Geographical Correlations between Indoor Radon Concentration and Risks of Lung Cancer, Non-Hodgkin’s Lymphoma, and Leukemia during 1999–2008 in Korea
Abstract
:1. Introduction
2. Materials and Methods
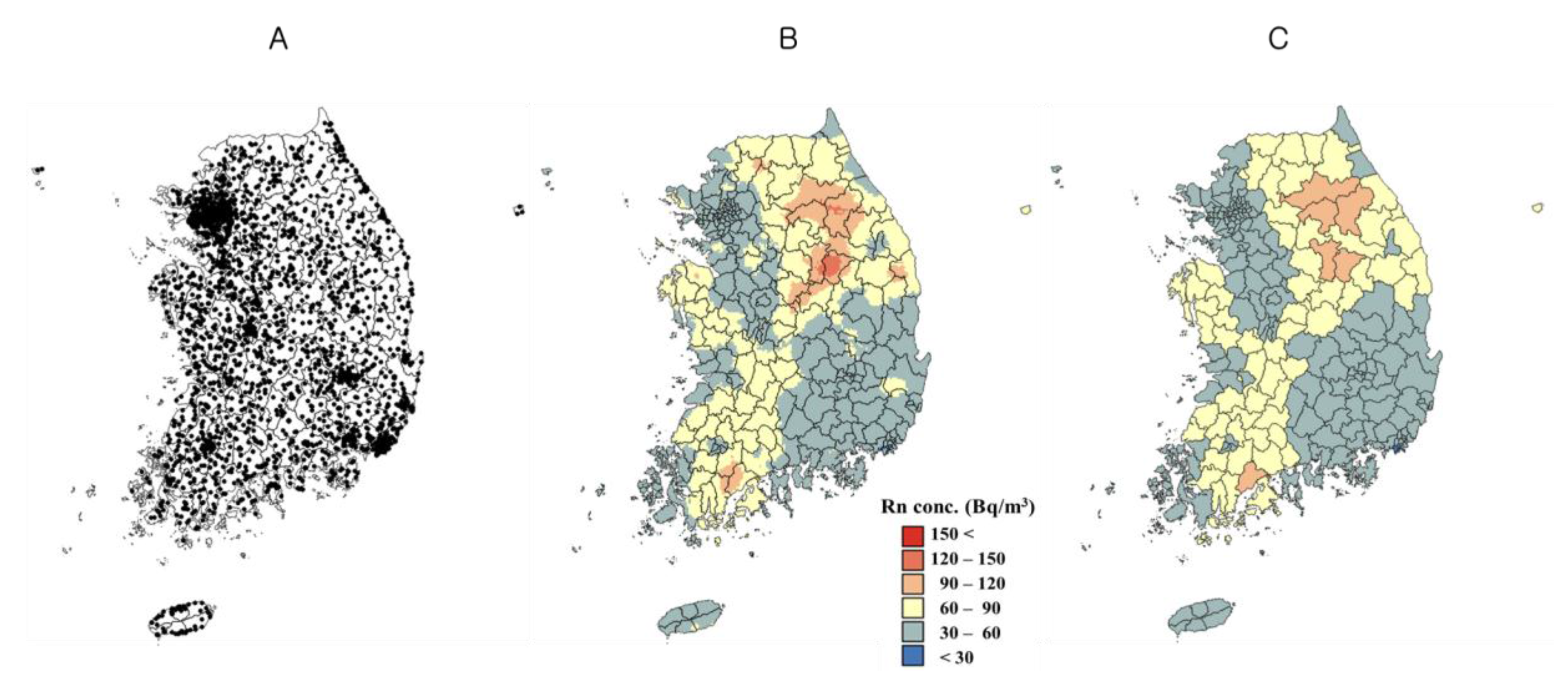
2.1. Indoor Radon Concentration
2.2. Incidences of Lung Cancer, NHL, and Leukemia
2.3. Confounding Factors
2.4. Bayesian Hierarchical Modeling for Radon Exposure and Cancers
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gillmore, G.K.; Phillips, P.S.; Denman, A.R. The effects of geology and the impact of seasonal correction factors on indoor radon levels: A case study approach. J. Environ. Radioact. 2005, 84, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Lee, S.C.; Lee, D.M.; Chang, B.U.; Rho, B.H.; Kang, H.D. Nationwide survey of radon levels in Korea. Health Phys. 2003, 84, 354–360. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines Approved by the Guidelines Review Committee. In WHO Handbook on Indoor Radon: A Public Health Perspective; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Kim, Y.; Chang, B.U.; Park, H.M.; Kim, C.K.; Tokonami, S. National radon survey in Korea. Radiat. Protect. Dosim. 2011, 146, 6–10. [Google Scholar] [CrossRef] [PubMed]
- National Library of Korea, Sejong. Available online: http://sejong.nl.go.kr/search/searchDetail.do?rec_key=SH1_KMO201437741&upperMenuId=&menuId (accessed on 24 March 2017).
- Yoon, J.Y.; Lee, J.D.; Joo, S.W.; Kang, D.R. Indoor radon exposure and lung cancer: A review of ecological studies. Ann. Occup. Environ. Med. 2016, 25, 15. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.; Hill, D.; Auvinen, A.; Barros-Dios, J.M.; Baysson, H.; Bochicchio, F.; Deo, H.; Falk, R.; Forastiere, F.; Hakama, M.; et al. Radon in homes and risk of lung cancer: Collaborative analysis of individual data from 13 European case-control studies. BMJ (Clin. Res. Ed.) 2005, 330, 223. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.; Hill, D.; Deo, H.; Auvinen, A.; Barros-Dios, J.M.; Baysson, H.; Bochicchio, F.; Falk, R.; Farchi, S.; Figueiras, A.; et al. Residential radon and lung cancer—Detailed results of a collaborative analysis of individual data on 7148 persons with lung cancer and 14,208 persons without lung cancer from 13 epidemiologic studies in Europe. Scand. J. Work Environ. Health 2006, 32, 1–83. [Google Scholar] [PubMed]
- Lecomte, J.F.; Solomon, S.; Takala, J.; Jung, T.; Strand, P.; Murith, C.; Kiselev, S.; Zhuo, W.; Shannoun, F.; Janssens, A. ICRP Publication 126: Radiological Protection against Radon Exposure. Ann. ICRP 2014, 43, 5–73. [Google Scholar] [CrossRef] [PubMed]
- Kendall, G.M.; Fell, T.P. Doses to the red bone marrow of young people and adults from radiation of natural origin. J. Radiol. Prot. Off. J. Soc. Radiol. Prot. 2011, 31, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.A.; Lee, W.K.; Lim, D.; Park, S.H.; Baik, S.J.; Kong, K.A.; Jung-Choi, K.; Park, H. Risks of Lung Cancer due to Radon Exposure among the Regions of Korea. J. Korean Med. Assoc. 2015, 30, 542–548. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Information Center. Available online: http://www.cancer.go.kr/mbs/cancer/subview.jsp?id=cancer_040102000000 (accessed on 13 March 2017).
- Alexander, D.D.; Mink, P.J.; Adami, H.O.; Chang, E.T.; Cole, P.; Mandel, J.S.; Trichopoulos, D. The non-Hodgkin lymphomas: A review of the epidemiologic literature. Int. J. Cancer 2007, 120 (Suppl. S12), 1–39. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.; Ha, M.; Song, I. Trends in major cancer mortality in Korea, 1983–2012, with a joinpoint analysis. Cancer Epidemiol. 2015, 39, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Goovaerts, P. Geostatistical interpolation of positively skewed and censored data in a dioxin-contaminated site. Environ. Sci. Technol. 2000, 34, 4228–4235. [Google Scholar] [CrossRef]
- Deutsch, C.V.; Journel, A.G. GSLIB: Geostatistical Software Library and User’s Guide, 2nd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 1998. [Google Scholar]
- Webster, R.; Oliver, M.A. Geostatistics for Environmental Scientists, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- National Cancer Center. Available online: http://ncc.re.kr/cancerStatsList.ncc?sea (accessed on 24 March 2017).
- Armitage, P.; Berry, G.; Matthews, J.N.S. Statistical Methods in Medical Research, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Korean Statistical Information Service (KOSIS). Available online: http://kosis.kr/statisticsList/statisticsList_01List.jsp?vwcd=MT_ZTITLE&parentId=A (accessed on 24 March 2017).
- Son, M. The relationships of occupational class, educational level and deprivation with mortality in Korea. J. Prev. Med. Public Health 2002, 35, 76–82. [Google Scholar]
- Townsend, P.; Phillimore, P.; Beattie, A. Health and Deprivation: Inequality and the North, 1st ed.; Routledge: London, UK, 1988. [Google Scholar]
- Community Health Survey. Available online: https://chs.cdc.go.kr/chs/index.do;jsessionid=Q2lc5BDm1npaX7hPnkfAYdAtxtwqiJRnq6RGHVCUp0j6GY4ngSwHzT4KwGya0uMx.KCDCWAS02_servlet_PUB2 (accessed on 24 March 2017).
- Lawson, A.B. Bayesian Disease Mapping: Hierarchical Modeling in Spatial Epidemiology, 2nd ed.; CRC Press: New York, NY, USA, 2009. [Google Scholar]
- Zhu, L.; Gorman, D.M.; Horel, S. Hierarchical Bayesian spatial models for alcohol availability, drug “hot spots” and violent crime. Int. J. Health Geograph. 2006, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Besag, J.; York, J.; Mollie, A. Bayesian Image-Restoration, with 2 Applications in Spatial Statistics. Ann. Inst. Stat. Math. 1991, 43, 1–20. [Google Scholar] [CrossRef]
- Spiegelhalter, D.; Andrew, T.; Best, N.; Lunn, D. WinBUGS User Manual Version 1.4.1; Medical Research Council Biostatistics Unit: Cambridge, UK, 2003; Available online: https://www.mrc-bsu.cam.ac.uk/wp-content/uploads/manual14.pdf (accessed on 24 March 2017).
- Brooks, S.P.; Gelman, A. General Methods for Monitoring Convergence of Iterative Simulations. J. Comput. Graph. Stat. 1997, 7, 434–455. [Google Scholar]
- Chahine, T.; Schultz, B.D.; Zartarian, V.G.; Xue, J.; Subramanian, S.V.; Levy, J.I. Modeling joint exposures and health outcomes for cumulative risk assessment: The case of radon and smoking. Int. J. Environ. Res. Public Health 2011, 8, 3688–3711. [Google Scholar] [CrossRef] [PubMed]
- Schnelzer, M.; Hammer, G.P.; Kreuzer, M.; Tschense, A.; Grosche, B. Accounting for smoking in the radon-related lung cancer risk among German uranium miners: Results of a nested case-control study. Health Phys. 2010, 98, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Qin, L.; Cao, Y.; Li, J.; Zhang, J.; Nie, J.; An, Y. Environmental radon exposure and childhood leukemia. J. Toxicol. Environ. Health Part B Crit. Rev. 2012, 15, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Raaschou-Nielsen, O. Indoor radon and childhood leukaemia. Radiat. Prot. Dosim. 2008, 132, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Hauri, D.; Spycher, B.; Huss, A.; Zimmermann, F.; Grotzer, M.; von der Weid, N.; Weber, D.; Spoerri, A.; Kuehni, C.E.; Roosli, M. Domestic radon exposure and risk of childhood cancer: A prospective census-based cohort study. Environ. Health Perspect. 2013, 121, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Ko, Y.H.; Lee, S.S.; Huh, J.; Kang, C.S.; Kim, C.W.; Kang, Y.K.; Go, J.H.; Kim, M.K.; Kim, W.S.; et al. WHO Classification of Malignant Lymphomas in Korea: Report of the Third Nationwide Study. Korean J. Pathol. 2011, 45, 254–260. [Google Scholar] [CrossRef]
- Crump, C.; Sundquist, K.; Sieh, W.; Winkleby, M.A.; Sundquist, J. Perinatal and family risk factors for non-Hodgkin lymphoma in early life: A Swedish national cohort study. J. Natl. Cancer Inst. 2012, 104, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Miligi, L.; Benvenuti, A.; Mattioli, S.; Salvan, A.; Tozzi, G.A.; Ranucci, A.; Legittimo, P.; Rondelli, R.; Bisanti, L.; Zambon, P.; et al. Risk of childhood leukaemia and non-Hodgkin’s lymphoma after parental occupational exposure to solvents and other agents: The SETIL Study. Occup. Environ. Med. 2013, 70, 648–655. [Google Scholar] [CrossRef] [PubMed]
- McNally, R.J.; Parker, L. Environmental factors and childhood acute leukemias and lymphomas. Leukemia Lymphoma 2006, 47, 583–598. [Google Scholar] [CrossRef] [PubMed]
- Zablotska, L.B.; Lane, R.S.; Frost, S.E.; Thompson, P.A. Leukemia, lymphoma and multiple myeloma mortality (1950–1999) and incidence (1969–1999) in the Eldorado uranium workers cohort. Environ. Res. 2014, 130, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Rericha, V.; Kulich, M.; Rericha, R.; Shore, D.L.; Sandler, D.P. Incidence of leukemia, lymphoma, and multiple myeloma in Czech uranium miners: A case-cohort study. Environ. Health Perspect. 2006, 114, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Peckham, E.C.; Scheurer, M.E.; Danysh, H.E.; Lubega, J.; Langlois, P.H.; Lupo, P.J. Residential Radon Exposure and Incidence of Childhood Lymphoma in Texas, 1995–2011. Int. J. Environ. Res. Public Health 2015, 12, 12110–12126. [Google Scholar] [CrossRef] [PubMed]
- Teras, L.R.; Diver, W.R.; Turner, M.C.; Krewski, D.; Sahar, L.; Ward, E.; Gapstur, S.M. Residential radon exposure and risk of incident hematologic malignancies in the Cancer Prevention Study-II Nutrition Cohort. Environ. Res. 2016, 148, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Krewski, D.; Lubin, J.H.; Zielinski, J.M.; Alavanja, M.; Catalan, V.S.; Field, R.W.; Klotz, J.B.; Letourneau, E.G.; Lynch, C.F.; Lyon, J.I.; et al. Residential radon and risk of lung cancer: A combined analysis of 7 North American case-control studies. Epidemiology 2005, 16, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Bradford, P.; Smith, D. Large Animal Internal Medicine, 4th ed.; Elsevier: Munich, Germany, 2009. [Google Scholar]
- Berkman, I.K.; Maria Glymour, M. Social Epidemiology, 2nd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2015. [Google Scholar]
- Cahoon, E.K.; Pfeiffer, R.M.; Wheeler, D.C.; Arhancet, J.; Lin, S.W.; Alexander, B.H.; Linet, M.S.; Freedman, D.M. Relationship between ambient ultraviolet radiation and non-Hodgkin lymphoma subtypes: A U.S. population-based study of racial and ethnic groups. Int. J. Cancer 2015, 136, E432–E441. [Google Scholar] [CrossRef] [PubMed]
- Szklo, M. Epidemiology: Beyond the Basics, 3rd ed.; Jones & Bartlett Learning: Burlington, MA, USA, 2014. [Google Scholar]
- Schwartz, G.G.; Klug, M.G. Incidence rates of chronic lymphocytic leukemia in US states are associated with residential radon levels. Future Oncol. 2016, 12, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Statistics Korea. National Index System. Available online: http://www.index.go.kr/potal/main/EachDtlPageDetail.do?idx_cd=1013 (accessed on 3 December 2017).
- Weinberg, C.R.; Umbach, D.M.; Greenland, S. When will nondifferential misclassification of an exposure preserve the direction of a trend? Am. J. Epidemiol. 1994, 140, 565–571. [Google Scholar] [CrossRef] [PubMed]
| Site (No. of Measurement Point) | Device | Comments | Annual Radon Concentration (Bq/m3) | ||
|---|---|---|---|---|---|
| Arithmetic Mean | Geometric Mean | Max. | |||
| 1st Survey (1989) | |||||
| Dwellings (530) | RadTrack | - indoor measurements only during the winter | 103.9 | 92.2 | 496 |
| 2nd Survey (1999–2000) | |||||
| Dwellings for three months (2953) | RadTrak | - calculation of annual average indoor concentration - distribution by season, house type, building age - focused on urban areas | 52.5 | 42.1 | 1350 |
| 3rd Survey (2002–2005) | |||||
| Dwellings for three months (Rn 970, Tn 450) | RadTrak, Radpot, thoron progeny monitor | - indoor Rn distribution map - distribution by season, house type, building age - more rural areas | 66.3 | 55.7 | 1186 |
| 4th Survey (2008–2009) | |||||
| Public buildings (1100) | Raduet | - 63% schools, 36.3% local governmental offices - measured on the first floor of the buildings | 79.3 | 60.5 | 1004 |
| Total (5553) | 62.1 | 49.0 | 1350 | ||
| Characteristics of the 234 Regions | Mean | Min. | Max. |
|---|---|---|---|
| Population (no. 2009) a | 172,857 | 10,325 | 1,073,149 |
| Male | 88,090.5 | 5393 | 539,655 |
| Female | 85,348.5 | 4935 | 533,494 |
| Median age (years, 2009) a | 42.8 | 32.8 | 59.2 |
| Male | 40.8 | 31.5 | 56.1 |
| Female | 44.8 | 33.1 | 62.6 |
| Aggregated indoor radon concentration (Bq/m3) | 57 | 24 | 231 |
| Deprivation index (z-score, 2005) | 0.06 | −7.82 | 7.28 |
| Smoking rate (%, 2009) b | 26.7 | 16.4 | 33.4 |
| Male | 50.4 | 31.9 | 62.4 |
| Female | 6.0 | 0.4 | 12.5 |
| Age-standardized incidence ratio c | |||
| Lung cancer | |||
| Male (no. of cases) e | 1.05 (494) | 0.51 (26) | 1.53 (1617) |
| Female (no. of cases) e | 1.00 (185) | 0.48 (11) | 1.42 (736) |
| Non-Hodgkin’s lymphoma, all | |||
| Male (no. of cases) e | 1.01 (55) | 0.44 (3) | 1.70 (256) |
| Female (no. of cases) e | 0.99 (43) | 0.46 (3) | 2.00 (211) |
| Non-Hodgkin’s lymphoma, children and adolescents d | |||
| Male (no. of cases) e | 1.00 (12) | 0.00 (0) | 3.93 (69) |
| Female (no. of cases) e | 0.98 (9) | 0.00 (0) | 3.42 (51) |
| Leukemia, all | |||
| Male (no. of cases) e | 0.98 (68) | 0.46 (4) | 1.48 (283) |
| Female (no. of cases) e | 0.98 (50) | 0.32 (0) | 1.65 (232) |
| Leukemia, children and adolescents d | |||
| Male (no. of cases) e | 0.94 (4) | 0.00 (0) | 4.99 (25) |
| Female (no. of cases) e | 0.99 (2) | 0.00 (0) | 6.13 (14) |
| Per 10 Bq/m3 Increase in Radon Concentration | Crude | Adjusted a | |||
|---|---|---|---|---|---|
| Cancer Type | Relative Risk | 95% Credible Intervals | Relative Risk | 95% Credible Intervals | |
| Male | Lung cancer | 1.03 | (1.02, 1.05) | 1.01 | (1.00, 1.02) |
| NHL, all | 1.01 | (0.99, 1.03) | 1.00 | (0.98, 1.02) | |
| NHL in children and adolescents b | 0.98 | (0.94, 1.02) | 0.97 | (0.93, 1.02) | |
| Leukemia, all | 0.98 | (0.96, 1.00) | 0.98 | (0.96, 1.00) | |
| Leukemia in children and adolescents b | 0.96 | (0.89, 1.03) | 1.00 | (0.92, 1.08) | |
| Female | Lung cancer | 1.01 | (0.99, 1.02) | 1.01 | (0.99, 1.02) |
| NHL, all | 1.03 | (1.01, 1.06) | 1.04 | (1.02, 1.07) | |
| NHL in children and adolescents b | 1.04 | (0.99, 1.10) | 1.07 | (1.01, 1.13) | |
| Leukemia, all | 0.98 | (0.96, 1.01) | 0.98 | (0.95, 1.00) | |
| Leukemia in children and adolescents b | 1.00 | (0.91, 1.10) | 0.98 | (0.88, 1.08) | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, M.; Hwang, S.-s.; Kang, S.; Park, N.-W.; Chang, B.-U.; Kim, Y. Geographical Correlations between Indoor Radon Concentration and Risks of Lung Cancer, Non-Hodgkin’s Lymphoma, and Leukemia during 1999–2008 in Korea. Int. J. Environ. Res. Public Health 2017, 14, 344. https://doi.org/10.3390/ijerph14040344
Ha M, Hwang S-s, Kang S, Park N-W, Chang B-U, Kim Y. Geographical Correlations between Indoor Radon Concentration and Risks of Lung Cancer, Non-Hodgkin’s Lymphoma, and Leukemia during 1999–2008 in Korea. International Journal of Environmental Research and Public Health. 2017; 14(4):344. https://doi.org/10.3390/ijerph14040344
Chicago/Turabian StyleHa, Mina, Seung-sik Hwang, Sungchan Kang, No-Wook Park, Byung-Uck Chang, and Yongjae Kim. 2017. "Geographical Correlations between Indoor Radon Concentration and Risks of Lung Cancer, Non-Hodgkin’s Lymphoma, and Leukemia during 1999–2008 in Korea" International Journal of Environmental Research and Public Health 14, no. 4: 344. https://doi.org/10.3390/ijerph14040344






