Molecular Characterization of Mycolactone Producing Mycobacteria from Aquatic Environments in Buruli Ulcer Non-Endemic Areas in Côte d’Ivoire
Abstract
:1. Introduction
2. Materials and Methods
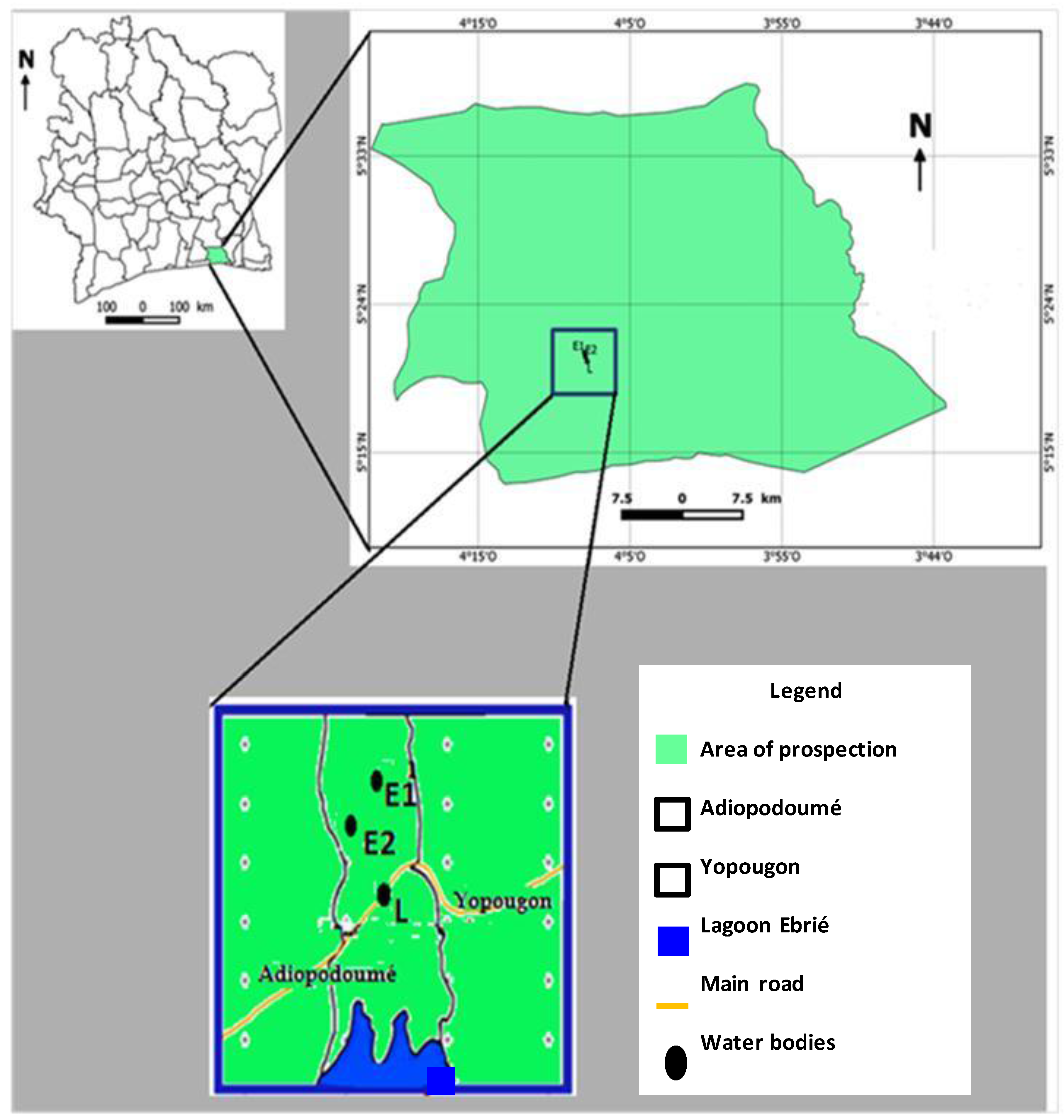
2.1. Study Site
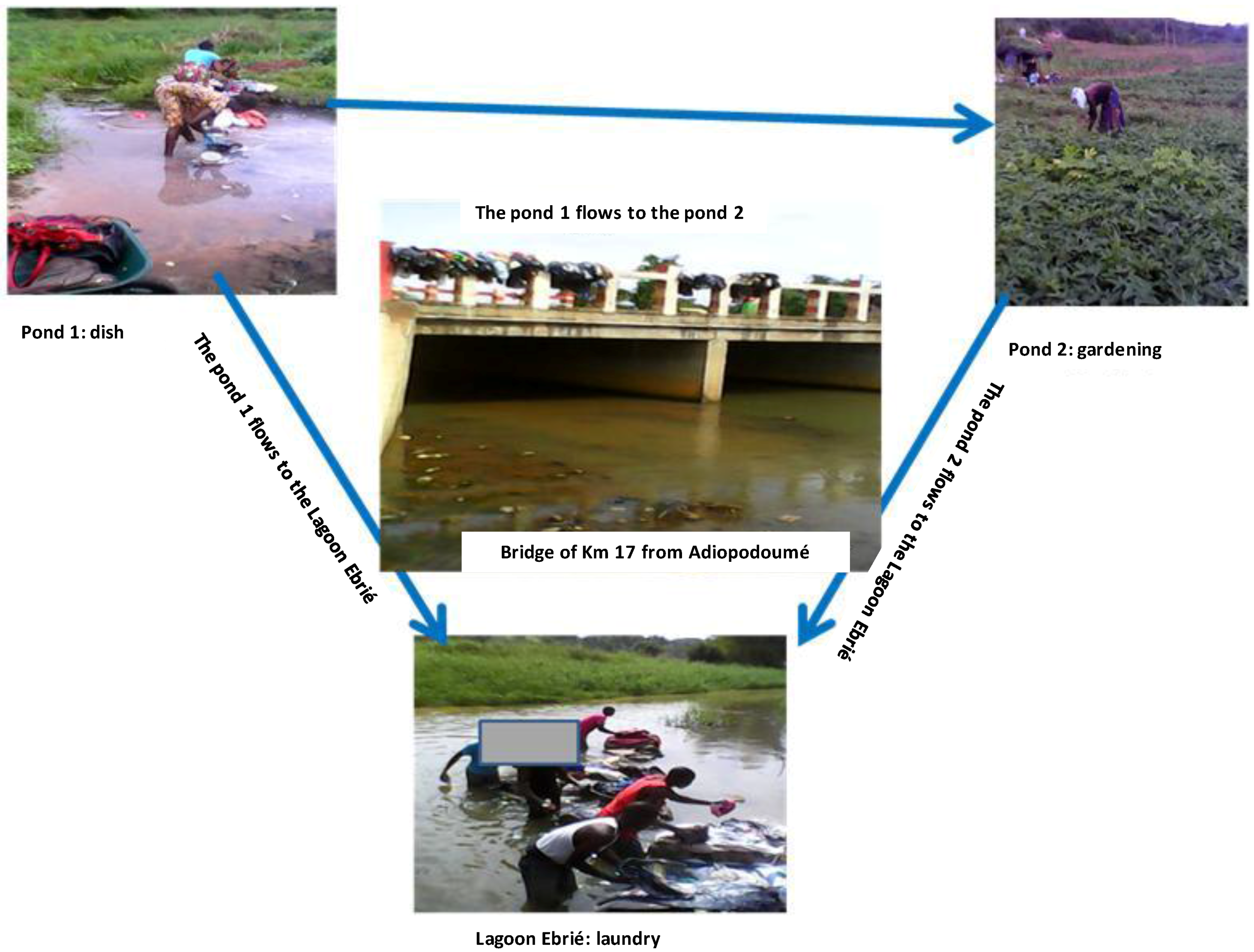
2.2. Aquatic Environmental Samples Collection
2.3. Molecular Characterization of Mycolactone Producing Mycobacteria
2.4. Statistical Analysis
3. Results
3.1. Distribution of Mycobacteria in Aquatic Environment from Adiopodoumé
3.2. Distribution of Mycolactone Producing Mycobacteria in Aquatic Environment from Adiopodoumé Using IS2404 Typing
3.3. Distribution of Mycolactone Producing Mycobacteria in Aquatic Environment from Adiopodoumé Using ER Typing
3.4. Discrimination between Mycolactone Producing Mycobacteria Species from the Adiopodoumé Aquatic Environment
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kazda, J. The Chronology of Mycobacteria and the Development of Mycobacterial Ecology. In The Ecology of Mycobacteria, 1st ed.; Springer Netherlands: Dordrecht, The Netherlands, 2009; pp. 1–11. [Google Scholar]
- N’Guessan, K.; Kouassi, Y.; Bouzid, S.; Ehuie, P.; Koffi, K.; Oniangue, C.; Aka, N.; Dosso, M. Value and limits of microscopy of exudates in Mycobacterium ulcerans cutaneous infection in Côte d’Ivoire. Bull. Soc. Pathol. Exot. 2001, 94, 9–10. [Google Scholar] [PubMed]
- Ranger, B.S.; Mahrous, E.A.; Mosi, L.; Adusumilli, S.; Lee, R.E.; Colorni, A.; Rhodes, M.; Small, P.L. Globally distributed mycobacterial fish pathogens produce a novel plasmid-encoded toxic macrolide, mycolactone F. Infect. Immun. 2006, 74, 6037–6045. [Google Scholar] [CrossRef] [PubMed]
- Merritt, R.W.; Walker, E.D.; Small, P.L.; Wallace, J.R.; Johnson, P.D.; Benbow, M.E.; Boakye, D.A. Ecology and transmission of Buruli ulcer disease: A systematic review. PLoS Negl. Trop. Dis. 2010, 4, e911. [Google Scholar] [CrossRef] [PubMed]
- Ablordey, A.; Amissah, D.A.; Aboagye, I.F.; Hatano, B.; Yamazaki, T.; Sata, T.; Ishikawa, K.; Katano, H. Detection of Mycobacterium ulcerans by the loop-mediated isothermal amplification method. PLoS Negl. Trop. Dis. 2012, 6, e1590. [Google Scholar] [CrossRef] [PubMed]
- Debacker, M.; Aguiar, J.; Steunou, C.; Zinsou, C.; Meyers, W.M.; Guédénon, A.; Scott, J.T.; Dramaix, M.; Portaels, F. Mycobacterium ulcerans Disease (Buruli Ulcer) in Rural Hospital, Southern Benin, 1997–2001. Emerg. Infect. Dis. 2004, 10, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Amofah, G.; Bonsu, F.; Tetteh, C.; Okrah, J.; Asamoa, K.; Asiedu, K.; Addy, J. Buruli ulcer in Ghana: Results of a national case search. Emerg. Infect. Dis. 2002, 8, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Ahoua, L.; Aka, N.; Ekaza, E.; Bouzid, S.; N’Guessan, R.; Dosso, M. Risk factors for Buruli ulcer in Côte d’Ivoire: Results of a case-control study. Afr. J. Biotechnol. 2009, 8, 536–546. [Google Scholar]
- Wagner, T.; Benbow, M.E.; Brenden, T.O.; Qi, J.; Johnson, R.C. Buruli ulcer disease prevalence in Benin, West Africa: Associations with land use/cover and the identification of disease clusters. Int. J. Health Geogr. 2008, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Williamson, H.R.; Benbow, M.E.; Nguyen, K.D.; Beachboard, D.C.; Kimbirauskas, R.K.; McIntosh, M.D.; Quaye, C.; Ampadu, E.O.; Boakye, D.; Merritt, R.W.; et al. Distribution of Mycobacterium ulcerans in Buruli ulcer endemic and non-endemic aquatic sites in Ghana. PLoS Negl. Trop. Dis. 2008, 2, e205. [Google Scholar] [CrossRef] [PubMed]
- Kanga, J.M.; Kacou, D.E. Aspects épidémiologiques de l’ulcère de Buruli en Côte d’Ivoire: Résultats d’une enquête nationale. Bull. Soc. Pathol. Exot. 2001, 94, 46–51. [Google Scholar] [PubMed]
- Résumé du bilan 2013 du Programme National de Lutte contre l'ulcère de Buruli. Aspect clinique, épidémiologique et thérapeutique; PNLUB: Abidjan, Côte d’Ivoire, 2014.
- Aiga, H.; Amano, T.; Cairncross, S.; Adomako, J.; Nanas, O.K.; Coleman, S. Assessing water-related risk factors for Buruli ulcer: A case-control study in Ghana. Am. J. Trop. Med. Hyg. 2004, 71, 387–392. [Google Scholar] [PubMed]
- Brou, T.; Broutin, H.; Elguero, E.; Asse, H.; Guegan, J.F. Landscape diversity related to Buruli ulcer disease in Côte d’Ivoire. PLoS Negl. Trop. Dis. 2008, 2, e271. [Google Scholar] [CrossRef] [PubMed]
- Raghunathan, P.L.; Whitney, E.A.; Asamoa, K.; Stienstra, Y.; Taylor, T.H., Jr.; Amofah, G.K.; Ofori-Adjei, D.; Dobos, K.; Guarner, J.; Martin, S.; et al. Risk factors for Buruli ulcer disease (Mycobacterium ulcerans Infection): Results from a case-control study in Ghana. Clin. Infect. Dis. 2005, 40, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Ekaza, E.; Kacou-N’douba, A.; Oniangue, N.C.; Ehuie, P.; N’Guessan, K.R.; Aka, N.; Bouzid, S.A.; Faye-Kette, H.; Dosso, M. Apport de l’amplification génique dans la détection de Mycobacterium ulcerans dans les exsudats et les biopsies cutanées en Côte d’Ivoire. Bull. Soc. Pathol. Exot. 2004, 97, 95–96. [Google Scholar] [PubMed]
- Stinear, T.P.; Seemann, T.; Pidot, S.; Frigui, W.; Reysset, G.; Garnier, T.; Meurice, G.; Simon, D.; Bouchier, C.; Ma, L.; et al. Reductive evolution and niche adaptation inferred from the genome of Mycobacterium ulcerans, the causative agent of Buruli ulcer. Genome Res. 2007, 17, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Yoder, S.; Argueta, C.; Holtzman, A.; Aronson, T.; Berlin, O.G.W.; Tomasek, P.; Glover, N.; Froman, S.; Stelma, G.J. PCR comparison of Mycobacterium avium isolates obtained from patients and foods. Appl. Environ. Microbiol. 1999, 65, 2650–2653. [Google Scholar] [PubMed]
- Lavender, C.J.; Stinear, T.P.; Johnson, P.D.; Azuolas, J.; Benbow, M.E.; Wallace, J.R.; Fyfe, J.A. Evaluation of VNTR typing for the identification of Mycobacterium ulcerans in environmental samples from Victoria, Australia. FEMS Microbiol. Lett. 2008, 287, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, J.A.; Lavender, C.J.; Johnson, P.D.R.; Globan, M.; Sievers, A.; Azuolas, J.; Stinear, T.P. Development and application of two multiplex real-time PCR assays for the detection of Mycobacterium ulcerans in clinical and environmental samples. Appl. Environ. Microbiol. 2007, 73, 4733–4740. [Google Scholar] [CrossRef] [PubMed]
- Narh, C.A.; Mosi, L.; Quaye, C.; Dassi, C.; Konan, D.O.; Tay, S.C.K.; de Souza, D.K.; Boakye, D.A.; Bonfoh, B. Source Tracking Mycobacterium ulcerans Infections in the Ashanti Region, Ghana. PLoS Negl. Trop. Dis. 2015, 9, e0003437. [Google Scholar] [CrossRef] [PubMed]
- Portaels, F.; World Health Organization. Laboratory Diagnosis of Buruli Ulcer: A Manual for Health Care Providers; World Health Organization: Geneva, Switzerland, 2014; Available online: http://www.who.int/iris/handle/10665/111738 (accessed on 17 January 2017).
- Williamson, H.; Phillips, R.; Sarfo, S.; Wansbrough-Jones, M.; Small, P. Genetic Diversity of PCR-Positive, Culture-Negative and Culture-Positive Mycobacterium ulcerans Isolated from Buruli Ulcer Patients in Ghana. PLoS ONE 2014, 9, e88007. [Google Scholar]
- Aka, N.; Ekaza, E.; Coulibaly-Ngolo, M.D.; Kouadio, K.; Koffi, L.; Coulibaly, B.; Kodia, M.; Ngazoa-Kakou, S.; N’Guessan, K.R.; Yapo-Crézoit, A.; et al. Buruli ulcer in Côte d’Ivoire: a new disease focus identified in Adiopodoumé, Yopougon commune (Abidjan, Km 17). In Abstracts of the annual meeting of the WHO Global Ulcer Initiative, 22–24 March 2010; WHO Headquarters: Geneva, Switzerland, 2010; p. 161. [Google Scholar]
- Hughes, M.S.; Skuce, R.A.; Beck, L.A.; Neill, S.D. Identification of mycobacteria from animals by restriction enzyme analysis and direct DNA cycle sequencing of polymerase chain reaction-amplified 16S rRNA gene sequences. J. Clin. Microbiol. 1993, 31, 3216–3222. [Google Scholar]
- Radomski, N. Sources des mycobactéries non tuberculeuses dans les bassins versants. Ph.D. Thesis, Université Paris-Est, Paris, France, 2011. [Google Scholar]
- Kirschner, P.; Bottger, E.C. Species identification of mycobacteria using rDNA sequencing. In Mycobacteria Protocols (Methods in Molecular Biology); Parish, T.S.N., Ed.; Humana Press Inc.: Totowa, NJ, USA, 1998; Volume 101, pp. 349–361. [Google Scholar]
- Ngazoa-Kakou, E.S.; Ekaza, E.; Aka, N.; Coulibaly-N’Golo, D.; Coulibaly, B.; Dosso, M. Evaluation of real-time PCR for Mycobacterium ulcerans in endemic region in Côte d’Ivoire. Afr. J. Microbiol. Res. 2011, 5, 2211–2216. [Google Scholar]
- Stinear, T.P.; Pryor, M.J.; Porter, J.L.; Cole, S.T. Functional analysis and annotation of the virulence plasmid pMUM001 from Mycobacterium ulcerans. Microbiology 2005, 151, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Marsollier, L.; Stinear, T.; Aubry, J.; Saint André, J.P.; Robert, R.; Legras, P.; Manceau, A.-L.; Audrain, C.; Bourdon, S.; Kouakou, H.; et al. Aquatic Plants Stimulate the Growth of and Biofilm Formation by Mycobacterium ulcerans in Axenic Culture and Harbor These Bacteria in the Environment. Appl. Environ. Microbiol. 2004, 70, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
| Primer Name | Forward and Reverse Primer Sequences | Size of Amplicon (bp) | References |
|---|---|---|---|
| 16S rRNA | PA: 5′-AGAGTTTGATCCTGGCTCAG-3′ MSHA: 5′-AAAAAGCGACAAACCTACGAG-3′ | 620 | [25] |
| IS2404 Nested 1 | pGp1: 5′-AGGGCAGCGCGGTGATACGG-3′ pGp2: 5′-CAGTGGATTGGTGCCGATCGAG-3′ | 400 | [5] |
| IS2404 Nested 2 | pGp3: 5′-GGCGCAGATCAACTTCGCGGT-3′ pGp4: 5′-CTGCGTGGTGCTTTACGCGC-3′ | 210 | [5] |
| ER | LMF: 5′-GAGATCGGTCCCGACGTCTAC-3′ LMR: 5′-GGCTTGACTCATGTCACGTAAG-3′ | 420 | [10] |
| Locus 6 | R-5′-GACATCGAAGAGGTGTGCCGTCT-3′ F-5′-GACCGTCATGTCGTTCGATCCTAGT-3′ | variable | [10] |
| Locus 19 | R-5′-TGGCGACGATCGAGTCTC-3′ F-5′-CCGACGGATGAATCTGTAGGT-3′ | variable | [10] |
| MIRU1 | R-5′-GCCCTCGGGAATGTGGTT-3′ F-5′-GCTGGTTCATGCGTGGAAG-3′ | variable | [10] |
| ST1 | R-5′-CGCCACCCGCGGACACAGTCG-3′ F-5′-CTGAGGGGATTTCACGACCAG-3′ | variable | [10] |
| Environmental Samples | 16S rRNA Positives (%) |
|---|---|
| Plant biofilms | 12/18 (66.7 a) |
| Water filtrates | 12/12 (100 a) |
| Plant detritus | 8/15 (53.3 a) |
| Soils | 8/15 (53.3 a) |
| Environmental Samples | IS2404 Positives (%) |
|---|---|
| Plant biofilms | 8/12 (66.7 a) |
| Water filtrates | 6/12 (50 a) |
| Plant detritus | 5/8 (62.5 a) |
| Soils | 4/8 (50 a) |
| Environmental Samples | ER-Positives (%) |
|---|---|
| Plant biofilms | 2/8 (25 a) |
| Water filtrates | 2/6 (33.3 a) |
| Plant detritus | 5/5 (100 a) |
| Soils | 1/4 (25 a) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tano, M.B.; Dassi, C.; Mosi, L.; Koussémon, M.; Bonfoh, B. Molecular Characterization of Mycolactone Producing Mycobacteria from Aquatic Environments in Buruli Ulcer Non-Endemic Areas in Côte d’Ivoire. Int. J. Environ. Res. Public Health 2017, 14, 178. https://doi.org/10.3390/ijerph14020178
Tano MB, Dassi C, Mosi L, Koussémon M, Bonfoh B. Molecular Characterization of Mycolactone Producing Mycobacteria from Aquatic Environments in Buruli Ulcer Non-Endemic Areas in Côte d’Ivoire. International Journal of Environmental Research and Public Health. 2017; 14(2):178. https://doi.org/10.3390/ijerph14020178
Chicago/Turabian StyleTano, Marcellin B., Christelle Dassi, Lydia Mosi, Marina Koussémon, and Bassirou Bonfoh. 2017. "Molecular Characterization of Mycolactone Producing Mycobacteria from Aquatic Environments in Buruli Ulcer Non-Endemic Areas in Côte d’Ivoire" International Journal of Environmental Research and Public Health 14, no. 2: 178. https://doi.org/10.3390/ijerph14020178






