.59 | ||||||
| >15.65 | 1.40 | 8.72 | 4.15 | 149.57 | 2.57 | ||||||
| ΣDBP | 0.022 * | 0.138 | 0.273 | 0.140 | 0.919 | ||||||
| <15.86 | 1.36 | 8.59 | 4.07 | 156.15 | 2.62 | ||||||
| 15.86–52.78 | 1.38 | 8.64 | 4.00 | 150.01 | 2.39 | ||||||
| >52.78 | 1.41 | 8.85 | 4.16 | 149.04 | 2.59 | ||||||
| MEHP | 0.166 | 0.296 | 0.153 | 0.865 | 0.441 | ||||||
| <2.09 | 1.35 | 8.42 | 3.97 | 150.49 | 2.75 | ||||||
| 2.09–7.67 | 1.41 | 9.00 | 4.15 | 155.16 | 2.28 | ||||||
| >7.67 | 1.39 | 8.67 | 4.10 | 149.53 | 2.58 | ||||||
| MEHHP | 0.078 | 0.187 | 0.071 | 0.507 | 0.915 | ||||||
| <9.71 | 1.36 | 8.63 | 4.02 | 155.54 | 2.58 | ||||||
| 9.71–25.58 | 1.38 | 8.52 | 4.01 | 147.01 | 2.42 | ||||||
| >25.58 | 1.40 | 8.93 | 4.19 | 152.58 | 2.60 | ||||||
| MEOHP | 0.095 | 0.228 | 0.179 | 0.419 | 0.778 | ||||||
| <6.35 | 1.36 | 8.59 | 4.02 | 154.20 | 2.64 | ||||||
| 6.35–16.77 | 1.38 | 8.61 | 4.07 | 150.19 | 2.39 | ||||||
| >16.77 | 1.40 | 8.88 | 4.14 | 150.82 | 2.57 | ||||||
| ΣDEHP | 0.038 * | 0.101 | 0.182 | 0.489 | 0.821 | ||||||
| <19.09 | 1.35 | 8.51 | 4.04 | 154.24 | 2.64 | ||||||
| 19.09–47.87 | 1.39 | 8.68 | 4.03 | 149.74 | 2.38 | ||||||
| >47.87 | 1.40 | 8.89 | 4.16 | 151.22 | 2.59 | ||||||
| boys | |||||||||||
| MEP | 0.916 | 0.234 | 0.303 | 0.720 | 0.226 | ||||||
| <20.07 | 1.38 | 8.49 | 3.98 | 149.47 | 2.99 | ||||||
| 20.07–51.41 | 1.40 | 8.80 | 4.18 | 148.18 | 2.41 | ||||||
| >51.41 | 1.38 | 9.05 | 4.12 | 152.97 | 2.53 | ||||||
| MiBP | 0.421 | 0.137 | 0.039 * | 0.646 | 0.131 | ||||||
| <7.96 | 1.37 | 8.60 | 4.02 | 153.46 | 3.04 | ||||||
| 7.96–23.12 | 1.39 | 8.77 | 4.06 | 148.14 | 2.32 | ||||||
| >23.12 | 1.40 | 9.07 | 4.22 | 149.50 | 2.50 | ||||||
| MnBP | 0.679 | 8.50 | 0.279 | 4.02 | 0.028 * | 150.73 | 0.876 | 2.73 | 0.628 | ||
| <8.45 | 1.39 | 8.50 | 4.02 | 150.73 | 2.73 | ||||||
| 8.45–15.65 | 1.38 | 9.01 | 3.99 | 149.15 | 2.65 | ||||||
| >15.65 | 1.39 | 8.86 | 4.26 | 151.49 | 2.52 | ||||||
| ΣDBP | 0.440 | 0.097 | 0.010 * | 0.863 | 0.186 | ||||||
| <15.86 | 1.38 | 8.56 | 4.00 | 151.41 | 3.03 | ||||||
| 15.86–52.78 | 1.39 | 8.85 | 4.02 | 149.22 | 2.36 | ||||||
| >52.78 | 1.39 | 9.00 | 4.26 | 150.78 | 2.53 | ||||||
| MEHP | 0.777 | 0.213 | 0.191 | 0.498 | 0.348 | ||||||
| <2.09 | 1.36 | 8.22 | 3.97 | 144.37 | 3.03 | ||||||
| 2.09–7.67 | 1.42 | 9.29 | 4.14 | 155.52 | 2.32 | ||||||
| >7.67 | 1.38 | 8.74 | 4.14 | 149.61 | 2.65 | ||||||
| MEHHP | 0.471 | 0.092 | 0.025 * | 0.665 | 0.423 | ||||||
| <9.71 | 1.38 | 8.62 | 4.04 | 153.22 | 2.84 | ||||||
| 9.71–25.58 | 1.38 | 8.59 | 3.95 | 144.25 | 2.55 | ||||||
| >25.58 | 1.40 | 9.18 | 4.28 | 154.97 | 2.56 | ||||||
| MEOHP | 0.643 | 0.332 | 0.122 | 0.888 | 0.276 | ||||||
| <6.35 | 1.38 | 8.60 | 4.03 | 152.38 | 2.87 | ||||||
| 6.35–16.77 | 1.39 | 8.81 | 4.04 | 147.67 | 2.58 | ||||||
| >16.77 | 1.39 | 8.97 | 4.21 | 151.67 | 2.49 | ||||||
| ΣDEHP | 0.434 | 0.082 | 0.061 | 0.693 | 0.239 | ||||||
| <19.09 | 1.36 | 8.39 | 4.03 | 150.28 | 2.97 | ||||||
| 19.09–47.87 | 1.40 | 8.87 | 4.01 | 148.54 | 2.51 | ||||||
| >47.87 | 1.39 | 9.04 | 4.25 | 152.87 | 2.53 | ||||||
| girls | |||||||||||
| MEP | 0.553 | 0.122 | 0.309 | 0.237 | 0.387 | ||||||
| <20.07 | 1.36 | 8.83 | 3.98 | 156.46 | 2.27 | ||||||
| 20.07–51.41 | 1.38 | 8.46 | 4.12 | 153.02 | 2.56 | ||||||
| >51.41 | 1.39 | 8.42 | 4.09 | 148.50 | 2.53 | ||||||
| MiBP | 0.075 | 0.917 | 0.768 | 0.191 | 0.888 | ||||||
| <7.96 | 1.34 | 8.56 | 4.13 | 160.13 | 2.50 | ||||||
| 7.96–23.12 | 1.37 | 8.51 | 3.92 | 148.15 | 2.27 | ||||||
| >23.12 | 1.41 | 8.68 | 4.11 | 150.83 | 2.56 | ||||||
| MnBP | 0.049 * | 0.795 | 0.863 | 0.146 | 0.178 | ||||||
| <8.45 | 1.34 | 8.59 | 4.10 | 157.48 | 2.23 | ||||||
| 8.45–15.65 | 1.38 | 8.61 | 4.02 | 153.09 | 2.52 | ||||||
| >15.65 | 1.41 | 8.57 | 4.04 | 147.54 | 2.63 | ||||||
| ΣDBP | 0.023 * | 8.61 | 0.928 | 4.12 | 0.855 | 160.19 | 0.041 | 2.27 | 0.224 | ||
| <15.86 | 1.34 | 8.61 | 4.12 | 160.19 | 2.27 | ||||||
| 15.86–52.78 | 1.36 | 8.42 | 3.98 | 150.90 | 2.43 | ||||||
| >52.78 | 1.42 | 8.71 | 4.06 | 147.45 | 2.64 | ||||||
| MEHP | 0.160 | 0.868 | 0.383 | 0.418 | 0.721 | ||||||
| <2.09 | 1.34 | 8.56 | 3.98 | 154.64 | 2.56 | ||||||
| 2.09–7.67 | 1.40 | 8.64 | 4.15 | 154.71 | 2.24 | ||||||
| >7.67 | 1.39 | 8.58 | 4.07 | 149.45 | 2.50 | ||||||
| MEHHP | 0.127 | 0.892 | 0.473 | 0.259 | 0.459 | ||||||
| <9.71 | 1.35 | 8.63 | 4.01 | 157.00 | 2.41 | ||||||
| 9.71–25.58 | 1.39 | 8.44 | 4.09 | 150.60 | 2.25 | ||||||
| >25.58 | 1.40 | 8.66 | 4.09 | 150.11 | 2.65 | ||||||
| MEOHP | 0.098 | 0.689 | 0.540 | 0.377 | 0.578 | ||||||
| <6.35 | 1.35 | 8.58 | 4.01 | 155.51 | 2.47 | ||||||
| 6.35–16.77 | 1.37 | 8.39 | 4.10 | 153.06 | 2.18 | ||||||
| >16.77 | 1.41 | 8.77 | 4.08 | 149.95 | 2.67 | ||||||
| ΣDEHP | 0.057 | 0.753 | 0.674 | 0.248 | 0.554 | ||||||
| <19.09 | 1.34 | 8.58 | 4.04 | 156.57 | 2.44 | ||||||
| 19.09–47.87 | 1.37 | 8.38 | 4.05 | 151.63 | 2.18 | ||||||
| >47.87 | 1.41 | 8.74 | 4.08 | 149.71 | 2.64 |
| Concentrations (μg/L) | Free T4 (ng/dL) | p | Total T4 (μg/dL) | p | Free T3 (ng/dL) | p | Total T3 (μg/dL) | p | TSH (miu/mL) | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| all | |||||||||||
| MEP | 0.907 | 0.276 | 0.261 | 0.629 | 0.473 | ||||||
| <23.07 | 1.38 | 8.58 | 4.01 | 151.29 | 2.70 | ||||||
| 23.07~50.55 | 1.38 | 8.58 | 4.12 | 150.50 | 2.34 | ||||||
| 50.55< | 1.39 | 8.92 | 4.10 | 153.30 | 2.57 | ||||||
| MiBP | 0.289 | 0.431 | 0.528 | 0.128 | 0.248 | ||||||
| <9.49 | 1.37 | 8.65 | 4.07 | 156.52 | 2.71 | ||||||
| 9.49~24.19 | 1.37 | 8.57 | 4.05 | 149.26 | 2.40 | ||||||
| 24.19< | 1.40 | 8.87 | 4.11 | 149.49 | 2.49 | ||||||
| MnBP | 0.057 | 0.280 | 0.382 | 0.289 | 0.806 | ||||||
| <9.52 | 1.36 | 8.62 | 4.10 | 156.50 | 2.56 | ||||||
| 9.52~26.47 | 1.38 | 8.56 | 3.95 | 146.84 | 2.41 | ||||||
| 26.47< | 1.41 | 8.90 | 4.17 | 151.68 | 2.63 | ||||||
| ΣDBP | 0.130 | 0.174 | 0.699 | 0.220 | 0.620 | ||||||
| <23.24 | 1.36 | 8.51 | 4.09 | 155.54 | 2.62 | ||||||
| 23.24~52.28 | 1.39 | 8.75 | 4.03 | 149.98 | 2.47 | ||||||
| 52.28< | 1.40 | 8.82 | 4.11 | 149.74 | 2.52 | ||||||
| MEHP | 0.337 | 0.714 | 0.205 | 0.686 | 0.289 | ||||||
| <2.74 | 1.36 | 8.55 | 4.01 | 151.07 | 2.78 | ||||||
| 2.74~6.42 | 1.40 | 8.91 | 4.09 | 154.37 | 2.30 | ||||||
| 6.42< | 1.38 | 8.63 | 4.13 | 149.82 | 2.53 | ||||||
| MEHHP | 0.146 | 0.281 | 0.039 | 0.626 | 0.829 | ||||||
| <13.04 | 1.35 | 8.42 | 3.98 | 152.34 | 2.60 | ||||||
| 13.04~25.93 | 1.40 | 8.94 | 4.08 | 151.91 | 2.45 | ||||||
| 25.93< | 1.39 | 8.74 | 4.17 | 150.93 | 2.55 | ||||||
| MEOHP | 0.098 | 0.326 | 0.127 | 0.462 | 0.214 | ||||||
| <9.15 | 1.35 | 8.43 | 4.02 | 153.75 | 2.69 | ||||||
| 9.15~17.95 | 1.39 | 8.94 | 4.06 | 150.51 | 2.47 | ||||||
| 17.95< | 1.40 | 8.72 | 4.15 | 150.90 | 2.44 | ||||||
| ΣDEHP | 0.175 | 8.36 | 0.168 | 3.97 | 0.041 * | 151.62 | 0.885 | 2.65 | 0.339 | ||
| <25.34 | 1.35 | 8.36 | 3.97 | 151.62 | 2.65 | ||||||
| 25.34~49.92 | 1.41 | 8.98 | 4.11 | 152.34 | 2.50 | ||||||
| 49.92< | 1.38 | 8.75 | 4.15 | 151.21 | 2.45 | ||||||
| boys | |||||||||||
| MEP | 0.434 | 0.138 | 0.201 | 0.141 | 0.175 | ||||||
| <23.07 | 1.40 | 8.53 | 4.00 | 146.99 | 2.92 | ||||||
| 23.07~50.55 | 1.37 | 8.67 | 4.13 | 146.78 | 2.39 | ||||||
| 50.55< | 1.39 | 9.21 | 4.16 | 157.06 | 2.52 | ||||||
| MiBP | 0.785 | 0.223 | 0.087 | 0.722 | 0.187 | ||||||
| <9.49 | 1.38 | 8.58 | 3.98 | 151.79 | 2.96 | ||||||
| 9.49~24.19 | 1.40 | 8.87 | 4.18 | 150.36 | 2.33 | ||||||
| 24.19< | 1.38 | 9.03 | 4.15 | 148.80 | 2.55 | ||||||
| MnBP | 0.652 | 0.214 | 0.031 * | 0.885 | 0.280 | ||||||
| <9.52 | 1.38 | 8.62 | 4.03 | 152.36 | 2.79 | ||||||
| 9.52~26.47 | 1.37 | 8.65 | 3.96 | 143.43 | 2.65 | ||||||
| 26.47< | 1.40 | 9.12 | 4.27 | 154.03 | 2.45 | ||||||
| ΣDBP | 0.828 | 0.151 | 0.037 * | 0.960 | 0.203 | ||||||
| <23.24 | 1.38 | 8.50 | 3.99 | 150.23 | 2.85 | ||||||
| 23.24~52.28 | 1.40 | 9.05 | 4.13 | 152.76 | 2.56 | ||||||
| 52.28< | 1.39 | 8.95 | 4.20 | 148.43 | 2.42 | ||||||
| MEHP | 0.652 | 0.691 | 0.262 | 0.801 | 0.294 | ||||||
| <2.74 | 1.37 | 8.49 | 4.03 | 147.54 | 2.93 | ||||||
| 2.74~6.42 | 1.43 | 9.24 | 4.07 | 153.57 | 2.41 | ||||||
| 6.42< | 1.36 | 8.70 | 4.17 | 150.13 | 2.57 | ||||||
| MEHHP | 0.657 | 0.181 | 0.002 * | 0.346 | 0.249 | ||||||
| <13.04 | 1.37 | 8.39 | 3.90 | 145.66 | 2.85 | ||||||
| 13.04~25.93 | 1.40 | 9.02 | 4.10 | 152.47 | 2.56 | ||||||
| 25.93< | 1.39 | 8.98 | 4.28 | 152.83 | 2.49 | ||||||
| MEOHP | 0.816 | 0.572 | 0.037 * | 0.866 | 0.099 | ||||||
| <9.15 | 1.39 | 8.57 | 3.98 | 149.23 | 2.98 | ||||||
| 9.15~17.95 | 1.38 | 8.93 | 4.07 | 149.92 | 2.43 | ||||||
| 17.95< | 1.39 | 8.91 | 4.24 | 152.18 | 2.49 | ||||||
| ΣDEHP | 0.843 | 0.273 | 0.007 * | 0.401 | 0.108 | ||||||
| <25.34 | 1.37 | 8.38 | 3.90 | 146.20 | 2.93 | ||||||
| 25.34~49.92 | 1.42 | 9.08 | 4.13 | 151.74 | 2.55 | ||||||
| 49.92< | 1.37 | 8.91 | 4.23 | 152.87 | 2.44 | ||||||
| girls | |||||||||||
| MEP | 0.451 | 0.723 | 0.612 | 0.364 | 0.585 | ||||||
| <23.07 | 1.35 | 8.63 | 4.01 | 156.51 | 2.44 | ||||||
| 23.07~50.55 | 1.38 | 8.52 | 4.11 | 153.19 | 2.30 | ||||||
| 50.55< | 1.39 | 8.63 | 4.04 | 149.65 | 2.61 | ||||||
| MiBP | 0.090 | 0.722 | 0.861 | 0.075 | 0.945 | ||||||
| <9.49 | 1.35 | 8.73 | 4.19 | 162.26 | 2.41 | ||||||
| 9.49~24.19 | 1.34 | 8.28 | 3.92 | 148.20 | 2.47 | ||||||
| 24.19< | 1.42 | 8.75 | 4.08 | 150.00 | 2.45 | ||||||
| MnBP | 0.034* | 0.862 | 0.663 | 0.074 | 0.113 | ||||||
| <9.52 | 1.33 | 8.61 | 4.18 | 161.06 | 2.30 | ||||||
| 9.52~26.47 | 1.38 | 8.50 | 3.95 | 149.37 | 2.24 | ||||||
| 26.47< | 1.41 | 8.67 | 4.07 | 149.25 | 2.81 | ||||||
| ΣDBP | 0.060 | 0.775 | 0.450 | 0.108 | 0.384 | ||||||
| <23.24 | 1.33 | 8.52 | 4.22 | 162.42 | 2.32 | ||||||
| 23.24~52.28 | 1.38 | 8.51 | 3.95 | 147.69 | 2.38 | ||||||
| 52.28< | 1.41 | 8.72 | 4.04 | 150.80 | 2.60 | ||||||
| MEHP | 0.123 | 0.805 | 0.416 | 0.420 | 0.530 | ||||||
| <2.74 | 1.35 | 8.60 | 3.99 | 154.18 | 2.65 | ||||||
| 2.74~6.42 | 1.38 | 8.60 | 4.10 | 155.11 | 2.20 | ||||||
| 6.42< | 1.40 | 8.57 | 4.10 | 149.50 | 2.48 | ||||||
| MEHHP | 0.145 | 0.998 | 0.727 | 0.118 | 0.482 | ||||||
| <13.04 | 1.34 | 8.45 | 4.04 | 158.05 | 2.39 | ||||||
| 13.04~25.93 | 1.41 | 8.83 | 4.05 | 151.24 | 2.32 | ||||||
| 25.93< | 1.39 | 8.53 | 4.08 | 149.35 | 2.60 | ||||||
| MEOHP | 0.020* | 0.582 | 0.709 | 0.192 | 0.836 | ||||||
| <9.15 | 1.32 | 8.29 | 4.05 | 157.86 | 2.43 | ||||||
| 9.15~17.95 | 1.40 | 8.95 | 4.06 | 151.13 | 2.51 | ||||||
| 17.95< | 1.41 | 8.56 | 4.07 | 149.77 | 2.40 | ||||||
| ΣDEHP | 0.060 | 0.573 | 0.573 | 0.286 | 0.944 | ||||||
| <25.34 | 1.33 | 8.34 | 4.02 | 156.09 | 2.42 | ||||||
| 25.34~49.92 | 1.41 | 8.87 | 4.08 | 152.99 | 2.45 | ||||||
| 49.92< | 1.40 | 8.60 | 4.08 | 149.66 | 2.47 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, T.-I.; Chen, M.-H.; Lien, G.-W.; Chen, P.-S.; Lin, J.C.-C.; Fang, C.-C.; Chen, P.-C. Effects of Gender on the Association of Urinary Phthalate Metabolites with Thyroid Hormones in Children: A Prospective Cohort Study in Taiwan. Int. J. Environ. Res. Public Health 2017, 14, 123. https://doi.org/10.3390/ijerph14020123
Weng T-I, Chen M-H, Lien G-W, Chen P-S, Lin JC-C, Fang C-C, Chen P-C. Effects of Gender on the Association of Urinary Phthalate Metabolites with Thyroid Hormones in Children: A Prospective Cohort Study in Taiwan. International Journal of Environmental Research and Public Health. 2017; 14(2):123. https://doi.org/10.3390/ijerph14020123
Chicago/Turabian StyleWeng, Te-I, Mei-Huei Chen, Guang-Wen Lien, Pai-Shan Chen, Jasper Chia-Cheng Lin, Cheng-Chung Fang, and Pau-Chung Chen. 2017. "Effects of Gender on the Association of Urinary Phthalate Metabolites with Thyroid Hormones in Children: A Prospective Cohort Study in Taiwan" International Journal of Environmental Research and Public Health 14, no. 2: 123. https://doi.org/10.3390/ijerph14020123






