Spatially Correlated Time Series and Ecological Niche Analysis of Cutaneous Leishmaniasis in Afghanistan
Abstract
:1. Introduction
2. Methods
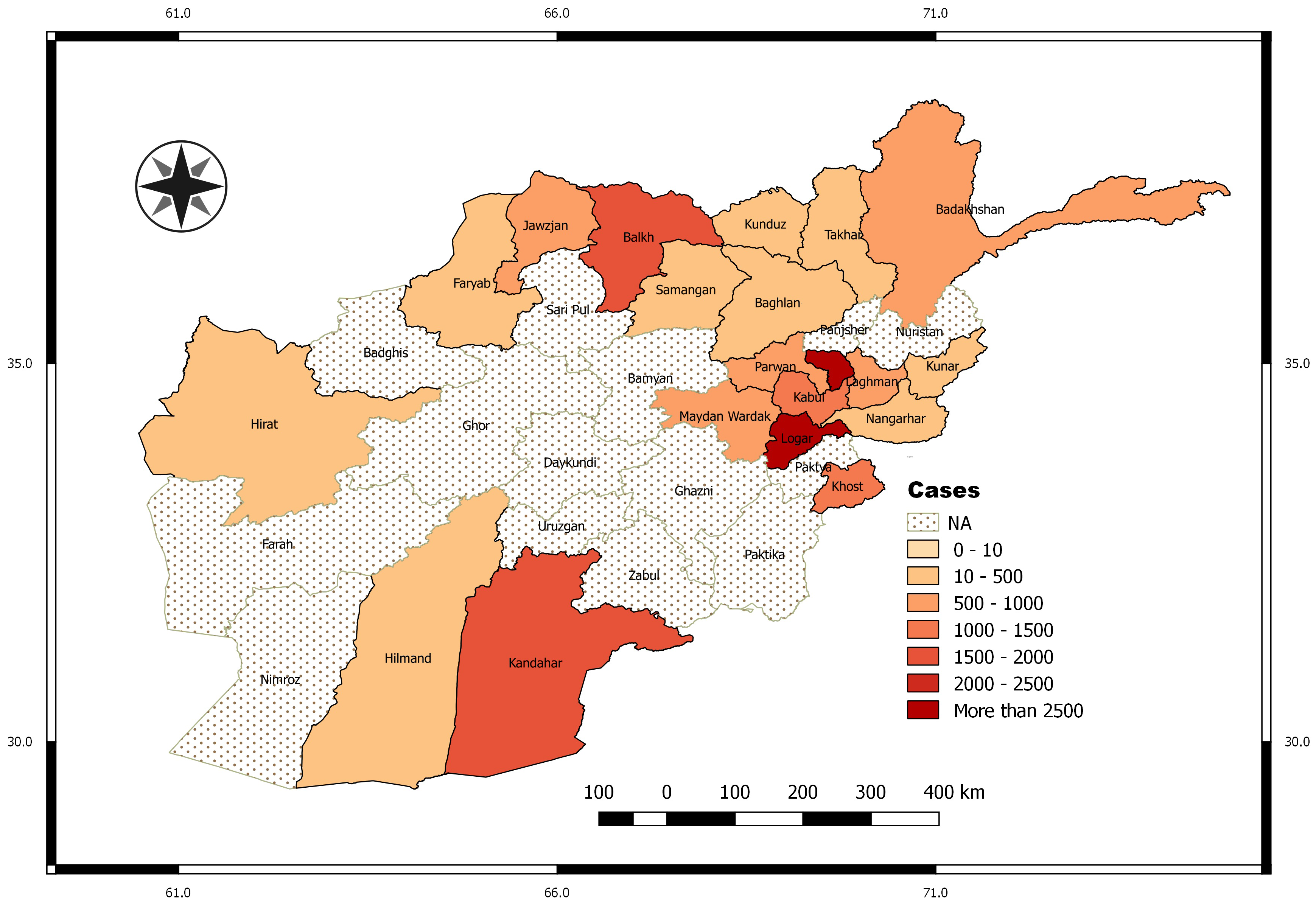
2.1. Leishmaniasis Data
2.2. Environmental Variables
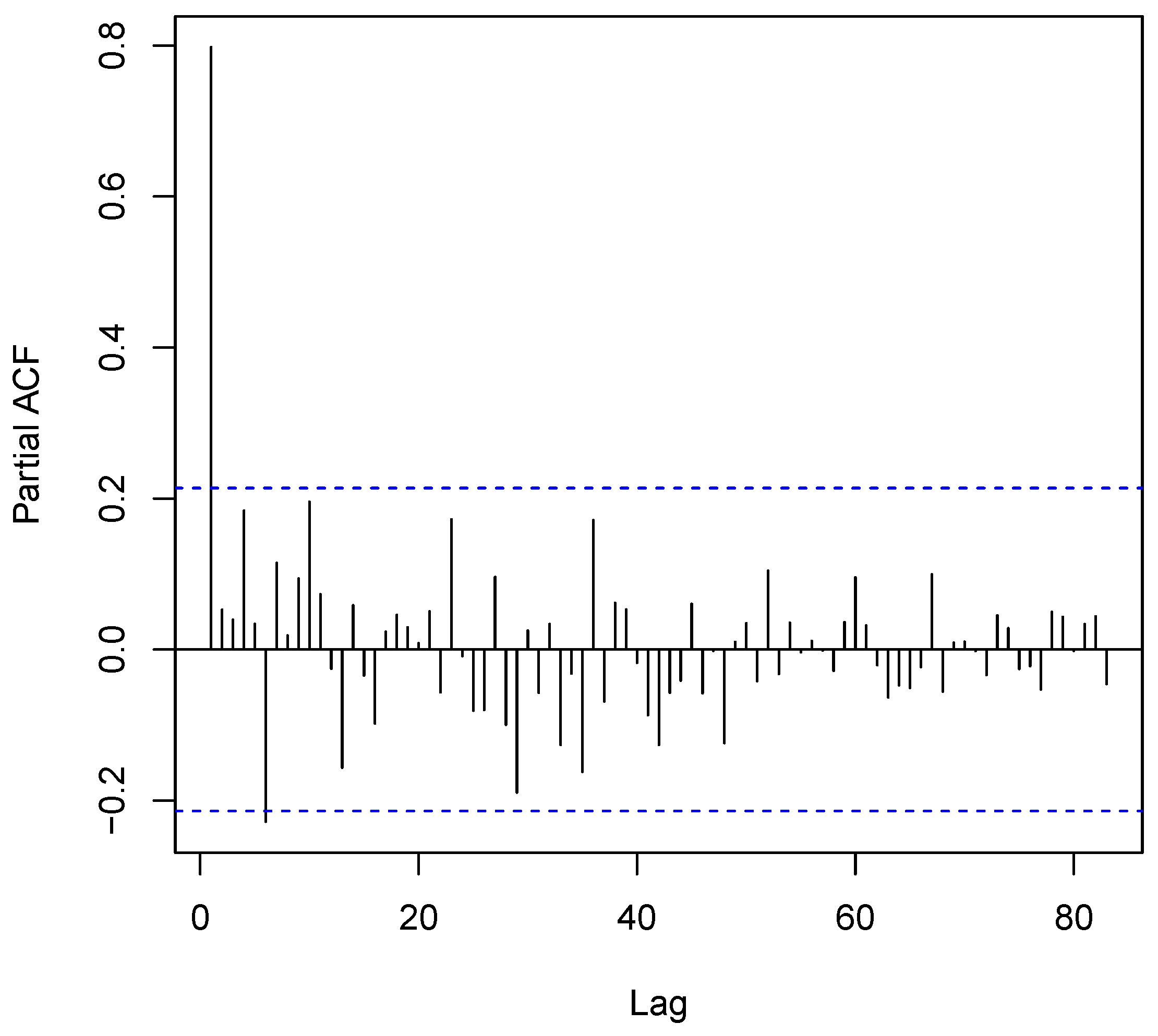
2.3. Multivariate Time Series Analysis
Model Selection
2.4. Ecological Niche Modelling
3. Results
3.1. Exploratory Data Analysis
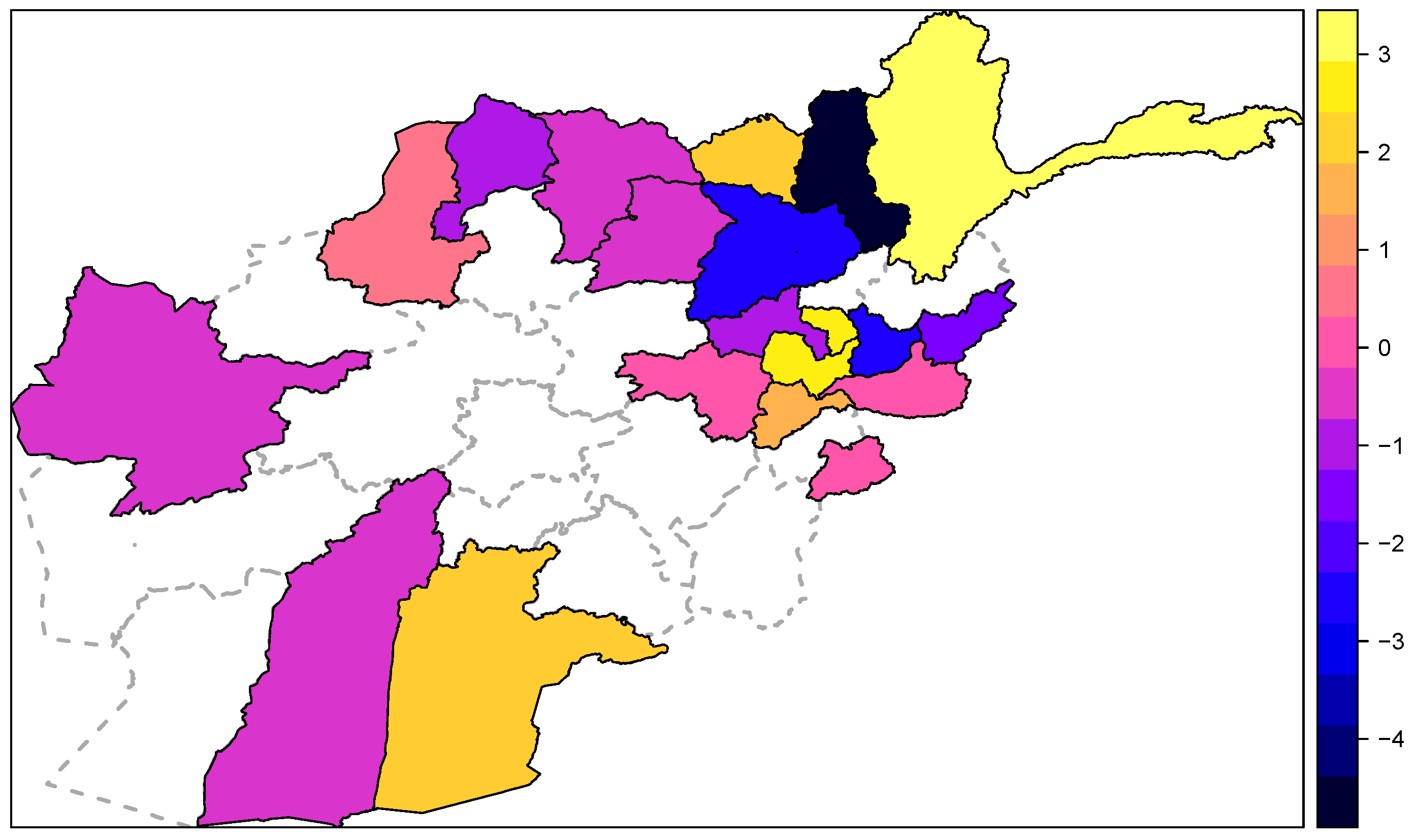
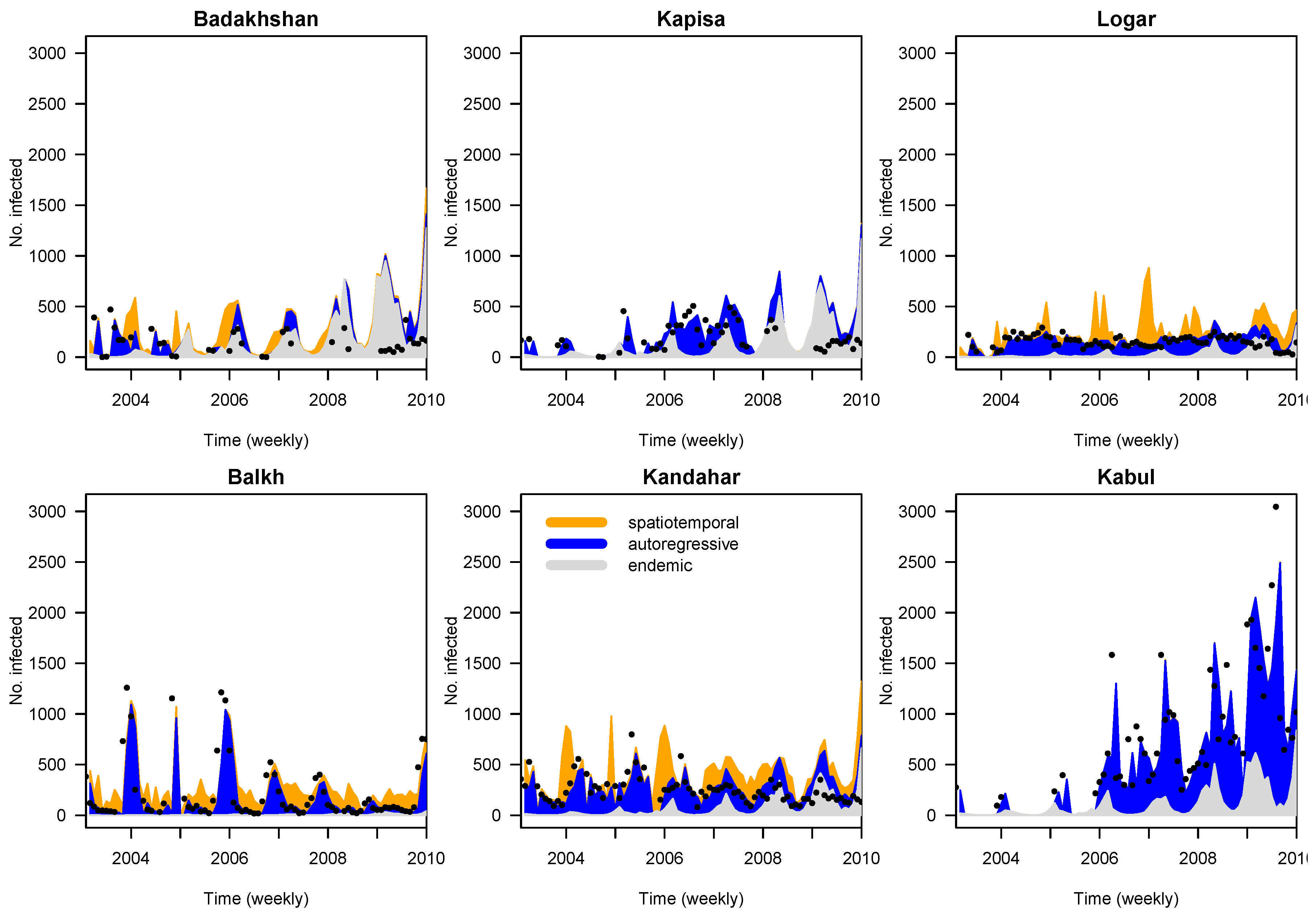
3.2. Time Series Analyses
- M1
- ; only seasonal variation in the endemic component, where
- M2
- ; seasonal variation in the endemic component, autoregressive in the epidemic component, spatiotemporal component, but without adjusting for covariates in the epidemic component, where , and , using the weights =1/# neighbors of region j in the spatiotemporal component.
- M3
- ; seasonal variation in the endemic component with random effects, autoregressive in the epidemic component with covariate adjustment and spatiotemporal component with random effects,
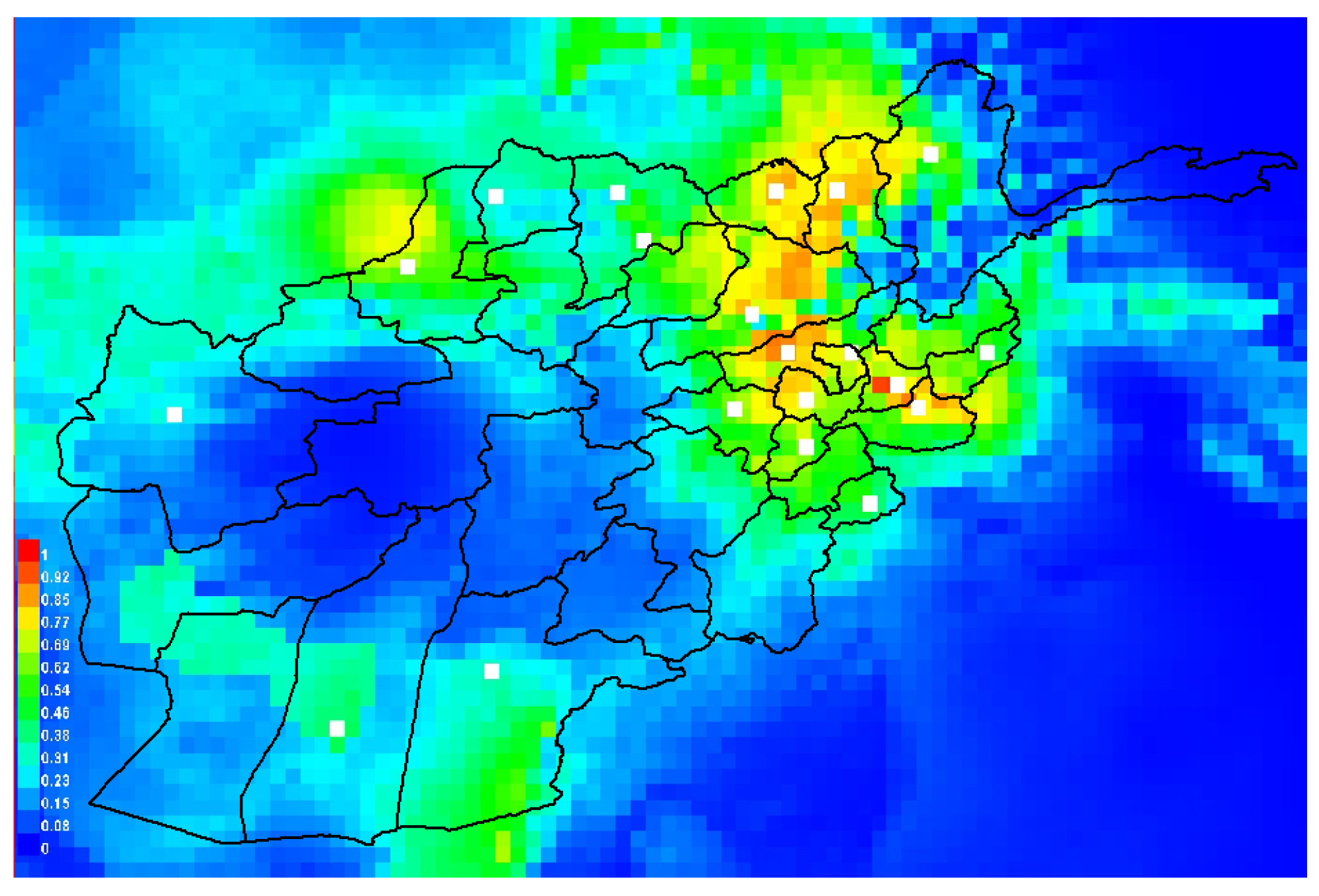
3.3. Suitability Index and Relative Importance of Environmental Layers for the Occurrences of Leishmaniasis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; Boer, M.; The WHO Leishmaniasis Control Team. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Dostálová, A.; Volf, P. Leishmania development in sand flies: Parasite-vector interactions overview. Parasites Vectors 2012, 5. [Google Scholar] [CrossRef] [PubMed]
- Adegboye, O.A.; Kotze, D. Disease mapping of Leishmaniasis outbreak in Afghanistan: Spatial hierarchical Bayesian analysis. Asian Pac. J. Trop. Dis. 2012, 2, 253–259. [Google Scholar] [CrossRef]
- Reithinger, R.; Aadil, K.; Kolaczinski, J.; Mohsen, M.; Hami, S. Social impact of Leishmaniasis, Afghanistan. Emerg. Infect. Dis. 2005, 11, 634–636. [Google Scholar] [CrossRef] [PubMed]
- Bates, P.A. Transmission of Leishmania Metacyclic Promastigotes by Phlebotomine Sand Flies. Int. J. Parasitol. 2007, 37, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Reithinger, R.; Mohsen, M.; Aadil, K.; Sidiqi, M.; Erasmus, P.; Coleman, P.G. Anthroponotic cutaneous Leishmaniasis, Kabul, Afghanistan. Emerg. Infect. Dis. 2003, 9, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Ashford, R.; Kohestany, K.; Karimzad, M. Cutaneous leishmaniasis in Kabul: Observations on a prolonged epidemic. Ann. Trop. Med. Parasitol. 1992, 86, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Reithinger, R.; Mohsen, M.; Leslie, T. Risk factors for anthroponotic cutaneous Leishamaniasis at the household level in Kabul, Afghanistan. PLoS Negl. Trop. Dis. 2010, 4, e639. [Google Scholar] [CrossRef] [PubMed]
- Killick-Kendrick, R.; Killick-Kendrick, M.; Tang, Y. Anthroponotic cutaneous leishmaniasis in Kabul: The high susceptibility of Phlebotomus sergenti to Leishmania tropica. Trans. R. Soc. Trop. Med. Hyg. 1995, 89, 477. [Google Scholar] [CrossRef]
- Faulde, M.; Schrader, J.; Heyl, G.; Amirih, M. Differences in transmission seasons as an epidemiological tool for characterization of anthroponotic and zoonotic cutaneous leishmaniasis in northern Afghanistan. Acta Trop. 2008, 105, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Faulde, M.; Schrader, J.; Heyl, G.; Amirih, M.; Hoerauf, A. Zoonotic cutaneous leishmaniasis outbreak in Mazar-e Sharif, northern Afghanistan: An epidemiological evaluation. Int. J. Med. Microbiol. 2008, 298, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Adegboye, O.A. Bayesian spatial analysis and disease mapping of Leishmaniasis outbreak in Afghanistan. In Proceedings of the 58th World Statistics Congress of the International Statistical Institute, Dublin, Ireland, 4–11 August 2011.
- Paul, M.; Held, L. Predictive assessment of a non-linear random effects model for multivariate time series of infectious disease counts. Stat. Med. 2011, 30, 1118–1136. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.T. Ecologic niche modeling and spatial patterns of disease transmission. Emerg. Infect. Dis. 2006, 12, 1822–1826. [Google Scholar] [CrossRef] [PubMed]
- Adegboye, O.; Kotze, D. Epidemiological analysis of spatially misaligned data: A case of highly pathogenic avian influenza virus outbreak in Nigeria. Epidemiol. Infect. 2014, 142, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Wang, Z.; Liu, Y.; Wang, H.; Xue, F.; Liu, Y. Ecological niche modeling for predicting the potential risk areas of severe fever with thrombocytopenia syndrome. Int. J. Infect. Dis. 2014, 26, 1–8. [Google Scholar] [CrossRef]
- Samy, A.M.; van de Sande, W.W.; Fahal, A.H.; Peterson, A.T. Mapping the potential risk of mycetoma infection in sudan and south sudan using ecological niche modeling. PLoS Negl. Trop. Dis. 2014, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pigott, D.M.; Golding, N.; Mylne, A.; Huang, Z.; Henry, A.J.; Weiss, D.J.; Brady, O.J.; Kraemer, M.U.; Smith, D.L.; Moyes, C.L.; et al. Mapping the zoonotic niche of ebola virus disease in Africa. eLife 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Chalghaf, B.; Chlif, S.; Mayala, B.; Ghawar, W.; Bettaieb, J.; Harrabi, M.; Benie, G.B.; Michael, E.; Salah, A.B. Ecological Niche Modeling for the Prediction of the Geographic Distribution of Cutaneous Leishmaniasis in Tunisia. Am. J. Trop. Med. Hyg. 2016, 4, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Hook, S.; Hulley, G. MOD11 L2 MODIS/Terra Land Surface Temperature/Emissivity 5-Min L2 Swath 1 km V006. NASA EOSDIS Land Processes DAAC, 2015. Available online: https://doi.org/10.5067/MODIS/MOD11_L2.006 (accessed on 24 January 2016). [Google Scholar]
- NASA JPL. ASTER Global Emissivity Dataset, 1-km, HDF5. NASA EOSDIS Land Processes DAAC, 2014. Available online: https://doi.org/10.5067/Community/ASTER_GED/AG1km.003 (accessed on 24 January 2016). [Google Scholar]
- Tropical Rainfall Measuring Mission (TRMM). TRMM (TMPA/3B43) Rainfall Estimate L3 1 Month 0.25 Degree × 0.25 Degree V7, Version, Greenbelt, MD, Goddard Earth Sciences Data and Information Services Center (GES DISC) 2011. Available online: http://disc.gsfc.nasa.gov/datacollection/TRMM_3B43_7.html (accessed on 24 January 2016).
- Held, L.; Hohle, M.; Hofmann, M. A statistical framework for the analysis of multivariate infectious disease surveillance counts. Stat. Model. 2005, 5, 187–199. [Google Scholar] [CrossRef] [Green Version]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2008. [Google Scholar]
- Höhle, M. surveillance: An R package for the monitoring of infectious diseases. Comput. Stat. 2007, 22, 571–582. [Google Scholar] [CrossRef]
- Michael Höhle, Sebastian Meyer and Michaela Paul. surveillance: Temporal and Spatio-Temporal Modeling and Monitoring of Epidemic Phenomena. R Package Version 1.12.1. 2016. Available online: https://CRAN.R-project.org/package=surveillance (accessed on 24 January 2016).
- Waltari, E.; Hijmans, R.J.; Peterson, A.T.; Nyári, Á.S.; Perkins, S.L.; Guralnick, R.P. Locating pleistocene refugia: Comparing phylogeographic and ecological niche model predictions. PLoS ONE 2007, 2, e563. [Google Scholar] [CrossRef] [PubMed]
- Soberon, J. Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodivers. Inform. 2005, 2, 1–10. [Google Scholar] [CrossRef]
- Phillips, S.; Dudik, M.; Schapire, R. Maxent Software, Version 3.3.3e; 2010. Available online: http://www.cs.princeton.edu/~schapire/maxent/ (accessed on 24 January 2016).
- Steven, J.P.; Miroslav, D.; Robert, E.S. A maximum entropy approach to species distribution modeling. In Proceedings of the Twenty-First International Conference on Machine Learning, Banff, AB, Canada, 4–8 July 2004; pp. 655–662.
- Steven, J.P.; Robert, P.A.; Robert, E.S. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression; John Wiley & Sons: New York, NY, USA, 2004. [Google Scholar]
- Jane, E.; Steven, J.P.; Trevor, H.; Miroslav, D.; Yung, E.C.; Colin, J.Y. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar]
- Peterson, A.T.; Jeffrey, S. Lutzomyia vectors for cutaneous leishmaniasis in Southern Brazil: Ecological niche models, predicted geographic distributions, and climate change effects. Int. J. Parasitol. 2003, 9, 919–931. [Google Scholar] [CrossRef]
- González, C.; Wang, O.; Strutz, S.E.; González-Salazar, C.; Sánchez-Cordero, V.; Sarkar, S. Climate change and risk of leishmaniasis in North America: Predictions from ecological niche models of vector and reservoir species. PLoS Negl. Trop. Dis. 2010, 1, e585. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; Mansourian, A.; Pilesjö, P.; Bazmani, A. Environmental modelling of visceral leishmaniasis by susceptibility-mapping using neural networks: A case study in north-western Iran. Geospat. Health 2014, 1, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Toumi, A.; Chlif, S.; Bettaieb, J.; Alaya, N.B.; Boukthir, A.; Ahmadi, Z.E.; Salah, A.B. Temporal dynamics and impact of climate factors on the incidence of zoonotic cutaneous leishmaniasis in central Tunisia. PLoS Negl. Trop. Dis. 2012, 5, e1633. [Google Scholar] [CrossRef] [PubMed]
- Gàlvez, R.; Descalzo, M.A.; Miró, G.; Jiménez, M.I.; Martín, O.; Dos Santos-Brandao, F.; Guerrero, I.; Cubero, E.; Molina, R. Seasonal trends and spatial relations between environmental/meteorological factors and leishmaniosis sand fly vector abundances in Central Spain. Acta Trop. 2010, 1, 95–102. [Google Scholar] [CrossRef] [PubMed]
- El-Shazly, M.M.; Mustafa, M.S.; Alia, Z. Seasonal abundance, number of annual generations, and effect of an entomopathogenic fungus on Phlebotomus papatasi (Diptera: Psychodidae). Environ. Entomol. 2012, 1, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Elnaiem, D.E.A.; Schorscher, J.; Bendall, A.; Obsomer, V.; Osman, M.E.; Mekkawi, A.M.; Connor, S.J.; Ashford, R.W.; Thomson, M.C. Risk mapping of visceral leishmaniasis: The role of local variation in rainfall and altitude on the presence and incidence of kala-azar in eastern Sudan. Am. J. Trop. Med. Hyg. 2003, 1, 10–17. [Google Scholar]
- Nygren, D.; Stoyanov, C.; Lewold, C.; Mansson, F.; Miller, J.; Kamanga, A.; Shiff, C. Remotely-sensed, nocturnal, dew point correlates with malaria transmission in Southern Province, Zambia: A time-series study. Malar. J. 2014, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Comfort, A.B.; van Dijk, J.H.; Mharakurwa, S.; Stillman, K.; Johns, B.; Hathi, P.; Korde, S.; Craig, A.S.; Nachbar, N.; Derriennic, Y.; et al. Association between malaria control and paediatric blood transfusions in rural Zambia: An interrupted time-series analysis. Malar. J. 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Midekisa, A.; Senay, G.; Menebry, G.; Semuniguse, P.; Wimberly, M. Remote sensing-based time series models for malaria early warning in the highlands of Ethiopia. Malar. J. 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Bi, Y.; Ho, S.; Liu, W.; Liang, S.; Goggins, W.B.; Chan, E.; Zhou, S.; Sung, J. One-year delayed effect of fog on malaria transmission: A time-series analysis in the rain forest area of Mengla County, south-west China. Malar. J. 2012, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewnard, J.; Jirmanus, L.; Jnior, N.; Machado, P.; Glesby, M.; Edgar, M.C.; Albert, S.; Daniel, M.W. Forecasting temporal dynamics of cutaneous leishmaniasis in northeast Brazil. PLoS Negl. Trop. Dis. 2014, 8, e3283. [Google Scholar] [CrossRef] [PubMed]
- Held, L.; Paul, M. Modeling seasonality in space-time infectious disease surveillance data. Biom. J. 2012, 54, 824–843. [Google Scholar] [CrossRef] [PubMed]
- Alegana, V.A.; Atkinson, P.M.; Wright, J.A.; Kamwi, R.; Uusiku, P.; Katokele, S.; Snow, R.W.; Noor, A.M. Estimation of malaria incidence in northern Namibia in 2009 using Bayesian conditional-autoregressive spatial temporal models. Spat. Spatio-Tempor. Epidemiol. 2015, 7, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, G.S.; Kesari, S.; Jeyaram, A.; Kumar, V.; Das, P. Influence of topography on the endemicity of Kala-azar: A study based on remote sensing and geographical information system. Geospat. Health 2010, 2, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Kassem, H.A.; Siri, J.; Kamal, H.A.; Wilson, M.L. Environmental factors underlying spatial patterns of sand flies (Diptera: Psychodidae) associated with leishmaniasis in southern Sinai, Egypt. Acta Trop. 2012, 1, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Machado-Coelho, G.L.; Assunção, R.; Mayrink, W.; Caiaffa, W.T. American cutaneous leishmaniasis in Southeast Brazil: Space-time clustering. Int. J. Epidemiol. 1999, 5, 982–989. [Google Scholar] [CrossRef]
- World Health Organisation. Fact Sheet-Leishmaniasis; World Health Organization: Geneva, Switzerland, 2006; Available online: http://www.who.int/mediacentre/factsheets/fs375/en/ (accessed on 2 September 2016).
- Alten, B.; Maia, C.; Afonso, M.O.; Campino, L.; Jiménez, M.; González, E.; Molina, R.; Bañuls, A.L.; Prudhomme, J.; Vergnes, B.; et al. Seasonal dynamics of phlebotomine sand fly species proven vectors of Mediterranean leishmaniasis caused by Leishmania infantum. PLoS Negl. Trop. Dis. 2016, 10, e4458. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.S.; Caddy, A.J.; McKinnon, K.A.; Fogg, L.F.; Roscoe, M.; Bailey, J.W.; O’Dempsey, T.J.; Beeching, N.J. Outbreak of zoonotic cutaneous leishmaniasis with local dissemination in Balkh, Afghanistan. J. R. Army Med. Corps 2012, 3, 225–228. [Google Scholar] [CrossRef]
- Abdel-Dayem, M.S.; Annajar, B.B.; Hanafi, H.A.; Obenauer, P.J. The potential distribution of Phlebotomus papatasi (Diptera: Psychodidae) in Libya based on ecological niche model. J. Med. Entomol. 2012, 3, 739–745. [Google Scholar] [CrossRef]

| Parameter | M1 | M2 | M3 |
|---|---|---|---|
| Endemic component | |||
| Intercept () | 6.940 (6.660, 7.219) | 5.978 (5.701, 6.255) | |
| Trend () | 0.031 (0.024, 0.036) | 0.003 (−0.004, 0.011) | 0.061 (0.049, 0.072) |
| Sine () | 0.349 (0.004, 0.374) | 1.139 (−0.050, 0.356) | 1.567 (1.026, 1.601) |
| Cosine | 0.997 (0.101, 0.485) | 1.436 (0.866, 1.392) | 0.577 (0.567, 1.142) |
| IID Random term a | 2.357 (1.244, 3.471) | ||
| Epidemic Autoregressive component | |||
| Intercept | −0.065 (−0248, 0.122) | 0.250 (1.575, 2.074) | |
| LogALT | 0.064 (0.030, 0.175) | ||
| Precipitation | 0.011 (0.007, 0.017) | ||
| Temperature | 0.050 (0.021, 0.089) | ||
| Wind | 0.003 (−0.052, 0.057) | ||
| Spatiotemporal | |||
| Spatiotemporal | −2.20 (−3.219, 3.820) | −5.003 (−7.037, −2.969) | |
| Overdispersion | |||
| Overdispersion ψ | 7.597 (7.027, 8.166) | 5.204 (4.789, 5.617) | 3.284 (3.007, 3.561) |
| AIC | 13,303.79 | 12,815.86 | |
| LogS | 6.700 | 6.348 | 6.178 |
| RPS | 120.128 | 93.568 | 90.204 |
| Variable | Description | Percent Contribution | Jackknife Rank |
|---|---|---|---|
| afg-prec4 | Mean precipitation for April | 54.5 | 1 |
| afg-prec9 | Mean precipitation for September | 15.3 | 9 |
| afg-tmean1 | Mean temperature for January | 9.9 | 2 |
| afg-tmean7 | Mean temperature for July | 7.1 | 11 |
| afg-prec10 | Mean precipitation for October | 5.6 | 7 |
| afg-prec6 | Mean precipitation for June | 2.1 | 10 |
| afg-prec7 | Mean precipitation for July | 1.7 | 4 |
| afg-prec8 | Mean precipitation for August | 1.4 | 8 |
| afg-prec12 | Mean precipitation for December | 1.2 | 5 |
| afg-prec2 | Mean precipitation for February | 0.6 | 3 |
| afg-prec11 | Mean precipitation for November | 0.6 | 6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adegboye, O.A.; Adegboye, M. Spatially Correlated Time Series and Ecological Niche Analysis of Cutaneous Leishmaniasis in Afghanistan. Int. J. Environ. Res. Public Health 2017, 14, 309. https://doi.org/10.3390/ijerph14030309
Adegboye OA, Adegboye M. Spatially Correlated Time Series and Ecological Niche Analysis of Cutaneous Leishmaniasis in Afghanistan. International Journal of Environmental Research and Public Health. 2017; 14(3):309. https://doi.org/10.3390/ijerph14030309
Chicago/Turabian StyleAdegboye, Oyelola A., and Majeed Adegboye. 2017. "Spatially Correlated Time Series and Ecological Niche Analysis of Cutaneous Leishmaniasis in Afghanistan" International Journal of Environmental Research and Public Health 14, no. 3: 309. https://doi.org/10.3390/ijerph14030309






