Correlation between Prenatal Exposure to Polybrominated Diphenyl Ethers (PBDEs) and Infant Birth Outcomes: A Meta-Analysis and an Experimental Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Analysis of the Results of Epidemiological Studies
2.1.1. Search Strategy
2.1.2. Selection Criteria
2.1.3. Data Extraction and Quality Assessment
2.1.4. Data Analysis
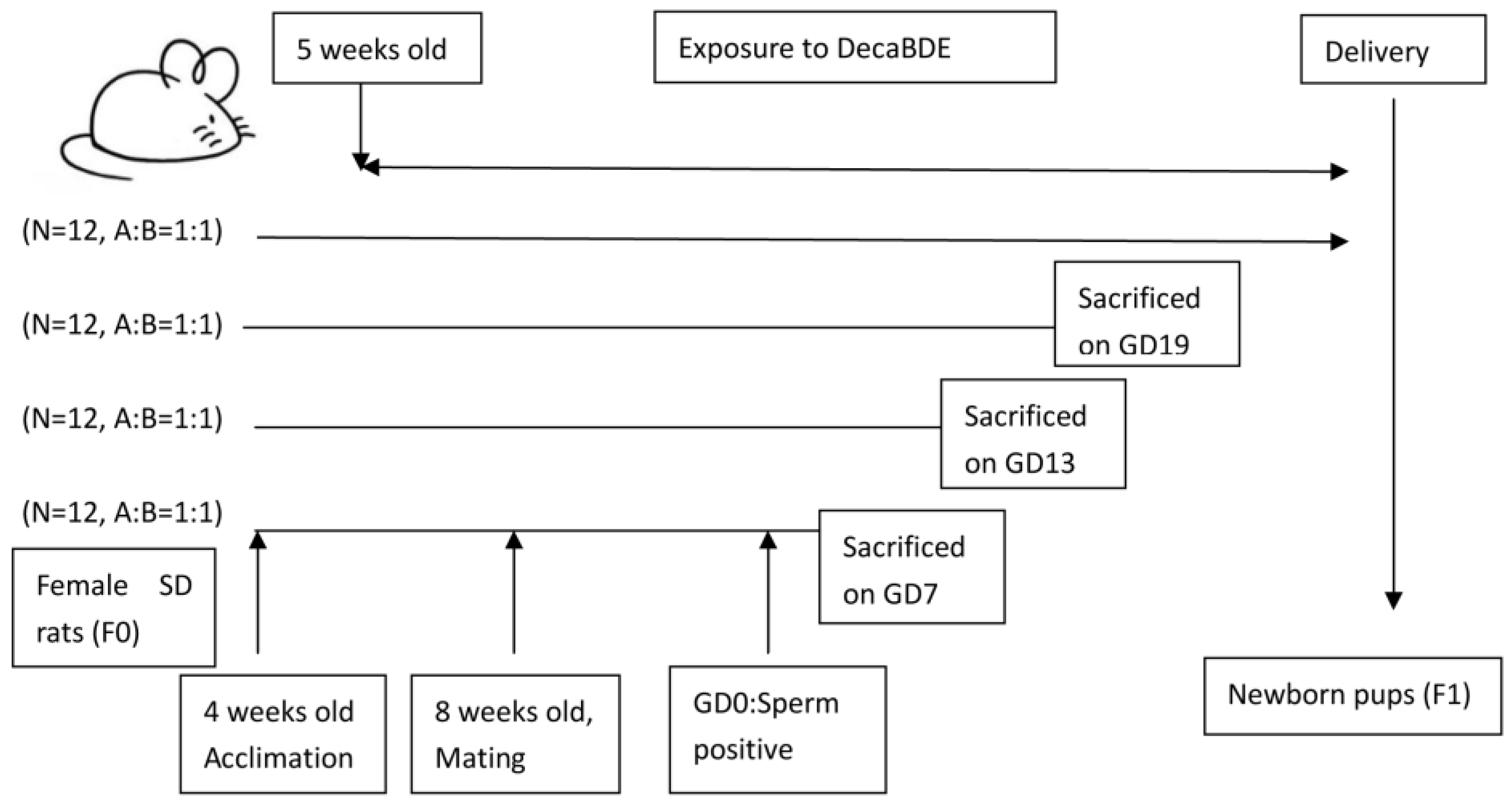
2.2. Animal Study Design and Samples
2.2.1. Animals and Housing
2.2.2. Experimental Design
2.2.3. Data Analysis
3. Results
3.1. Analysis of the Results of Human Studies
3.1.1. The Correlation between PEP and Birth Weight
3.1.2. The Correlation between PEP and Other Birth Outcomes
3.2. Experimental Animal Study
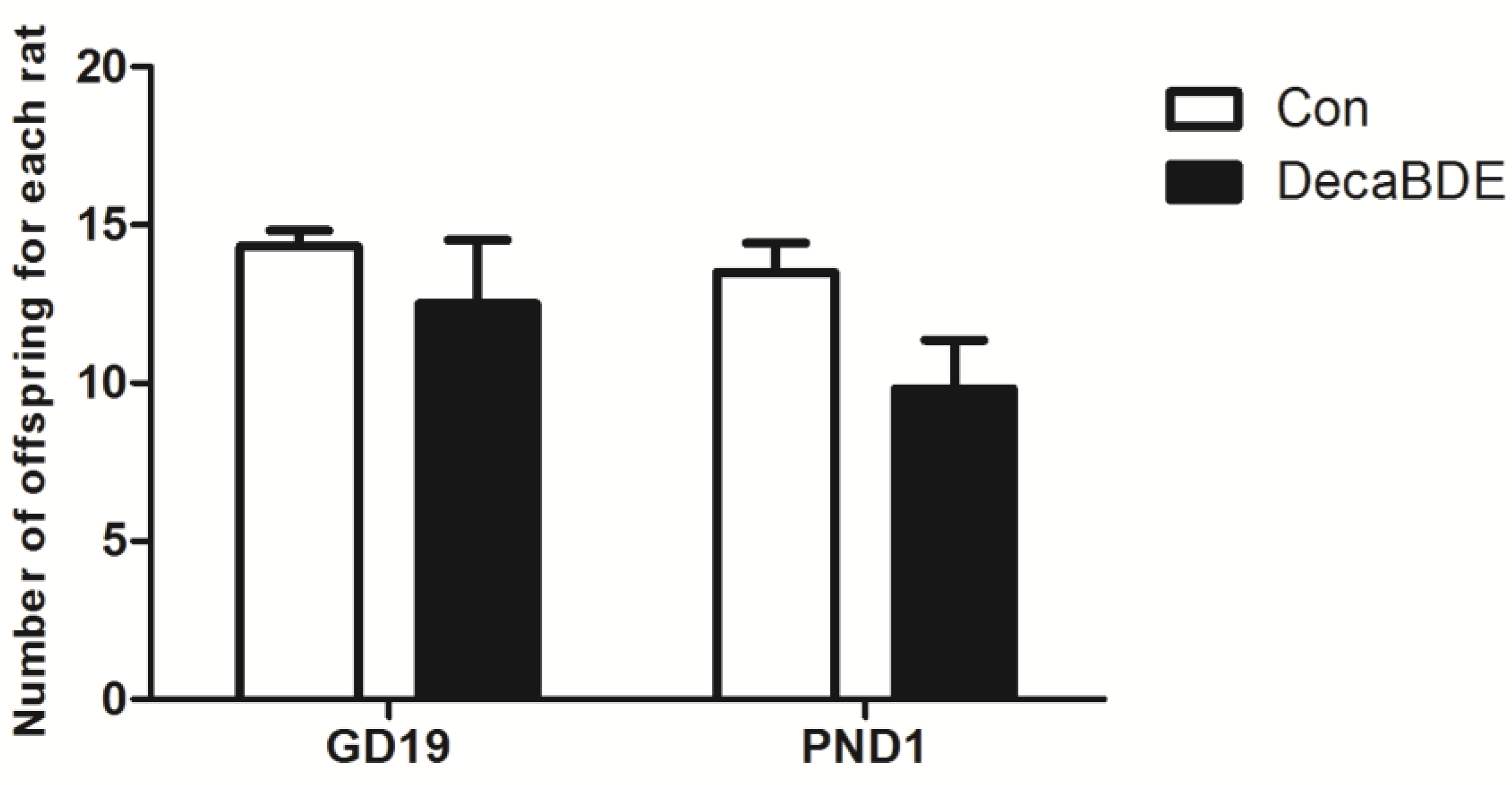
3.2.1. The Effect of PEP on the Number of Embryos or Fetuses during Pregnancy and Pups after Delivery
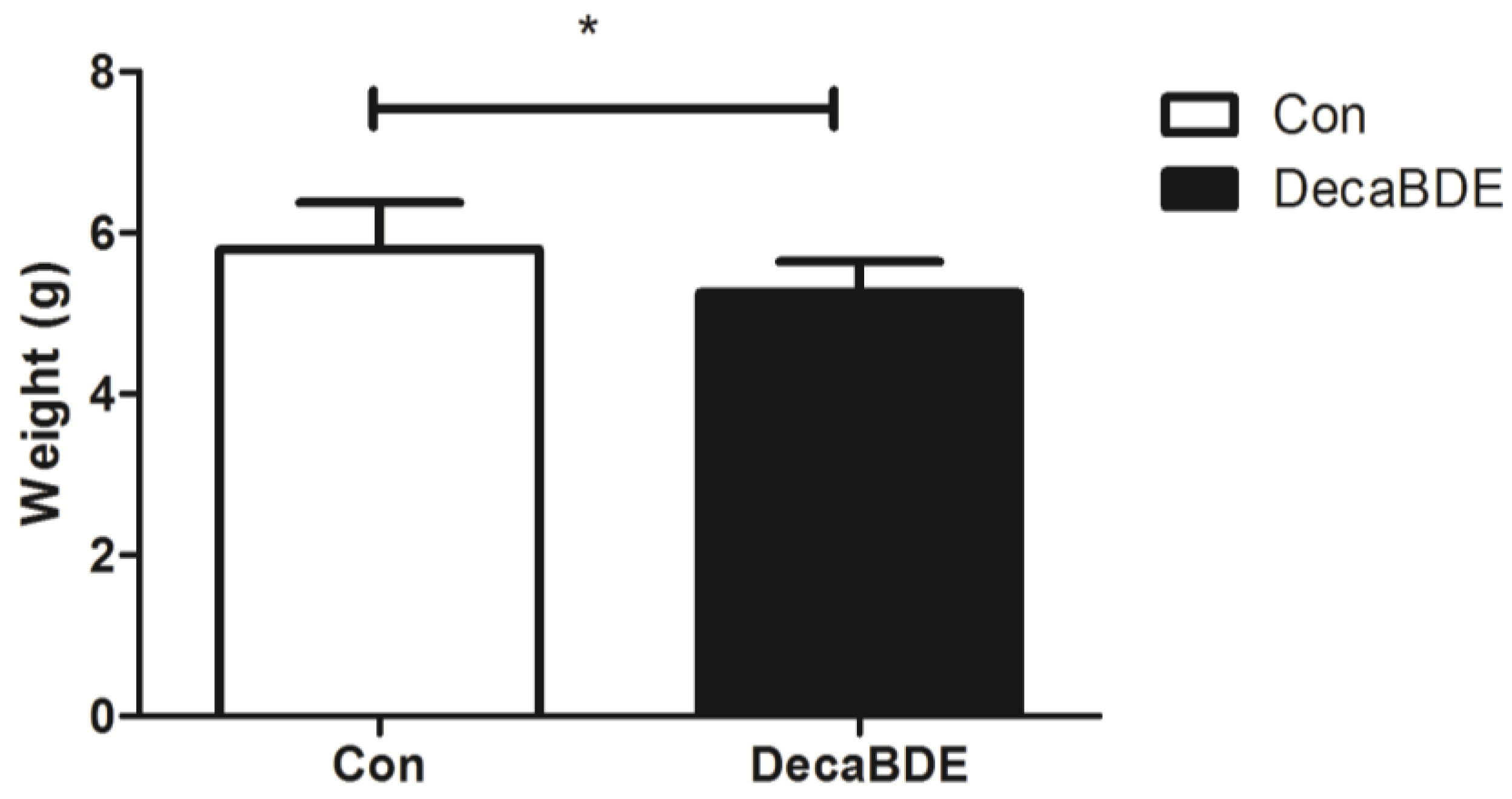
3.2.2. The Effect of PEP on the Birth Outcomes of Newborn Pups
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sjodin, A.; Patterson, D.G., Jr.; Bergman, A. A review on human exposure to brominated flame retardants—Particularly polybrominated diphenyl ethers. Environ. Int. 2003, 29, 829–839. [Google Scholar] [CrossRef]
- Lorber, M. Exposure of Americans to polybrominated diphenyl ethers. J. Expo. Sci. Environ. Epidemiol. 2008, 18, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.S.; Alves, A.; Madeira, L.M. Chemical and photochemical degradation of polybrominated diphenyl ethers in liquid systems—A review. Water Res. 2016, 88, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Restrepo, B.; Kannan, K.; Rapaport, D.P.; Rodan, B.D. Polybrominated diphenyl ethers and polychlorinated biphenyls in human adipose tissue from New York. Environ. Sci. Technol. 2005, 39, 5177–5182. [Google Scholar] [CrossRef] [PubMed]
- Fangstrom, B.; Strid, A.; Grandjean, P.; Weihe, P.; Bergman, A. A retrospective study of PDBEs and PCBs in human milk from the Faroe Islands. Environ. Health 2005, 4, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, M.Y.; Lee, S.; Kim, H.J.; Lee, J.J.; Choi, G.; Choi, S.; Kim, S.; Kim, S.Y.; Park, J.; Moon, H.B.; et al. Polybrominated diphenyl ethers in maternal serum, breast milk, umbilical cord serum, and house dust in a South Korean birth panel of mother-neonate pairs. Int. J. Environ. Res. Public Health 2016, 13, 767. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Yolton, K.; Rauch, S.A.; Webster, G.M.; Hornung, R.; Sjodin, A.; Dietrich, K.N.; Lanphear, B.P. Prenatal polybrominated diphenyl ether exposures and neurodevelopment in U.S. Children through 5 years of age: The home study. Environ. Health Perspect. 2014, 122, 856–862. [Google Scholar] [PubMed]
- Yanagisawa, R.; Koike, E.; Win-Shwe, T.T.; Yamamoto, M.; Takano, H. Impaired lipid and glucose homeostasis in hexabromocyclododecane-exposed mice fed a high-fat diet. Environ. Health Perspect. 2014, 122, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Dingemans, M.M.; van den Berg, M.; Westerink, R.H. Neurotoxicity of brominated flame retardants: (In)direct effects of parent and hydroxylated polybrominated diphenyl ethers on the (developing) nervous system. Environ. Health Perspect. 2011, 119, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Patisaul, H.B.; Roberts, S.C.; Mabrey, N.; McCaffrey, K.A.; Gear, R.B.; Braun, J.; Belcher, S.M.; Stapleton, H.M. Accumulation and endocrine disrupting effects of the flame retardant mixture Firemaster® 550 in rats: An exploratory assessment. J. Biochem. Mol. Toxicol. 2013, 27, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.R.; Wang, S.L.; Lee, W.J.; Wang, Y.F.; Papke, O. Levels of polybrominated diphenyl ethers (PDBEs) in breast milk from central Taiwan and their relation to infant birth outcome and maternal menstruation effects. Environ. Int. 2007, 33, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Harley, K.G.; Chevrier, J.; Aguilar Schall, R.; Sjodin, A.; Bradman, A.; Eskenazi, B. Association of prenatal exposure to polybrominated diphenyl ethers and infant birth weight. Am. J. Epidemiol. 2011, 174, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Loganath, A.; Chong, Y.S.; Obbard, J.P. Exposure to persistent organic pollutants in utero and related maternal characteristics on birth outcomes: A multivariate data analysis approach. Chemosphere 2009, 74, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.L.; Anthopolos, R.; Wolkin, A.; Stapleton, H.M. Associations of birth outcomes with maternal polybrominated diphenyl ethers and thyroid hormones during pregnancy. Environ. Int. 2015, 85, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, C.; Cui, C.; Ding, G.; Zhou, Y.; Jin, J.; Gao, Y.; Tian, Y. Prenatal exposure to polybrominated diphenyl ethers and birth outcomes. Environ. Pollut. 2015, 206, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Li, Z.; Du, L.; Zhang, H.; Zhou, Y.; Sun, W.; Xiao, X.; He, Y.; Sun, B.; Yu, Y.; et al. The effects of PBDE-209 exposure during pregnancy on placental ET-1 and eNOS expression and the birth weight of offspring. Int. J. Dev. Neurosci. 2015, 43, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, Y.J.; Lee, E.; Kim, M.S.; Kwack, S.J.; Kim, K.B.; Chung, K.K.; Kang, T.S.; Han, S.Y.; Lee, J.; et al. Effects of gestational exposure to decabromodiphenyl ether on reproductive parameters, thyroid hormone levels, and neuronal development in sprague-dawley rats offspring. J. Toxicol. Environ. Health A 2009, 72, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Lignell, S.; Aune, M.; Darnerud, P.O.; Hanberg, A.; Larsson, S.C.; Glynn, A. Prenatal exposure to polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) may influence birth weight among infants in a Swedish cohort with background exposure: A cross-sectional study. Environ. Health 2013, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Serme-Gbedo, Y.K.; Abdelouahab, N.; Pasquier, J.C.; Cohen, A.A.; Takser, L. Maternal levels of endocrine disruptors, polybrominated diphenyl ethers, in early pregnancy are not associated with lower birth weight in the canadian birth cohort geste. Environ. Health 2016, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, H.; Li, J.; Shan, Z.; Teng, W.; Teng, X. The correlation between polybrominated diphenyl ethers (PBDEs) and thyroid hormones in the general population: A meta-analysis. PLoS ONE 2015, 10, e0126989. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Dong, Y.; Chen, X.; Liu, Y.; Ma, D.; Liu, X.; Zheng, R.; Mao, X.; Chen, T.; He, W. Prevalence of suicide attempts among chinese adolescents: A meta-analysis of cross-sectional studies. Compr. Psychiatry 2015, 61, 78–89. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials 2015, 45, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.H.; Lim, J.E.; Park, H.; Jee, S.H. Body burden of persistent organic pollutants on hypertension: A meta-analysis. Environ. Sci. Pollut. Res. Int. 2016, 23, 14284–14293. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Miyaso, H.; Nakamura, N.; Matsuno, Y.; Kawashiro, Y.; Komiyama, M.; Mori, C. Postnatal exposure to low-dose decabromodiphenyl ether adversely affects mouse testes by increasing thyrosine phosphorylation level of cortactin. J. Toxicol. Sci. 2012, 37, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Miyaso, H.; Nakamura, N.; Naito, M.; Hirai, S.; Matsuno, Y.; Itoh, M.; Mori, C. Early postnatal exposure to a low dose of decabromodiphenyl ether affects expression of androgen and thyroid hormone receptor-alpha and its splicing variants in mouse sertoli cells. PLoS ONE 2014, 9, e114487. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.C.; Noyes, P.D.; Gallagher, E.P.; Stapleton, H.M. Species-specific differences and structure-activity relationships in the debromination of pbde congeners in three fish species. Environ. Sci. Technol. 2011, 45, 1999–2005. [Google Scholar] [CrossRef] [PubMed]
- Deis, J.N.; Spiro, D.M.; Jenkins, C.A.; Buckles, T.L.; Arnold, D.H. Parental knowledge and use of preventive asthma care measures in two pediatric emergency departments. J. Asthma 2010, 47, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.I.; Stapleton, H.M.; Mukherjee, B.; Hauser, R.; Meeker, J.D. Associations between brominated flame retardants in house dust and hormone levels in men. Sci. Total Environ. 2013, 445–446, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Huo, X.; Zhang, Y.; Li, W.; Zhang, J.; Xu, X. Polybrominated diphenyl ethers in human placenta associated with neonatal physiological development at a typical e-waste recycling area in China. Environ. Pollut. 2015, 196, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, H.M.; Eagle, S.; Anthopolos, R.; Wolkin, A.; Miranda, M.L. Associations between polybrominated diphenyl ether (PBDE) flame retardants, phenolic metabolites, and thyroid hormones during pregnancy. Environ. Health Perspect. 2011, 119, 1454–1459. [Google Scholar] [CrossRef] [PubMed]
- Shy, C.G.; Huang, H.L.; Chao, H.R.; Chang-Chien, G.P. Cord blood levels of thyroid hormones and IGF-1 weakly correlate with breast milk levels of PBDEs in Taiwan. Int. J. Hyg. Environ. Health 2012, 215, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Wen, S.; Li, J.; Zhong, Y.; Zhao, Y.; Wu, Y. The human body burden of polybrominated diphenyl ethers and their relationships with thyroid hormones in the general population in northern China. Sci. Total Environ. 2014, 466–467, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Xu, X.; Liu, J.; Guo, Y.; Li, Y.; Huo, X. Polybrominated diphenyl ethers in umbilical cord blood and relevant factors in neonates from Guiyu, China. Environ. Sci. Technol. 2010, 44, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, J.; Hu, G.; Luo, X.; Mai, B.; Dai, J. Tissue distribution and associated toxicological effects of decabrominated diphenyl ether in subchronically exposed male rats. ISRN Toxicol. 2011, 2011, 989251. [Google Scholar] [CrossRef] [PubMed]
- Gismondi, E.; Mazzucchelli, G.; De Pauw, E.; Joaquim-Justo, C.; Thome, J.P. Gender differences in responses in gammarus pulex exposed to BDE-47: A gel-free proteomic approach. Ecotoxicol. Environ. Saf. 2015, 122, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Wu, H.; Wei, L.; Zhao, J.; Yu, J. Proteomic and metabolomic analysis reveal gender-specific responses of mussel Mytilus galloprovincialis to 2,2′,4,4′-tetrabromodiphenyl ether (BDE 47). Aquat. Toxicol. 2013, 140–141, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Mazdai, A.; Dodder, N.G.; Abernathy, M.P.; Hites, R.A.; Bigsby, R.M. Polybrominated diphenyl ethers in maternal and fetal blood samples. Environ. Health Perspect. 2003, 111, 1249–1252. [Google Scholar] [CrossRef] [PubMed]
- Eskenazi, B.; Chevrier, J.; Rauch, S.A.; Kogut, K.; Harley, K.G.; Johnson, C.; Trujillo, C.; Sjodin, A.; Bradman, A. In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study. Environ. Health Perspect. 2013, 121, 257–262. [Google Scholar] [PubMed]
- Lin, S.M.; Chen, F.A.; Huang, Y.F.; Hsing, L.L.; Chen, L.L.; Wu, L.S.; Liu, T.S.; Chang-Chien, G.P.; Chen, K.C.; Chao, H.R. Negative associations between PBDE levels and thyroid hormones in cord blood. Int. J. Hyg. Environ. Health 2011, 214, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Taylor, M.M.; DeVito, M.J.; Crofton, K.M. Developmental exposure to brominated diphenyl ethers results in thyroid hormone disruption. Toxicol. Sci. 2002, 66, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, H.; Woo, G.H.; Inoue, K.; Takahashi, M.; Hirose, M.; Nishikawa, A.; Shibutani, M. Impaired oligodendroglial development by decabromodiphenyl ether in rat offspring after maternal exposure from mid-gestation through lactation. Reprod. Toxicol. 2011, 31, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.Y.; Vasilopoulou, E.; Kilby, M.D. The role of the placenta in thyroid hormone delivery to the fetus. Nat. Clin. Pract. Endocrinol. Metab. 2009, 5, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Butt, C.M.; Stapleton, H.M. Inhibition of thyroid hormone sulfotransferase activity by brominated flame retardants and halogenated phenolics. Chem. Res. Toxicol. 2013, 26, 1692–1702. [Google Scholar] [CrossRef] [PubMed]
- Butt, C.M.; Wang, D.; Stapleton, H.M. Halogenated phenolic contaminants inhibit the in vitro activity of the thyroid-regulating deiodinases in human liver. Toxicol. Sci. 2011, 124, 339–347. [Google Scholar] [CrossRef] [PubMed]
| Author | Year | Country | Median Serum PBDE b | Sample Size | PBDE Congeners | Gender of Infants | Effect Size β (95% CI) c |
|---|---|---|---|---|---|---|---|
| Harley | 2011 | USA Mexico | 22.9 | 286 | 47, 99, 100, 153 | M, F | −140.2 * (−254.1, −26.3) |
| Lignell | 2013 | Sweden | 1.67 a | 185 | 47, 99, 100, 153 | M | −126 * (−247.52, −4.48) |
| Lignell | 2013 | Sweden | 1.67 a | 161 | 47, 99, 100, 153 | F | 32 (−105.2, 169.2) |
| Miranda | 2015 | USA | 34.69 | 136 | 47, 99, 100, 153 | M, F | −31.9 (−89.2, 25.41) |
| Chen | 2015 | China | 12.84 | 104 | 47, 99, 100, 153, 28 | F | 55.51 (−185.17, 296.19) |
| Chen | 2015 | China | 12.84 | 111 | 47, 99, 100, 153, 28 | M | −103.29 (−346.254, 139.69) |
| Serme-Gbedo | 2016 | Canada | 0.19 | 349 | 47, 99, 100, 153 | M, F | −25.4 (−128.7, 77.9) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Peng, S.; Xiang, Y.; Yang, Y.; Li, J.; Shan, Z.; Teng, W. Correlation between Prenatal Exposure to Polybrominated Diphenyl Ethers (PBDEs) and Infant Birth Outcomes: A Meta-Analysis and an Experimental Study. Int. J. Environ. Res. Public Health 2017, 14, 268. https://doi.org/10.3390/ijerph14030268
Zhao X, Peng S, Xiang Y, Yang Y, Li J, Shan Z, Teng W. Correlation between Prenatal Exposure to Polybrominated Diphenyl Ethers (PBDEs) and Infant Birth Outcomes: A Meta-Analysis and an Experimental Study. International Journal of Environmental Research and Public Health. 2017; 14(3):268. https://doi.org/10.3390/ijerph14030268
Chicago/Turabian StyleZhao, Xuemin, Shiqiao Peng, Yang Xiang, Yali Yang, Jing Li, Zhongyan Shan, and Weiping Teng. 2017. "Correlation between Prenatal Exposure to Polybrominated Diphenyl Ethers (PBDEs) and Infant Birth Outcomes: A Meta-Analysis and an Experimental Study" International Journal of Environmental Research and Public Health 14, no. 3: 268. https://doi.org/10.3390/ijerph14030268






