Pollutants’ Release, Redistribution and Remediation of Black Smelly River Sediment Based on Re-Suspension and Deep Aeration of Sediment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
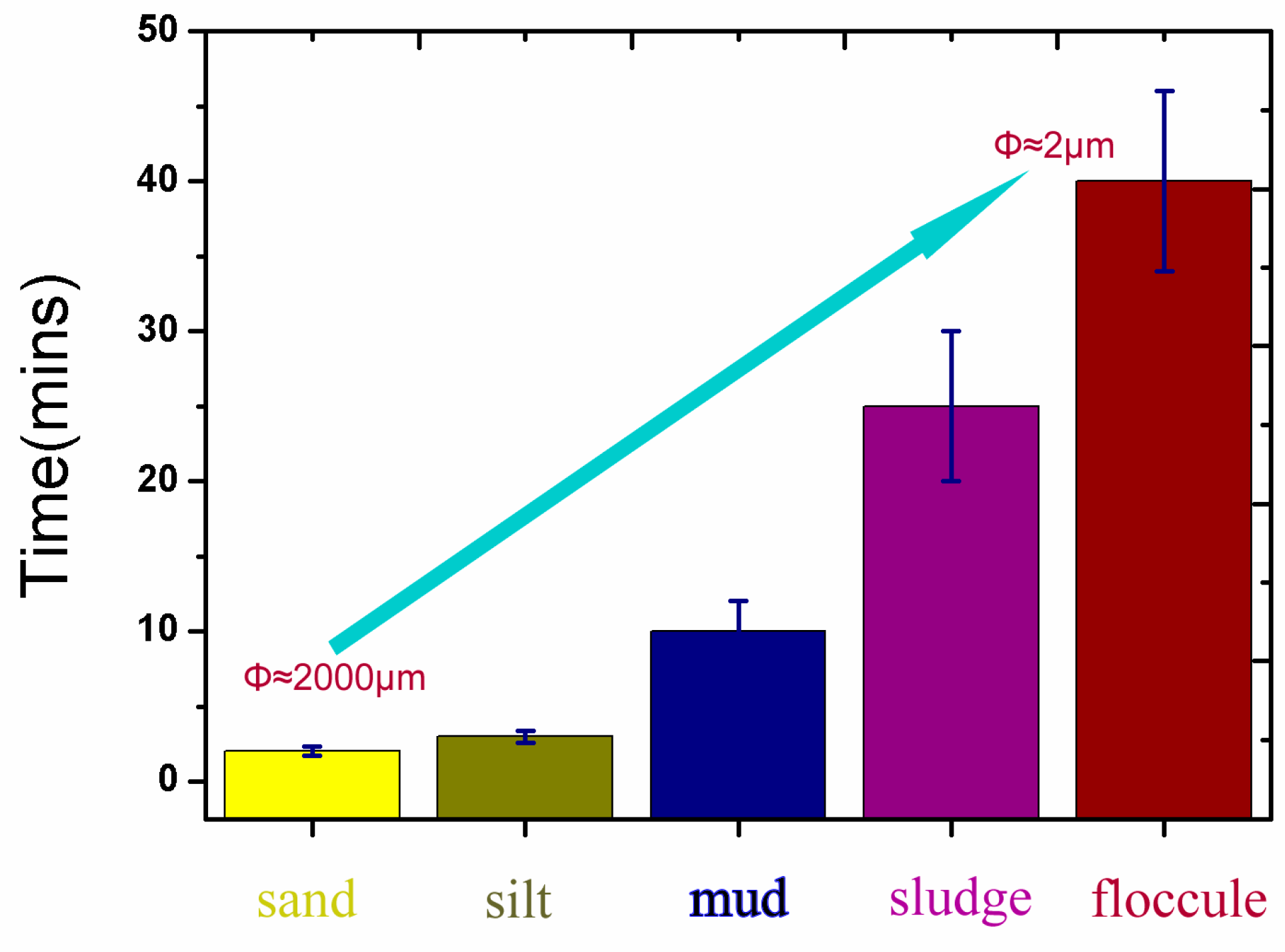
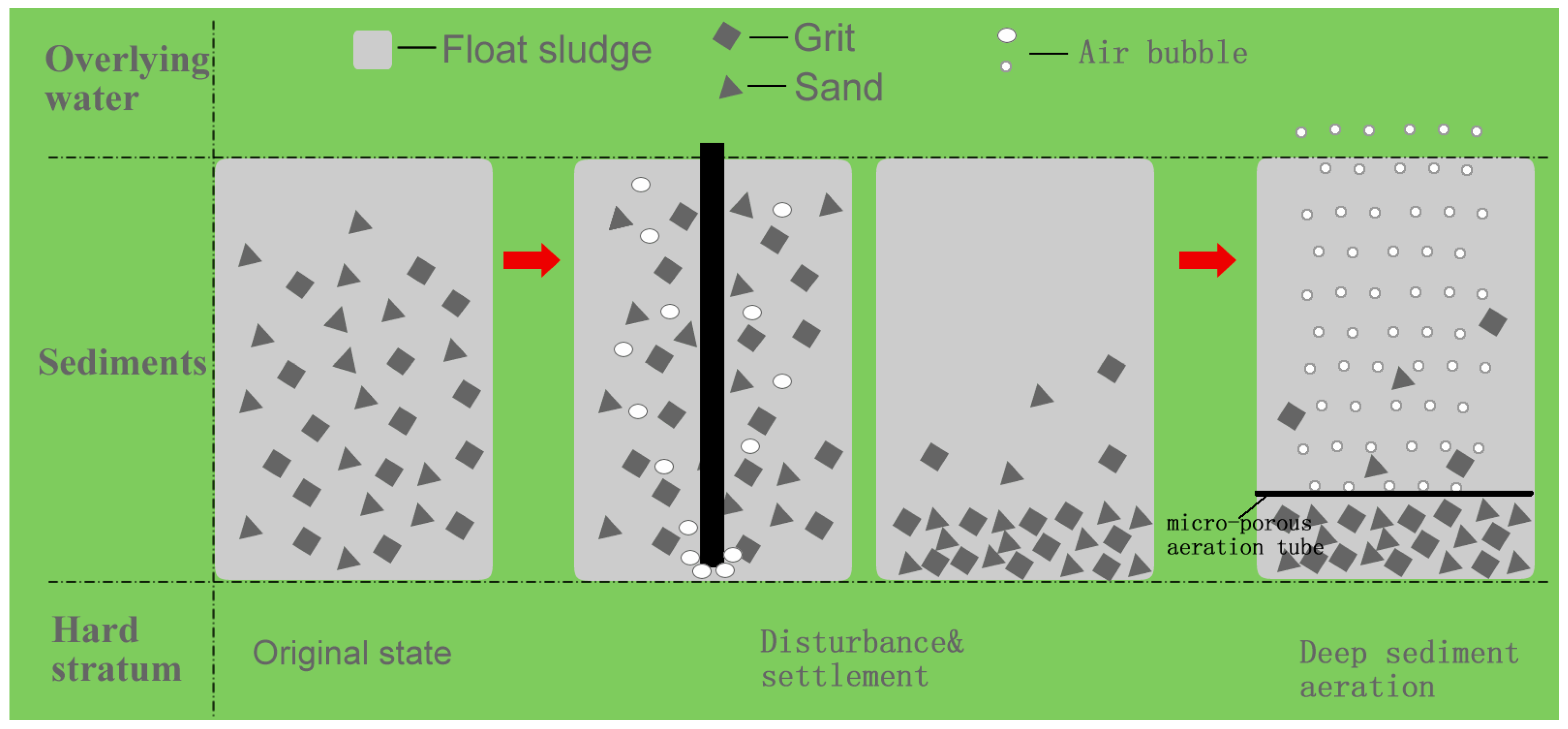
2.2. Sediment Disturbance and Deep Aeration
2.3. Water, Sediment Sampling, and Analysis
3. Results and Discussion
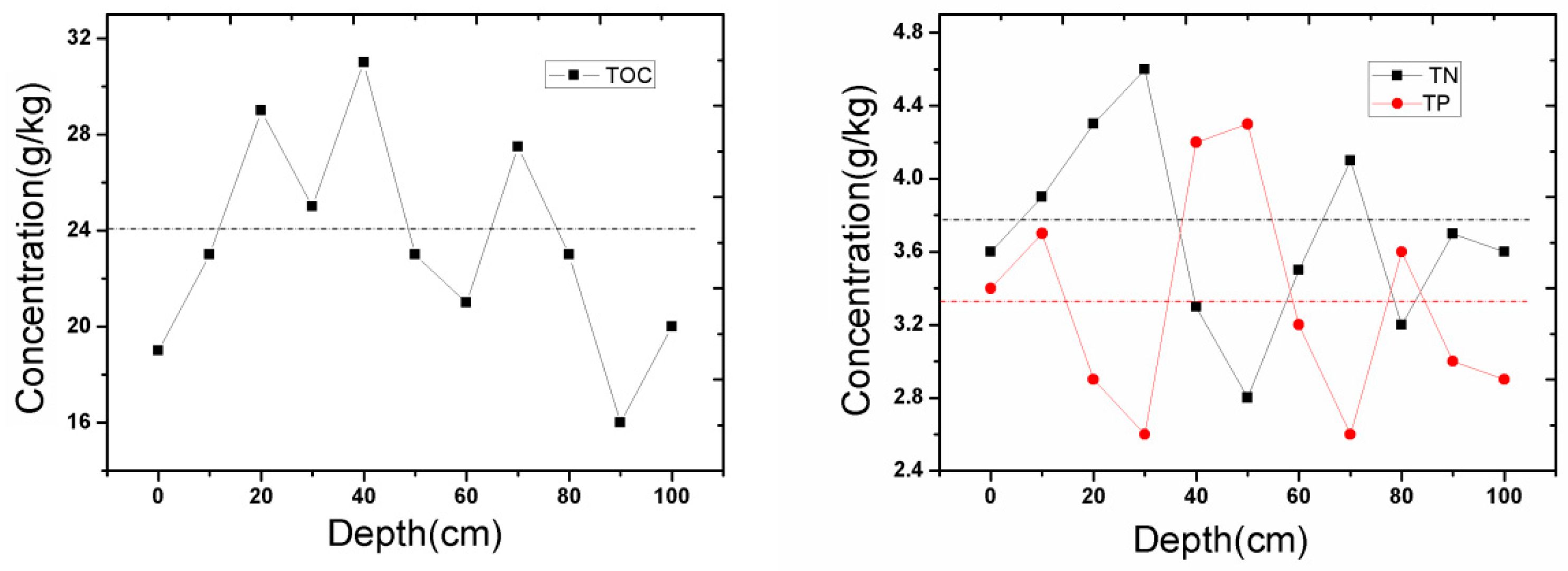
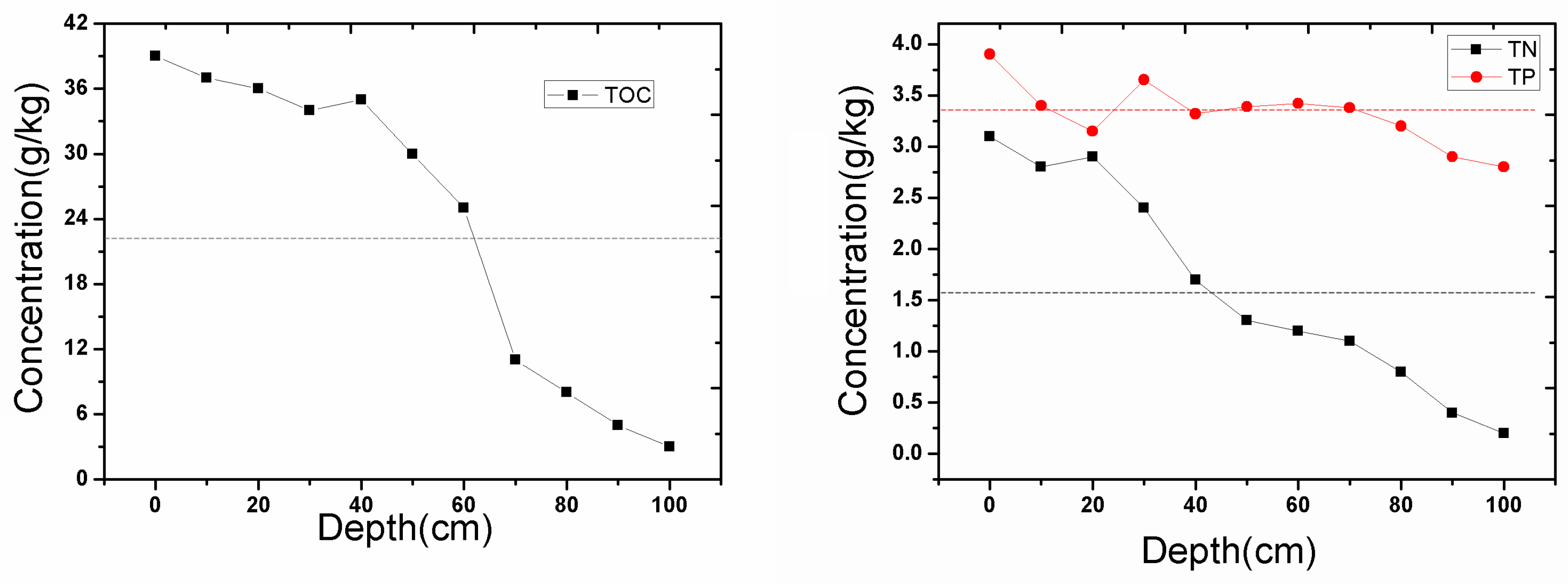
3.1. The Distribution of Pollutants in the Sediment
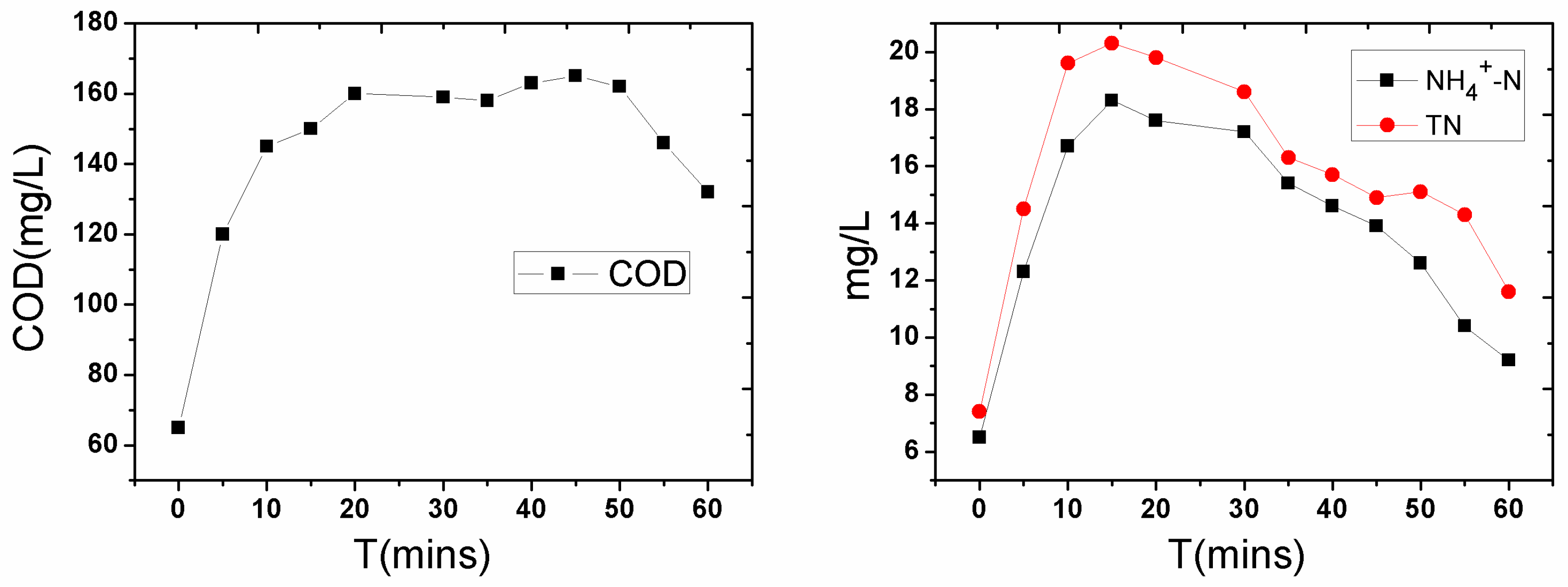
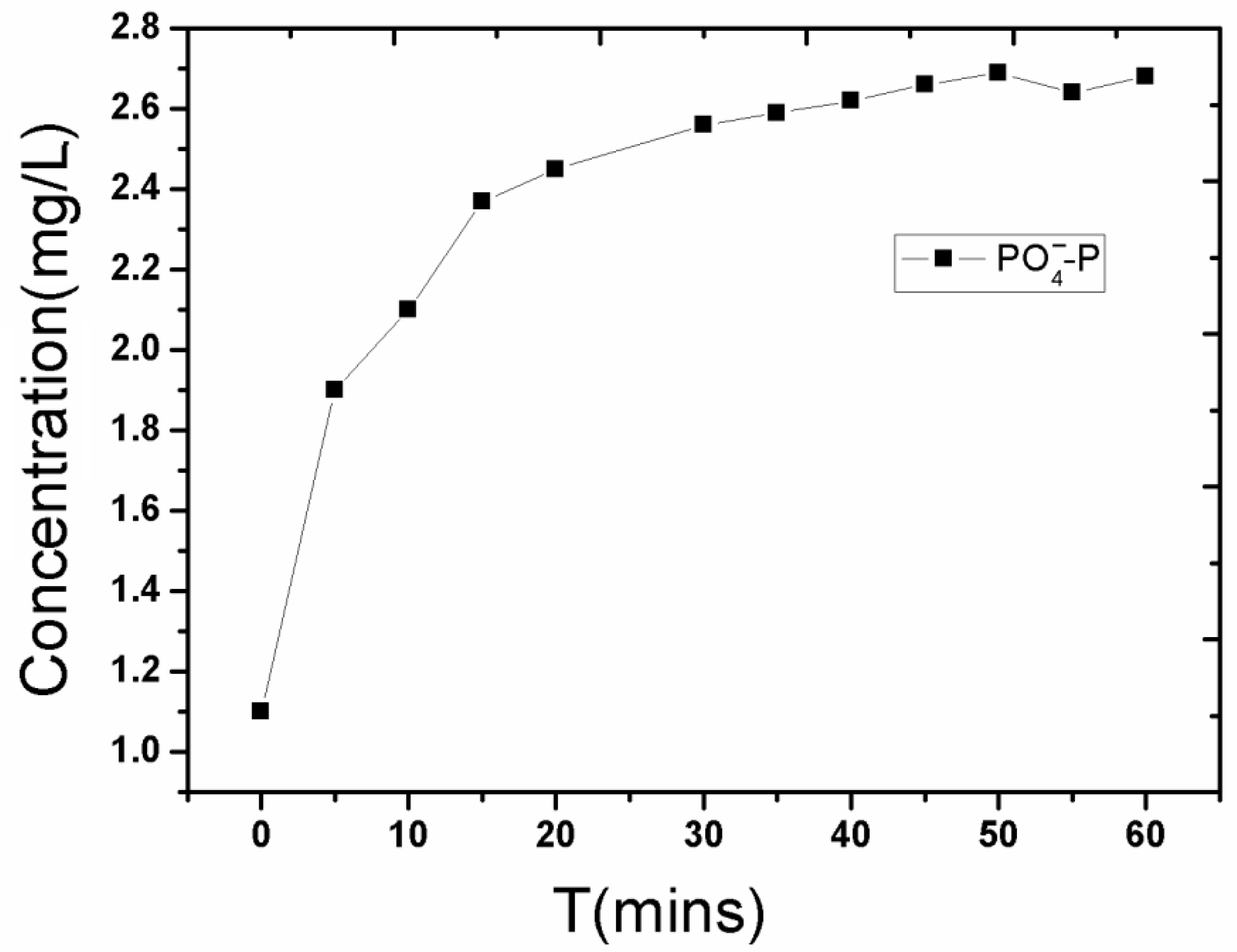
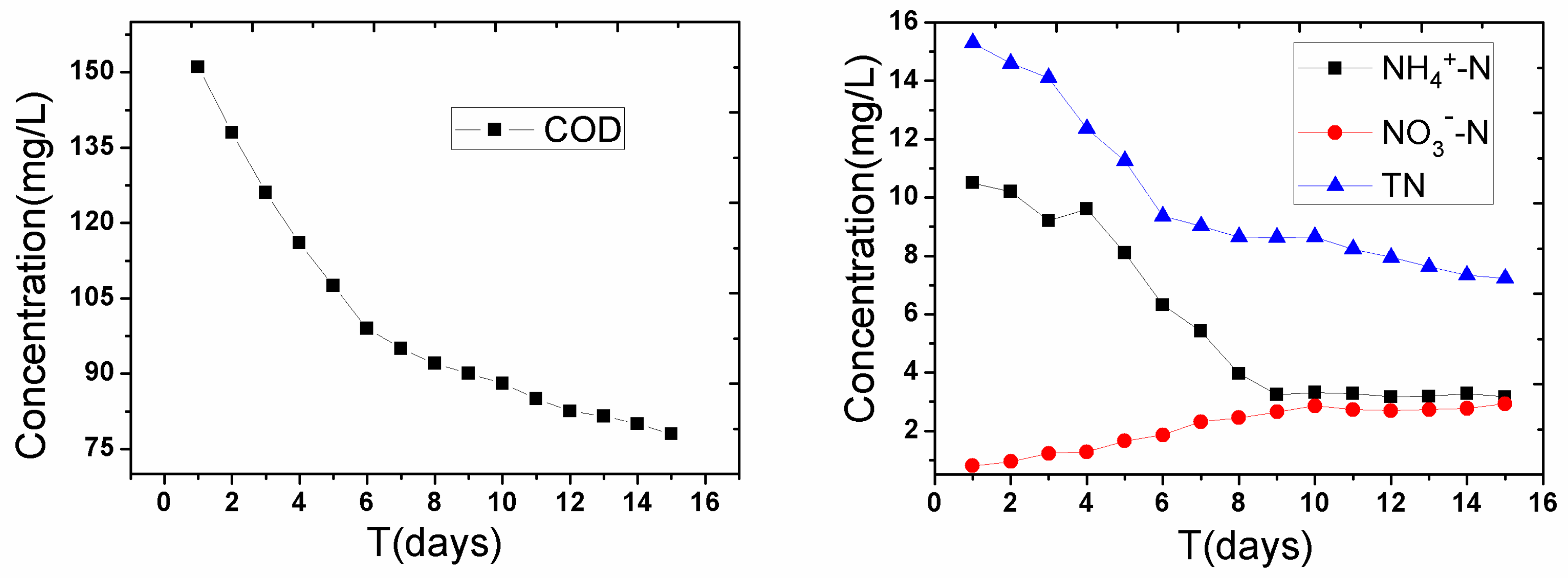
3.2. Disturbance of Water Contaminants after Severe Disturbance
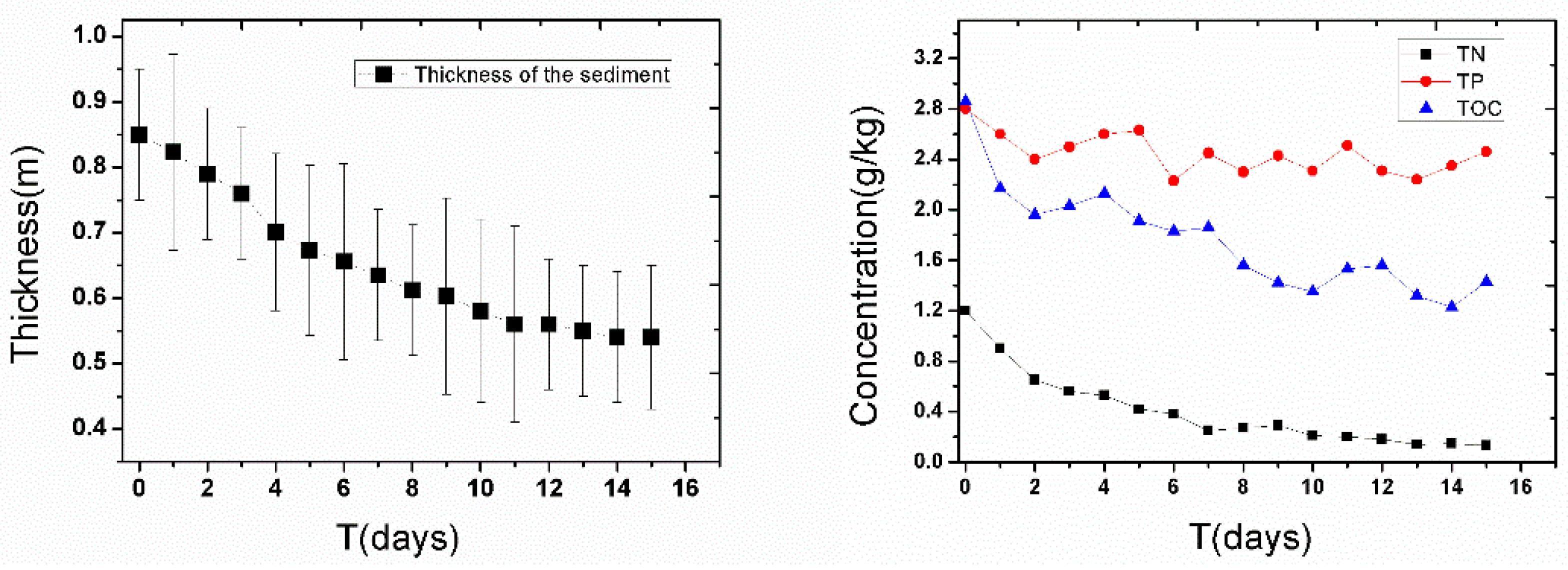
3.3. Distribution of Pollutants in Sediment after Settling for 24 h
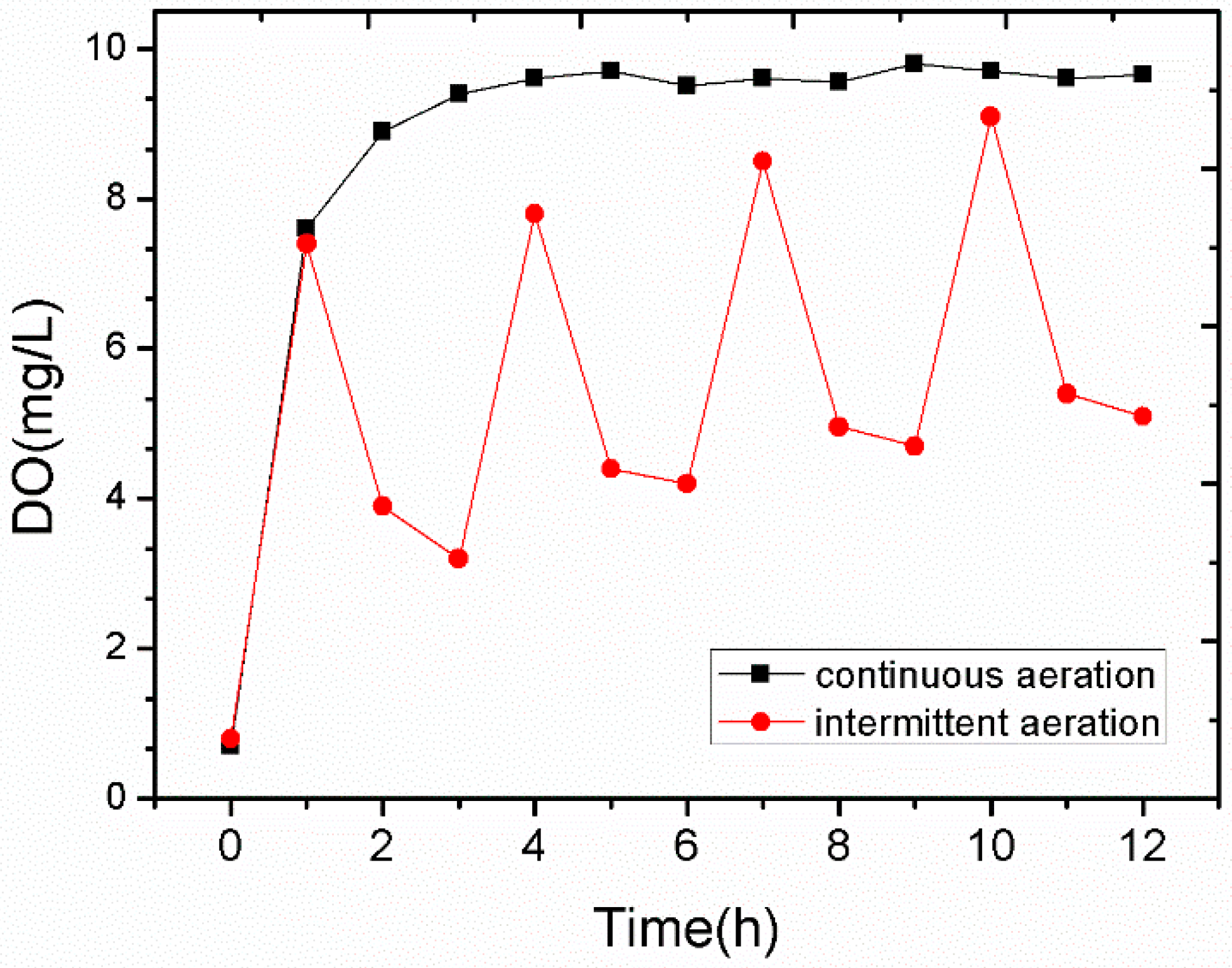
3.4. Effect of Deep Aeration after Re-Suspending the Sediment
4. Conclusions
- The exhaustive re-suspension of sediment promotes the fast release of pollutants in the sediment and uniformly distributes the sediment pollutants. The thickness of the sediments is also reduced.
- No chemical or biological substances are needed; this causes less destruction of the aquatic ecosystem and is cost effective.
- Deep sediment aeration results in the high degradation efficiency of pollutants and effectively inhibits the re-release of pollutants in common surface sediment aeration.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Luoma, S.N. Bioavailability of trace metals to aquatic organisms—A review. Sci. Total Environ. 1983, 28, 1–22. [Google Scholar] [CrossRef]
- DePinto, J.V.; Young, T.C.; Martin, S.C. Algal-available phosphorus in suspended sediments from lower great lakes tributaries. J. Great Lakes Res. 1981, 3, 311–325. [Google Scholar] [CrossRef]
- Fan, W.; Wang, W.X.; Chen, J.; Li, X.; Yen, Y.F. Cu, Ni and Pb speciation in surface sediments from a contaminated bay of northern China. Mar. Pollut. Bull. 2002, 44, 816–832. [Google Scholar] [CrossRef]
- Huang, J.; Ge, X.; Wang, D. Distribution of heavy metals in the water column, suspended particulate matters and the sediment under hydrodynamic conditions using an annular flume. J. Environ. Sci. 2012, 24, 2051–2059. [Google Scholar] [CrossRef]
- Wan, L.; Xu, L.; Fu, Y. Contamination and risk assessment of heavy metals in lake bed sediment of a large lake scenic area in China. Int. J. Environ. Res. Public Health 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- Rekha, P.; Suman Raj, D.S.; Aparna, C.; Hima Bindu, V.; Anjaneyulu, Y. Bioremediation of contaminated lake sediments and evaluation of maturity indicies as indicators of compost stability. Int. J. Environ. Res. Public Health 2005, 2, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Conaway, C.H.; Squire, S.; Mason, R.P.; Flegal, A.R. Mercury speciation in the San Francisco bay estuary. Mar. Chem. 2003, 80, 199–225. [Google Scholar] [CrossRef]
- Latimer, J.; Davis, W.; Keith, D. Mobilization of PAHs and PCBs from in-place contaminated marine sediments during simulated resuspension events. Estuar. Coast Shelf Sci. 1999, 49, 577–595. [Google Scholar] [CrossRef]
- Simpson, S.L.; Apte, S.C.; Batley, G.E. Effect of short-term resuspension events on trace metal speciation in polluted anoxic sediments. Environ. Sci. Technol. 1998, 325, 620–625. [Google Scholar] [CrossRef]
- Won, N.; Kim, K.-H.; Kang, J.H.; Park, S.R.; Lee, H.J. Exploring the impacts of anthropogenic disturbance on seawater and sediment microbial communities in Korean coastal waters using metagenomics analysis. Int. J. Environ. Res. Public Health 2017, 14. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Kan, A.T.; Fu, G.; Vignona, C.; Tomson, M. Adsorption–desorption behaviors of hydrophobic organic compounds in sediments of Lake Charles Louisiana, USA. Environ. Toxicol. Chem. 1999, 18, 1610–1616. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, R.S.S.; Hong, H.-S.; Poon, K.-F.; Lam, M.H.W. Field study on the desorption rates of polynuclear aromatic hydrocarbons from contaminated marine sediment. Environ. Toxicol. Chem. 2000, 19, 2431–2435. [Google Scholar] [CrossRef]
- Calmano, W.; Hong, J.; Forstner, U. Binding and mobilisation of heavy metals in contaminated sediments affected by pH and redox potential. Water Sci. Technol. 1993, 28, 223–235. [Google Scholar]
- Eggleton, J.; Thomas, K.V. A review of factors affecting the release and bioavailability of contaminants during sediment disturbance events. Environ. Int. 2004, 30, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Li, D.P.; Huang, Y.; Fan, C.X.; Yuan, Y. Contributions of phosphorus on sedimentary phosphorus bioavailability under sediment resuspension conditions. Chem. Eng. J. 2011, 168, 1049–1054. [Google Scholar]
- Fukue, M.; Uehara, K.; Sato, Y.; Mulligan, C. Re-suspension technique for improving organic rich sediment-water quality in a shallow sea area. Mar. Georesour. Geotec. 2012, 30, 222–233. [Google Scholar] [CrossRef]
- Gomes, H.I.; Dias-Ferreira, C.; Ribeiro, A.B. Overview of in situ and ex situ remediation technologies for PCB-contaminated soils and sediments and obstacles for full-scale application. Sci. Total Environ. 2013, 445, 237–260. [Google Scholar] [CrossRef] [PubMed]
- Utley, B.C.; Vellidis, G.; Lowrance, R. Factors affecting sediment oxygen demand dynamics in black water streams of Georgia’s coastal plain. J. Am. Water Resour. As. 2008, 44, 742–753. [Google Scholar] [CrossRef]
- Petersen, W.; Willer, E.; Willamowski, C. Remobilization of trace elements from polluted anoxic sediments after resuspension in oxic water. Water Air Soil Poll. 1997, 99, 515–522. [Google Scholar] [CrossRef]
- Pourabadehei, M.; Mulligan, C.N. Resuspension of sediment, a new approach for remediation of contaminated sediment. Environ. Pollut. 2016, 213, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Langstone, W.J.; Pope, N.D. Determinants of TBT adsorption and desorption in estuarine sediments. Mar. Pollut. Bull. 1995, 31, 32–43. [Google Scholar] [CrossRef]
- Roberts, D.A. Causes and ecological effects of resuspended contaminated sediments (RCS) in marine environments. Environ. Int. 2012, 40, 230–243. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 20th ed. Available online: https://books.google.com.hk/books/about/Standard_Methods_for_the_Examination_of.html?id=2BcoYAAACAAJ&redir_esc=y (accessed on 15 September 2016).
- Wang, Y.; Zhang, D.; Shen, Z.Y.; Chen, J.; Feng, C.H. Characterization and spacial distribution variability ofchromophoric dissolved organic matter (CDOM) in the Yangtze Estuary. Chemosphere 2014, 95, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Dong, J.; Wang, M.; Xie, H.; Xia, N.; Li, H.; Zhang, X.; Mou, X.; Wen, J.; Bao, Y. Effect of water-sediment regulation of the Xiaolangdi reservoir on the concentrations, characteristics, and fluxes of suspended sediment and organic carbon in the Yellow River. Sci. Total Environ. 2016, 571, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, A.; Makinia, J.; Pagilla, K. Impact of aeration conditions on the removal of low concentrations of nitrogen in a tertiary partially aerated biological filter. Ecol. Eng. 2012, 44, 44–52. [Google Scholar] [CrossRef]
- He, Y.; Chen, Y.; Zhang, Y.; Huang, M. Role of aerated turbulence in the fate of endogenous nitrogen from malodorous river Sediments. Environ. Eng. Sci. 2013, 30, 11–16. [Google Scholar] [CrossRef]
- Mountouris, A.; Voutsas, E.; Tassios, D. Bioconcentration of heavy metals in aquatic environments: The importance of bioavailability. Mar. Pollut. Bull. 2002, 44, 1136–1141. [Google Scholar] [CrossRef]
- Zhou, J.; Broodbank, N. Sediment-water interactions of pharmaceutical residues in the river environment. Water Res. 2014, 48, 61–70. [Google Scholar] [CrossRef]
- Cheng, X.; Zeng, Y.; Guo, Z.; Zhu, L. Diffusion of nitrogen and phosphorus across the sediment-water interface and in seawater at aquaculture areas of Daya Bay, China. Int. J. Environ. Res. Public Health. 2014, 11, 1557–1572. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Li, X.; Zhang, C.; Duan, Z. Pollutants’ Release, Redistribution and Remediation of Black Smelly River Sediment Based on Re-Suspension and Deep Aeration of Sediment. Int. J. Environ. Res. Public Health 2017, 14, 374. https://doi.org/10.3390/ijerph14040374
Zhu L, Li X, Zhang C, Duan Z. Pollutants’ Release, Redistribution and Remediation of Black Smelly River Sediment Based on Re-Suspension and Deep Aeration of Sediment. International Journal of Environmental Research and Public Health. 2017; 14(4):374. https://doi.org/10.3390/ijerph14040374
Chicago/Turabian StyleZhu, Lin, Xun Li, Chen Zhang, and Zengqiang Duan. 2017. "Pollutants’ Release, Redistribution and Remediation of Black Smelly River Sediment Based on Re-Suspension and Deep Aeration of Sediment" International Journal of Environmental Research and Public Health 14, no. 4: 374. https://doi.org/10.3390/ijerph14040374





