Parabens Accelerate Ovarian Dysfunction in a 4-Vinylcyclohexene Diepoxide-Induced Ovarian Failure Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animal Treatment
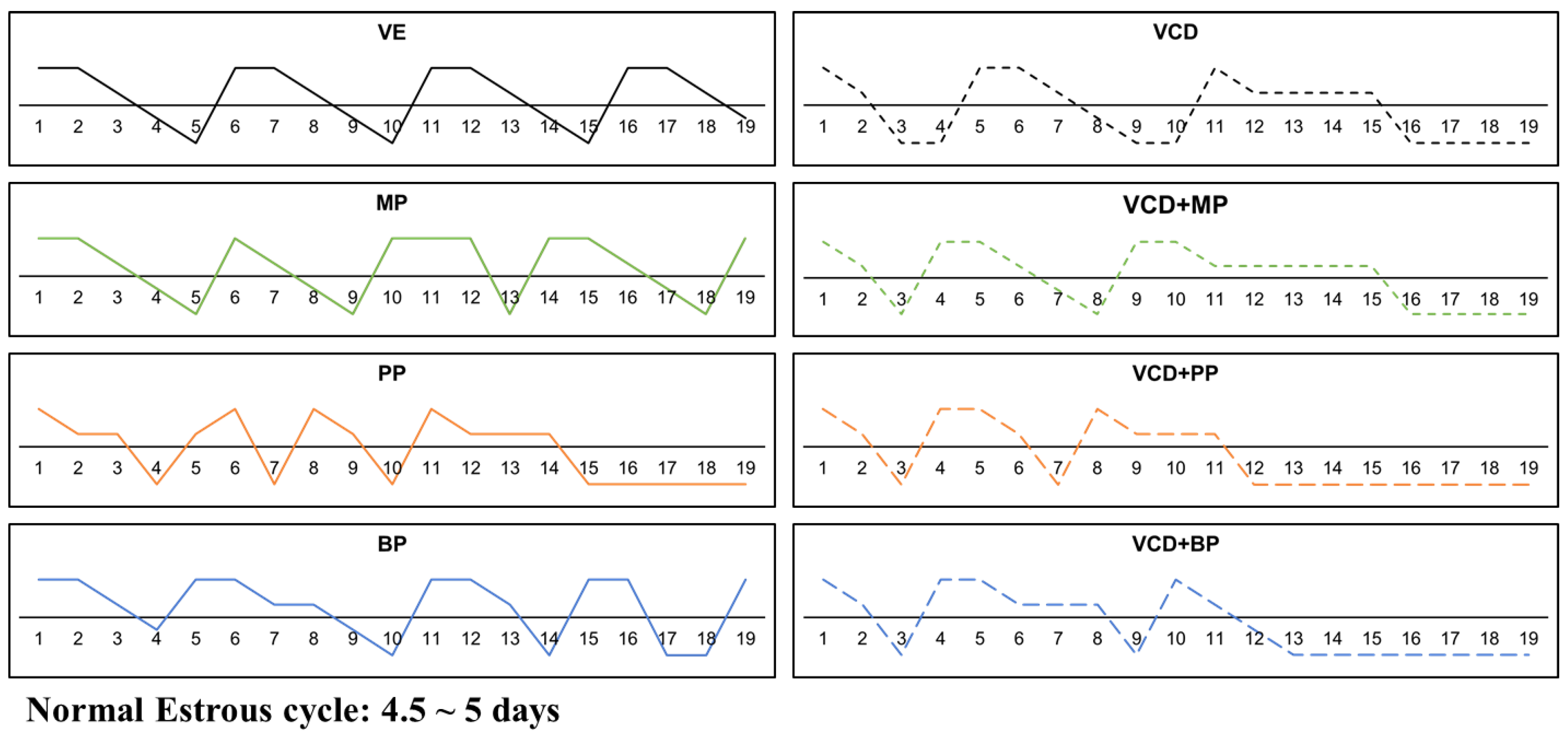
2.3. Determination of Estrus Cycle
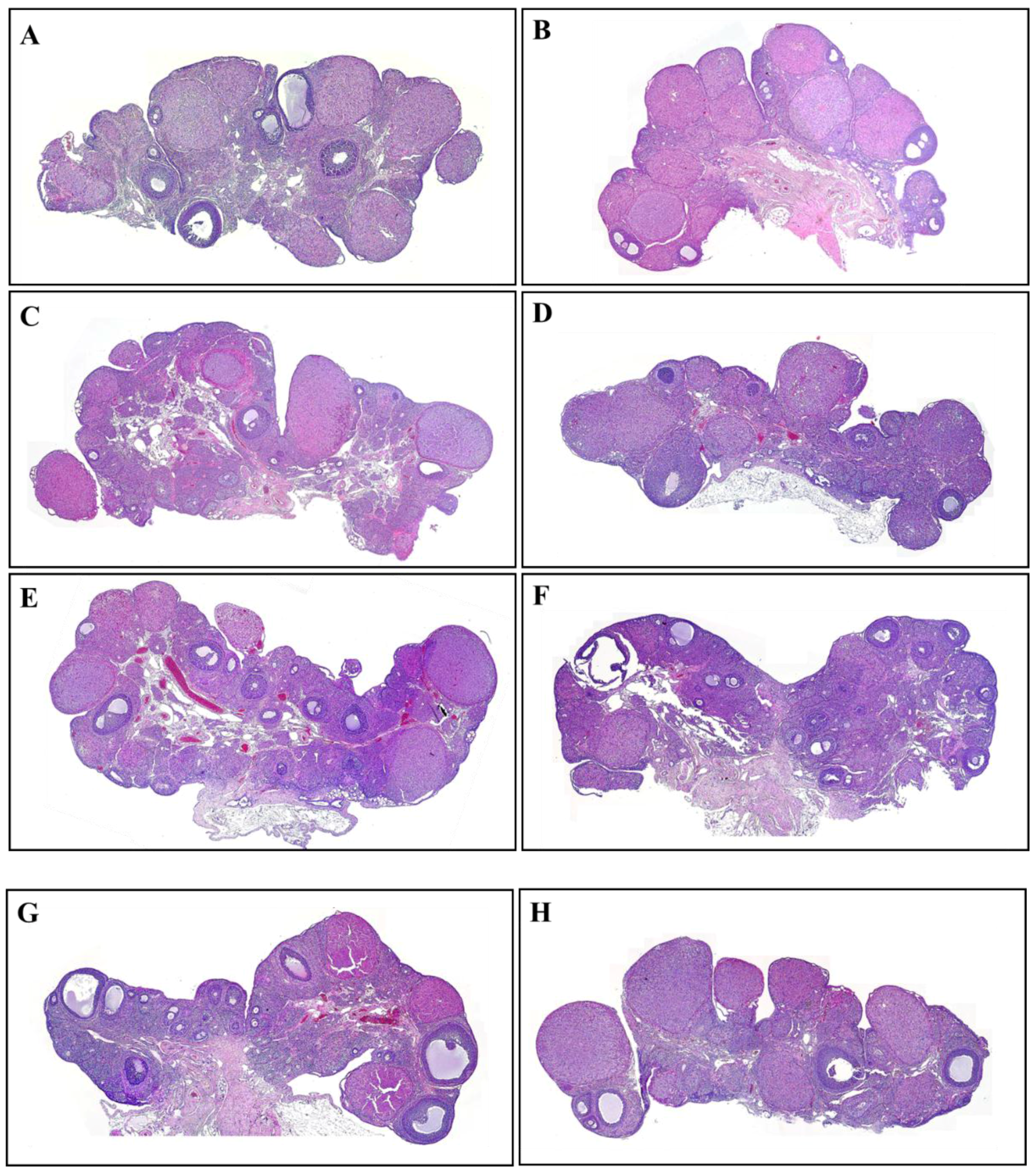
2.4. Hematoxylin and Eosin Staining
2.5. Quantitative Real-Time PCR
2.6. Serum Hormone Analysis
2.7. Data Analysis
3. Results
3.1. Estrus Cycle Changes after Response to the Administration of 4-Vinylcyclohexene Diepoxide and Parabens
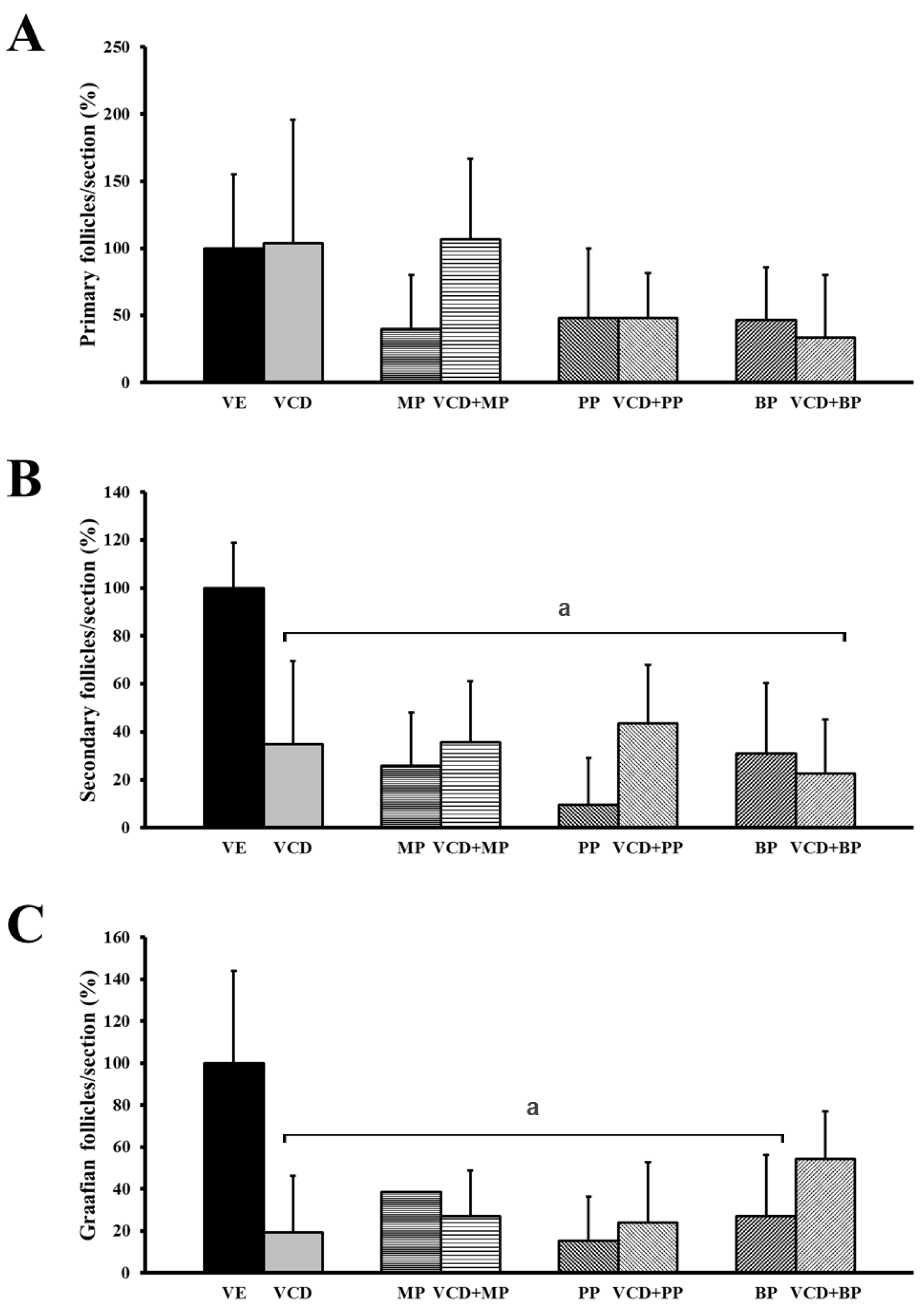
3.2. The Number and Development of Follicles Based on Histological Analysis
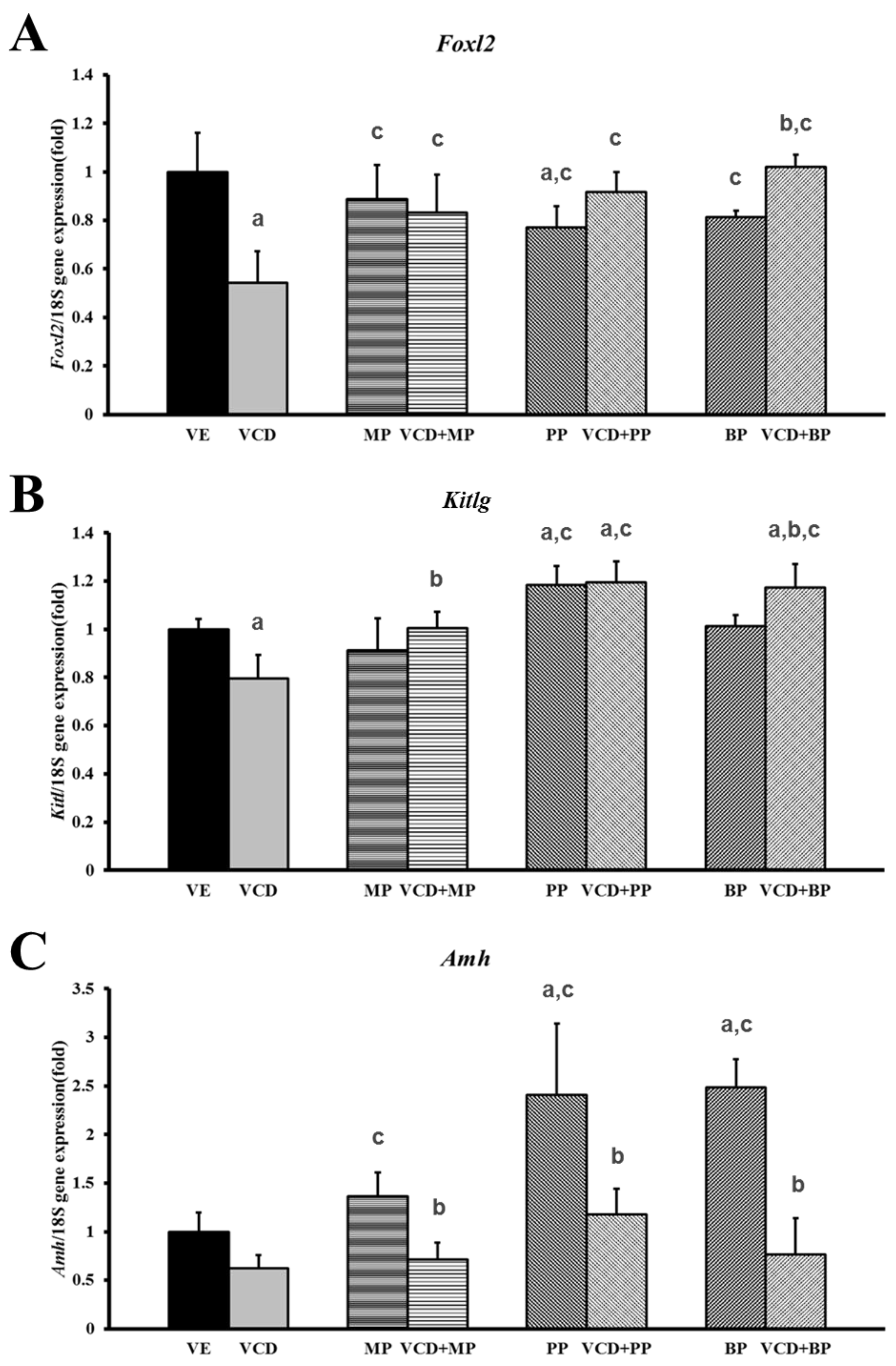
3.3. Effects of Parabens on Premature Ovarian Failure Model of Follicle Development-Related Genes
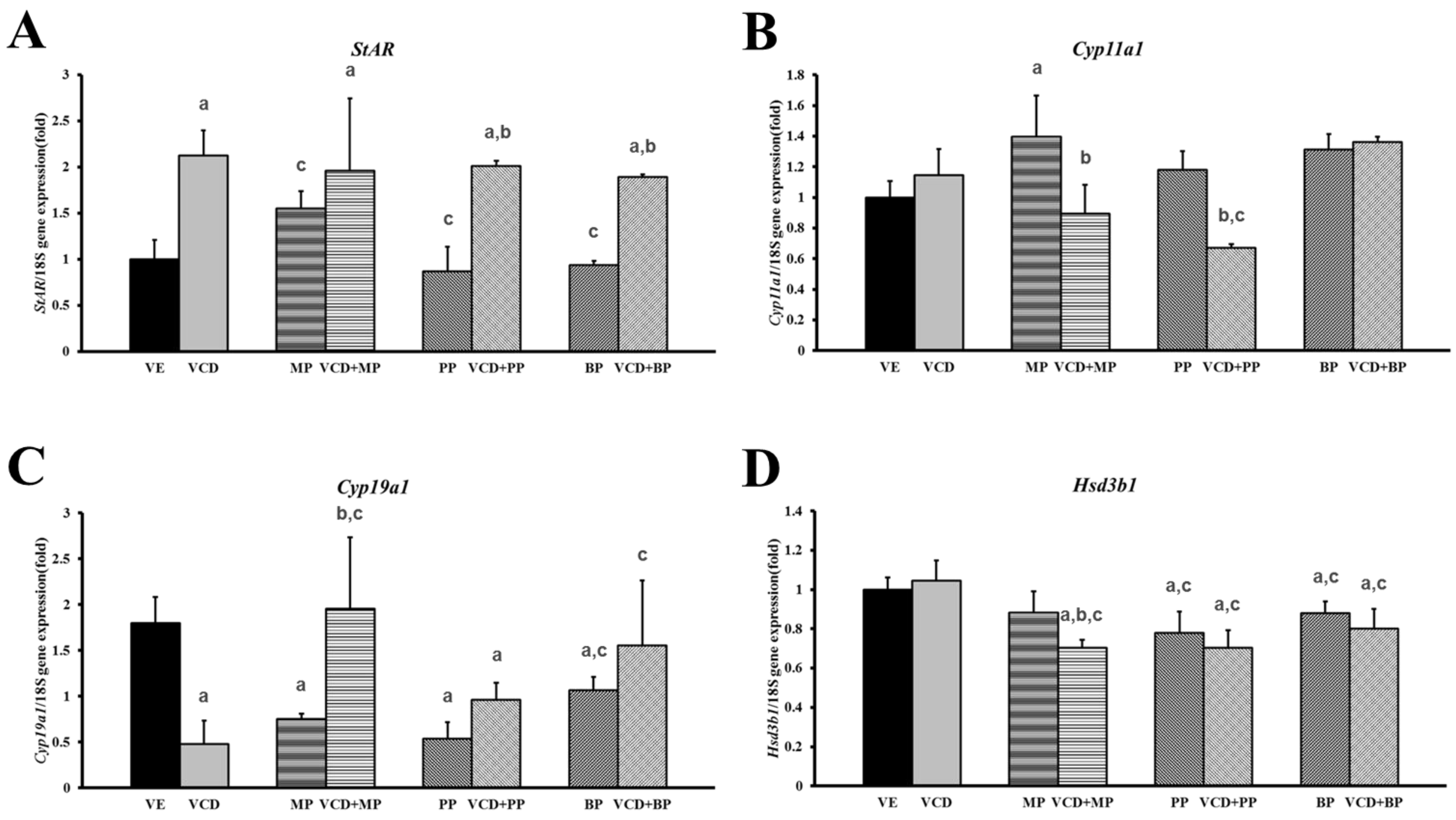
3.4. Changes in mRNA Expression Levels in the Premature Ovarian Failure Model of Steroidogenesis-Related Genes
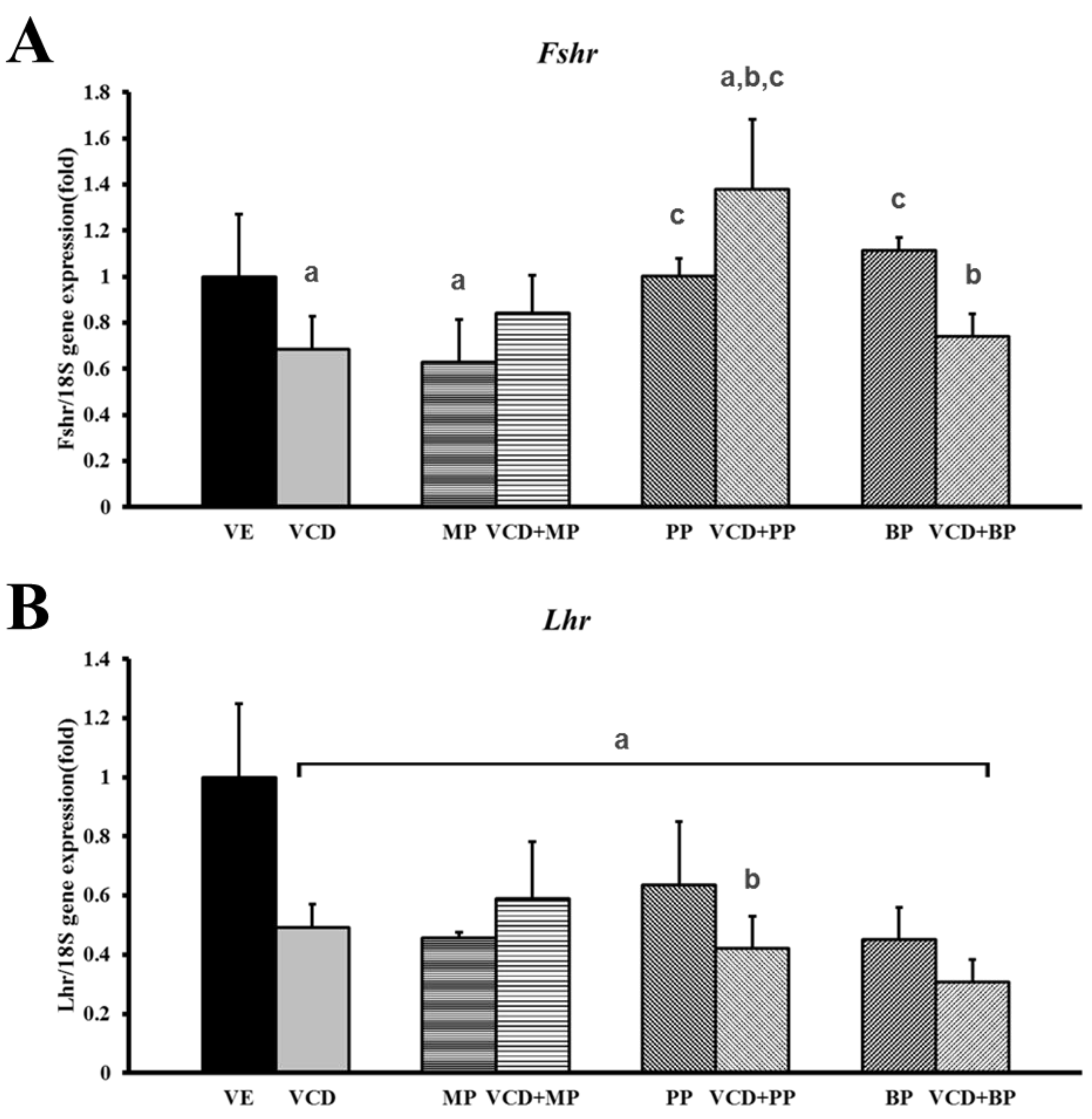
3.5. mRNA Expression of Hormone Receptors Following VCD and Paraben Treatment
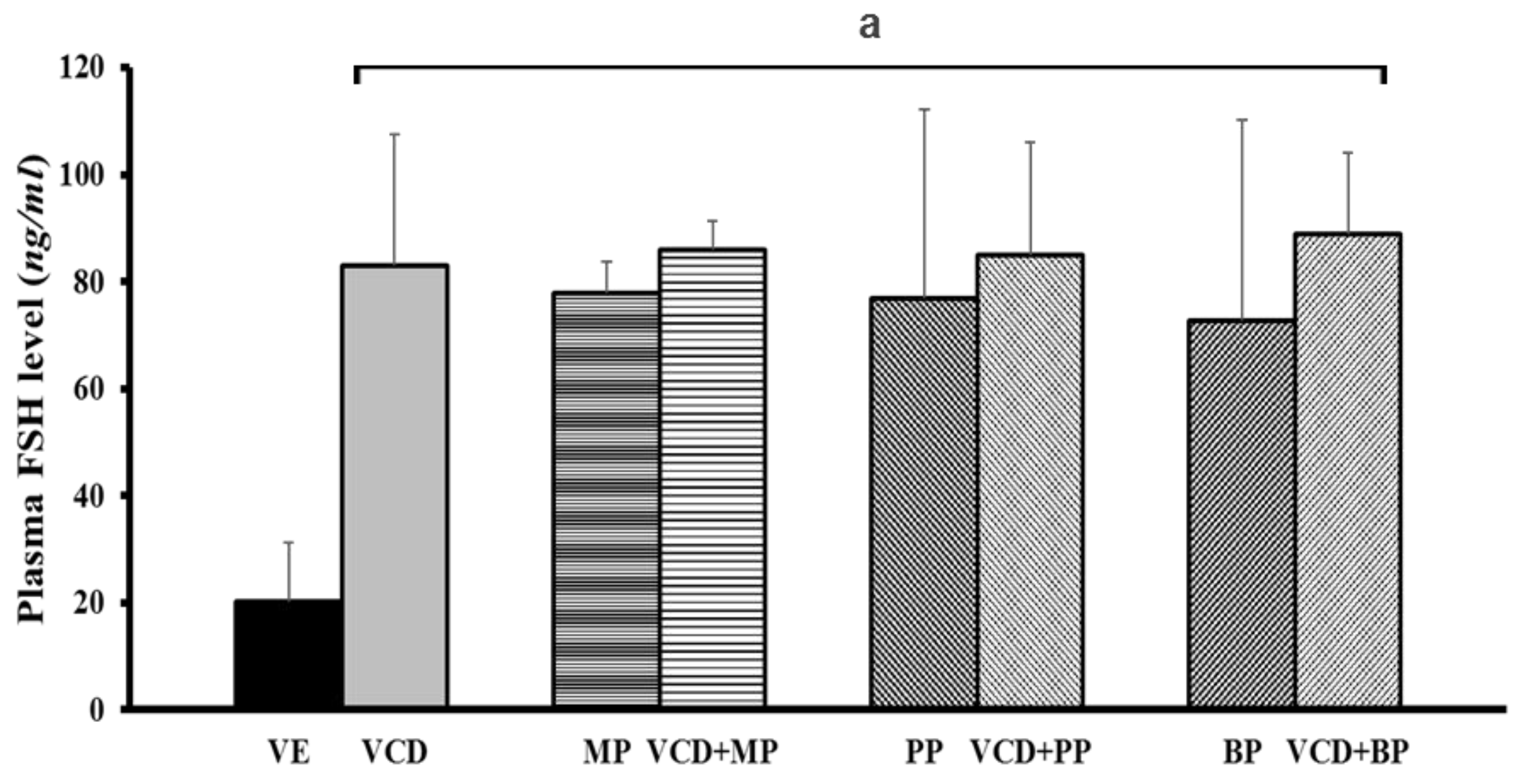
3.6. Follicle-Stimulating Hormone Levels in Each Experimental Group
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miyazaki, K.; Miki, F.; Uchida, S.; Masuda, H.; Uchida, H.; Maruyama, T. Serum estradiol level during withdrawal bleeding as a predictive factor for intermittent ovarian function in women with primary ovarian insufficiency. Endocr. J. 2015, 62, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Okeke, T.; Anyaehie, U.; Ezenyeaku, C. Premature menopause. Ann. Med. Health Sci. Res. 2013, 3, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, P.B.; Cannady, E.A.; Kroeger, N.A.; Sipes, I.G. Mechanisms of ovotoxicity induced by environmental chemicals: 4-Vinylcyclohexene diepoxide as a model chemical. Adv. Exp. Med. Biol. 2001, 500, 73–81. [Google Scholar] [PubMed]
- Wright, L.E.; Frye, J.B.; Lukefahr, A.L.; Marion, S.L.; Hoyer, P.B.; Besselsen, D.G.; Funk, J.L. 4-Vinylcyclohexene diepoxide (VCD) inhibits mammary epithelial differentiation and induces fibroadenoma formation in female sprague dawley rats. Reprod. Toxicol. 2011, 32, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Frye, J.B.; Lukefahr, A.L.; Wright, L.E.; Marion, S.L.; Hoyer, P.B.; Funk, J.L. Modeling perimenopause in sprague-dawley rats by chemical manipulation of the transition to ovarian failure. Comp. Med. 2012, 62, 193–202. [Google Scholar] [PubMed]
- Kappeler, C.J.; Hoyer, P.B. 4-Vinylcyclohexene diepoxide: A model chemical for ovotoxicity. Syst. Biol. Reprod. Med. 2012, 58, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.T.; An, B.S.; Yang, H.; Jung, E.M.; Hwang, I.; Jeung, E.B. Calbindin-D9K as a sensitive molecular biomarker for evaluating the synergistic impact of estrogenic chemicals on GH3 rat pituitary cells. Int. J. Mol. Med. 2012, 30, 1233–1240. [Google Scholar] [PubMed]
- Kim, Y.R.; Jung, E.M.; Choi, K.C.; Jeung, E.B. Synergistic effects of octylphenol and isobutyl paraben on the expression of calbindin-D(9)K in GH3 rat pituitary cells. Int. J. Mol. Med. 2012, 29, 294–302. [Google Scholar] [PubMed]
- Ahn, H.J.; An, B.S.; Jung, E.M.; Yang, H.; Choi, K.C.; Jeung, E.B. Parabens inhibit the early phase of folliculogenesis and steroidogenesis in the ovaries of neonatal rats. Mol. Reprod. Dev. 2012, 79, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Boberg, J.; Taxvig, C.; Christiansen, S.; Hass, U. Possible endocrine disrupting effects of parabens and their metabolites. Reprod. Toxicol. 2010, 30, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Jung, E.M.; An, B.S.; Hwang, I.; Vo, T.T.; Kim, S.R.; Lee, S.M.; Choi, K.C.; Jeung, E.B. Additional effects of bisphenol a and paraben on the induction of calbindin-D(9K) and progesterone receptor via an estrogen receptor pathway in rat pituitary GH3 cells. J. Physiol. Pharmacol. 2012, 63, 445–455. [Google Scholar] [PubMed]
- Shahed, A.; Young, K.A. Anti-mullerian hormone (AMH), inhibin-alpha, growth differentiation factor 9 (GDF9), and bone morphogenic protein-15 (BMP15) mrna and protein are influenced by photoperiod-induced ovarian regression and recrudescence in siberian hamster ovaries. Mol. Reprod. Dev. 2013, 80, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Zama, A.M.; Uzumcu, M. Epigenetic effects of endocrine-disrupting chemicals on female reproduction: An ovarian perspective. Front. Neuroendocrinol. 2010, 31, 420–439. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, S.M.; Keating, A.F.; Christian, P.J.; Sen, N.; Hoying, J.B.; Brooks, H.L.; Hoyer, P.B. Involvement of the kit/kitl signaling pathway in 4-vinylcyclohexene diepoxide-induced ovarian follicle loss in rats. Biol. Reprod. 2008, 79, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, I.B.; Laitinen, M.P.; Scott, J.E.; Louhio, H.; Velentzis, L.; Tuuri, T.; Aaltonen, J.; Ritvos, O.; Winston, R.M.; Hovatta, O. Kit ligand and c-kit are expressed during early human ovarian follicular development and their interaction is required for the survival of follicles in long-term culture. Reproduction 2006, 131, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Tuck, A.R.; Robker, R.L.; Norman, R.J.; Tilley, W.D.; Hickey, T.E. Expression and localisation of c-kit and kitl in the adult human ovary. J. Ovarian Res. 2015, 8, 31. [Google Scholar] [CrossRef]
- Kuo, F.T.; Bentsi-Barnes, I.K.; Barlow, G.M.; Pisarska, M.D. Mutant forkhead l2 (Foxl2) proteins associated with premature ovarian failure (pof) dimerize with wild-type Foxl2, leading to altered regulation of genes associated with granulosa cell differentiation. Endocrinology 2011, 152, 3917–3929. [Google Scholar] [CrossRef]
- Kocerha, J.; Prucha, M.S.; Kroll, K.J.; Steinhilber, D.; Denslow, N. Regulation of steroidogenic acute regulatory protein transcription in largemouth bass by orphan nuclear receptor signaling pathways. Endocrinology 2010, 151, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Trzeciak, W.H.; Ahmed, C.E.; Simpson, E.R.; Ojeda, S.R. Vasoactive intestinal peptide induces the synthesis of the cholesterol side-chain cleavage enzyme complex in cultured rat ovarian granulosa cells. Proc. Natl. Acad. Sci. USA 1986, 83, 7490–7494. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.T.; Jeung, E.B. An evaluation of estrogenic activity of parabens using uterine calbindin-d9k gene in an immature rat model. Toxicol. Sci. 2009, 112, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.T.; Yoo, Y.M.; Choi, K.C.; Jeung, E.B. Potential estrogenic effect(s) of parabens at the prepubertal stage of a postnatal female rat model. Reprod. Toxicol. 2010, 29, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Van Kempen, T.A.; Milner, T.A.; Waters, E.M. Accelerated ovarian failure: A novel, chemically induced animal model of menopause. Brain Res. 2011, 1379, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D. Exposure to environmental endocrine disrupting compounds and men’s health. Maturitas 2010, 66, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Flaws, J.A.; Doerr, J.K.; Sipes, I.G.; Hoyer, P.B. Destruction of preantral follicles in adult rats by 4-vinyl-1-cyclohexene diepoxide. Reprod. Toxicol. 1994, 8, 509–514. [Google Scholar] [CrossRef]
- Chen, H.; Perez, J.N.; Constantopoulos, E.; McKee, L.; Regan, J.; Hoyer, P.B.; Brooks, H.L.; Konhilas, J. A method to study the impact of chemically-induced ovarian failure on exercise capacity and cardiac adaptation in mice. J. Vis. Exp. 2014. [Google Scholar] [CrossRef] [PubMed]
- Lohff, J.C.; Christian, P.J.; Marion, S.L.; Arrandale, A.; Hoyer, P.B. Characterization of cyclicity and hormonal profile with impending ovarian failure in a novel chemical-induced mouse model of perimenopause. Comp. Med. 2005, 55, 523–527. [Google Scholar] [PubMed]
- Hu, X.; Roberts, J.R.; Apopa, P.L.; Kan, Y.W.; Ma, Q. Accelerated ovarian failure induced by 4-vinyl cyclohexene diepoxide in Nrf2 null mice. Mol. Cell. Biol. 2006, 26, 940–954. [Google Scholar] [CrossRef] [PubMed]
- Kezele, P.; Skinner, M.K. Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: Endocrine model of follicle assembly. Endocrinology 2003, 144, 3329–3337. [Google Scholar] [CrossRef] [PubMed]
- Wich, B.K.; Carnes, M. Menopause and the aging female reproductive system. Endocrinol. Metab. Clin. N. Am. 1995, 24, 273–295. [Google Scholar]
- McGee, E.A.; Hsueh, A.J. Initial and cyclic recruitment of ovarian follicles. Endocr. Rev. 2000, 21, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Parrott, J.A.; Skinner, M.K. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinology 1999, 140, 4262–4271. [Google Scholar] [CrossRef] [PubMed]
- Pisarska, M.D.; Barlow, G.; Kuo, F.T. Minireview: Roles of the forkhead transcription factor Foxl2 in granulosa cell biology and pathology. Endocrinology 2011, 152, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Mark-Kappeler, C.J.; Sen, N.; Keating, A.F.; Sipes, I.G.; Hoyer, P.B. Distribution and responsiveness of rat anti-mullerian hormone during ovarian development and vcd-induced ovotoxicity. Toxicol. Appl. Pharmacol. 2010, 249, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hannon, P.R.; Flaws, J.A. The effects of phthalates on the ovary. Front. Endocrinol. 2015, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Yeung, B.H.; Wan, H.T.; Law, A.Y.; Wong, C.K. Endocrine disrupting chemicals: Multiple effects on testicular signaling and spermatogenesis. Spermatogenesis 2011, 1, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Mayer, L.P.; Devine, P.J.; Dyer, C.A.; Hoyer, P.B. The follicle-deplete mouse ovary produces androgen. Biol. Reprod. 2004, 71, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Danilovich, N.; Roy, I.; Sairam, M.R. Emergence of uterine pathology during accelerated biological aging in fsh receptor-haploinsufficient mice. Endocrinology 2002, 143, 3618–3627. [Google Scholar] [CrossRef] [PubMed]
| Species | Gene | Primer Sequence (5’→3’) | Accession Number | Product Size | Location of Primer Start |
|---|---|---|---|---|---|
| Rat | Foxl2 | F: TCGCTAAGTTCCCGTTCTAC | XM_003750571.4 | 173 | 301 |
| R: GTAATTGCCCTTCTCGAACA | |||||
| Kitlg | F: TTCAAGGACTTCATGGTGGC | NM_021843.4 | 164 | 451 | |
| R: GCGGCTTTCCTATTACTGCT | |||||
| Amh | F: CTGGCTGAAGTGATATGGGA | NM_012902.1 | 198 | 391 | |
| R: CACAGTCAGCACCAAATAGC | |||||
| Star | F: GCGGAACATGAAAGGACTGA | NM_031558.3 | 184 | 51 | |
| R: TCCTTGCTGGATGTAGGACA | |||||
| Cyp11a1 | F: GCTTTGCCTTTGAGTCCATC | NM_017286.3 | 189 | 605 | |
| R: CATGGTCCTTCCAGGTCTTA | |||||
| Cyp19a1 | F: GGCAAGCACTCCTTATCAAACC | NM_017085.2 | 197 | 671 | |
| R: TCCACGTCTCTCAGCGAAAA | |||||
| Hsd3b1 | F: TGCCACTTGGTCACACTGTCA | NM_001007719.3 | 148 | 949 | |
| R: CCCTGTGCTGCTCCACTAGTGT | |||||
| Fshr | F: CTTGAAGCGGCAAATCTCTG | NM_199237.1 | 198 | 910 | |
| R: GAGCAGGTCACATCAACAAC | |||||
| Lhcgr | F: CTCACTGAAAACACTGCCCT | NM_001007719.3 | 198 | 817 | |
| R: ATGGCGGAATAAAGCGTCTC | |||||
| Rn18s | F: CTCAACACGGGAAACCTCAC | NR_046237.1 | 110 | 1251 | |
| R: CGCTCCACCAACTAAGAACG |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Lee, M.; Ahn, C.; Kang, H.Y.; Tran, D.N.; Jeung, E.-B. Parabens Accelerate Ovarian Dysfunction in a 4-Vinylcyclohexene Diepoxide-Induced Ovarian Failure Model. Int. J. Environ. Res. Public Health 2017, 14, 161. https://doi.org/10.3390/ijerph14020161
Lee J-H, Lee M, Ahn C, Kang HY, Tran DN, Jeung E-B. Parabens Accelerate Ovarian Dysfunction in a 4-Vinylcyclohexene Diepoxide-Induced Ovarian Failure Model. International Journal of Environmental Research and Public Health. 2017; 14(2):161. https://doi.org/10.3390/ijerph14020161
Chicago/Turabian StyleLee, Jae-Hwan, Myeongho Lee, Changhwan Ahn, Hee Young Kang, Dinh Nam Tran, and Eui-Bae Jeung. 2017. "Parabens Accelerate Ovarian Dysfunction in a 4-Vinylcyclohexene Diepoxide-Induced Ovarian Failure Model" International Journal of Environmental Research and Public Health 14, no. 2: 161. https://doi.org/10.3390/ijerph14020161






