Dendritic Architecture of Principal Basolateral Amygdala Neurons Changes Congruently with Endocrine Response to Stress
Abstract
:1. Introduction
2. Materials and Methods
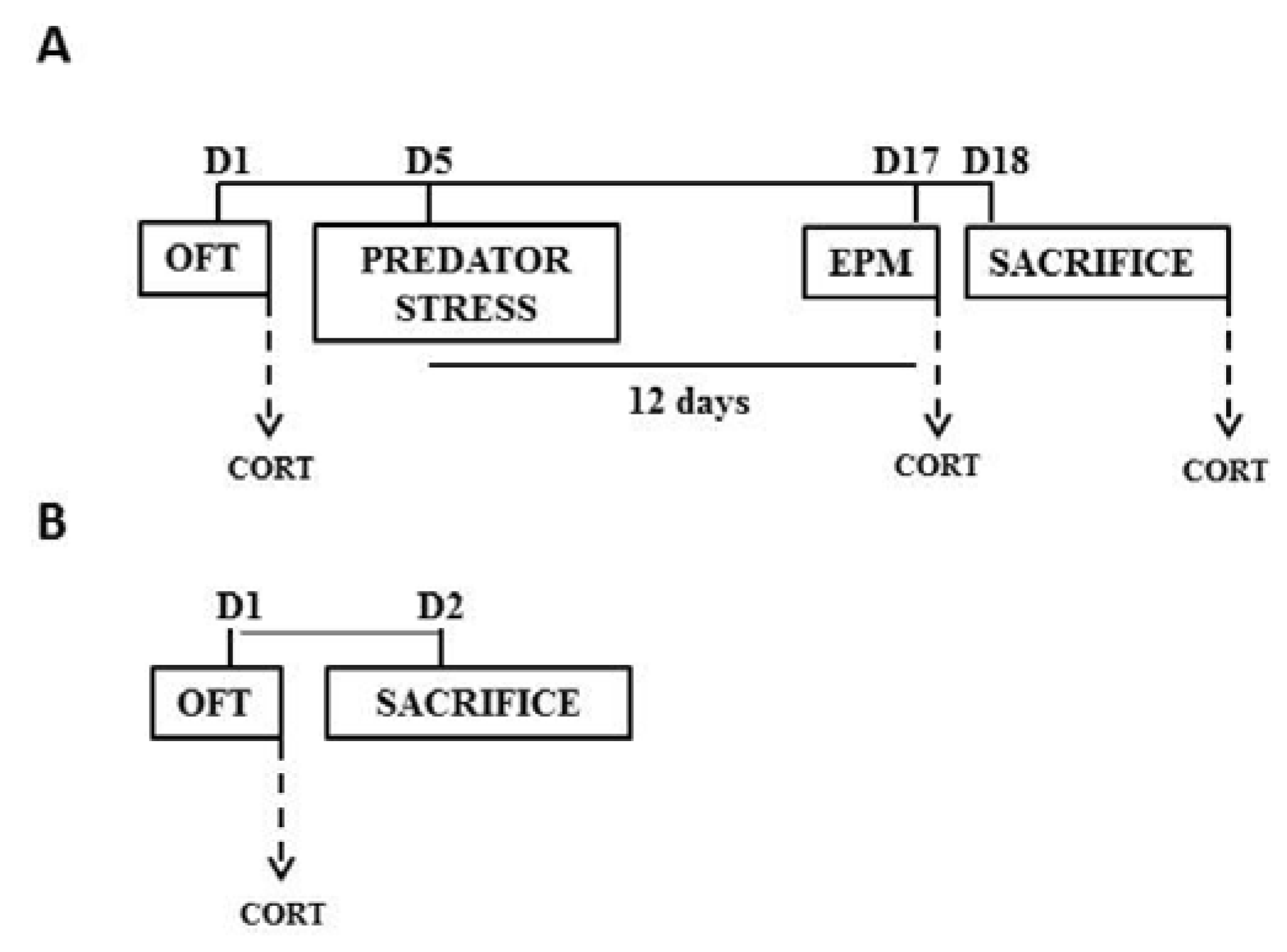
2.1. Animals and Experimental Groups
2.2. Open Field Test (OFT)
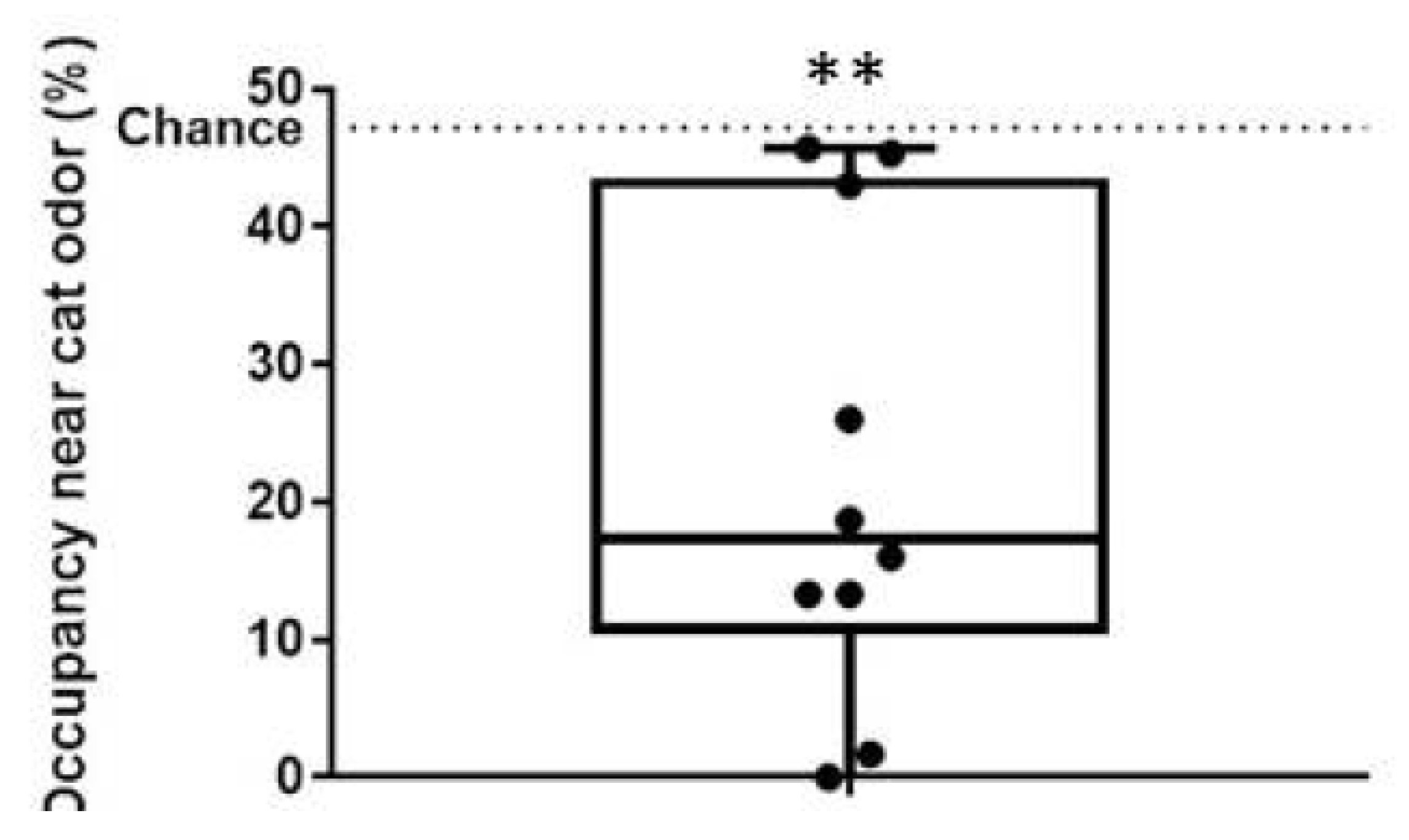
2.3. Predator Odor Exposure
2.4. Elevated Plus Maze (EPM)
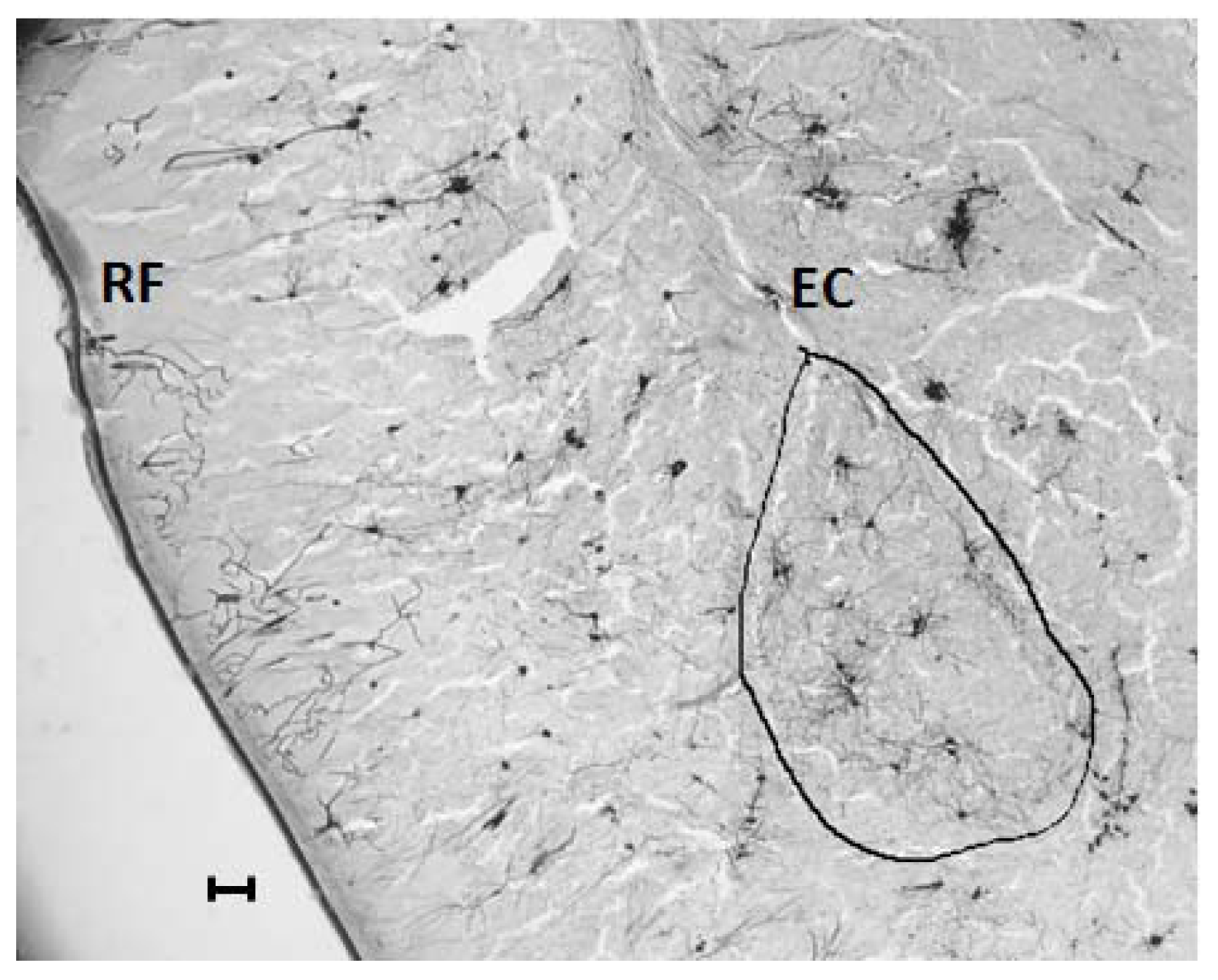
2.5. Morphological Analysis of BLA Neurons
2.6. Corticosterone Estimation
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [PubMed]
- Coderre, L.; Srivastava, A.K.; Chiasson, J.L. Role of glucocorticoid in the regulation of glycogen metabolism in skeletal muscle. Am. J. Physiol. 1991, 260, E927–E932. [Google Scholar] [PubMed]
- Myers, B.; McKlveen, J.M.; Herman, J.P. Glucocorticoid actions on synapses, circuits, and behavior: Implications for the energetics of stress. Front. Neuroendocrinol. 2014, 35, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Cockrem, J.F. Individual variation in glucocorticoid stress responses in animals. Gen. Comp. Endocrinol. 2013, 181, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Cockrem, J.F.; Barrett, D.P.; Candy, E.J.; Potter, M.A. Corticosterone responses in birds: Individual variation and repeatability in Adelie penguins (Pygoscelisadeliae) and other species, and the use of power analysis to determine sample sizes. Gener. Comp. Endocrinol. 2009, 163, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Dufty, A.M.; Clobert, J.; Møller, A.P. Hormones, developmental plasticity and adaptation. Trends Ecol. Evol. 2002, 17, 190–196. [Google Scholar] [CrossRef]
- Ricklefs, R.E.; Wikelski, M. The physiology/life-history nexus. Trends Ecol. Evol. 2002, 17, 462–468. [Google Scholar] [CrossRef]
- Hau, M.; Ricklefs, R.E.; Wikelski, M.; Lee, K.A. Corticosterone, testosterone and life-history strategies of birds. Proc. R. Soc. Lond. B Biol. Sci. 2010, 277, 3203–3212. [Google Scholar] [CrossRef] [PubMed]
- Adamec, R.E.; Blundell, J.; Burton, P. Relationship of the predatory attack experience to neural plasticity, pCREB expression and neuroendocrine response. Neurosci. Biobehav. Rev. 2006, 30, 356–375. [Google Scholar] [CrossRef] [PubMed]
- Bowen, M.T.; Dass, S.A.H.; Booth, J.; Suraev, A.; Vyas, A.; McGregor, I.S. Active coping toward predatory stress is associated with lower corticosterone and progesterone plasma levels and decreased methylation in the medial amygdala vasopressin system. Horm. Behav. 2014, 66, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R.; Vyas, A.; Chatterjee, G.; Chattarji, S. Chronic-stress induced modulation of different states of anxiety-like behavior in female rats. Neurosci. Lett. 2005, 383, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Vyas, A.; Chattarji, S. Modulation of different states of anxiety-like behavior by chronic stress. Behav. Neurosci. 2004, 118, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Vyas, A.; Mitra, R.; Chattarji, S. Enhanced anxiety and hypertrophy in basolateral amygdala neurons following chronic stress in rats. Ann. N. Y. Acad. Sci. 2003, 985, 554–555. [Google Scholar] [CrossRef]
- Liang, Z.; King, J.; Zhang, N. Neuroplasticity to a single-episode traumatic stress revealed by resting-state fMRI in awake rats. NeuroImage 2014, 103, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Adamec, R.; Walling, S.; Burton, P. Long-lasting, selective, anxiogenic effects of feline predator stress in mice. Physiol. Behav. 2004, 83, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Adamec, R.E.; Blundell, J.; Collins, A. Neural plasticity and stress induced changes in defense in the rat. Neurosci. Biobehav. Rev. 2001, 25, 721–744. [Google Scholar] [CrossRef]
- DeMaria, E.J.; Lilly, M.P.; Gann, D.S. Potentiated hormonal responses in a model of traumatic injury. J. Surg. Res. 1987, 43, 45–51. [Google Scholar] [CrossRef]
- Lynn, S.E.; Prince, L.E.; Phillips, M.M. A single exposure to an acute stressor has lasting consequences for the hypothalamo-pituitary-adrenal response to stress in free-living birds. Gen. Comp. Endocrinol. 2010, 165, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Vyas, A.; Mitra, R.; Rao, B.S.; Chattarji, S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 2002, 22, 6810–6818. [Google Scholar] [PubMed]
- Mitra, R.; Jadhav, S.; McEwen, B.S.; Vyas, A.; Chattarji, S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc. Natl. Acad. Sci. USA 2005, 102, 9371–9376. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R.; Sapolsky, R.M. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc. Natl. Acad. Sci. USA 2008, 105, 5573–5578. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Benjamin, J.; Kaplan, Z.; Kotler, M. Administration of high-dose ketoconazole, an inhibitor of steroid synthesis, prevents posttraumatic anxiety in an animal model. Eur. Neuropsychopharmacol. 2000, 10, 429–435. [Google Scholar] [CrossRef]
- Mitra, R.; Ferguson, D.; Sapolsky, R.M. Mineralocorticoid receptor overexpression in basolateral amygdala reduces corticosterone secretion and anxiety. Biol. Psychiatry 2009, 66, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R.; Sapolsky, R.M. Expression of chimeric estrogen-glucocorticoid-receptor in the amygdala reduces anxiety. Brain Res. 2010, 1342, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R.; Ferguson, D.; Sapolsky, R.M. SK2 potassium channel overexpression in basolateral amygdala reduces anxiety, stress-induced corticosterone secretion and dendritic arborization. Mol. Psychiatry 2009, 14, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R.; Sapolsky, R.M. Gene therapy in rodent amygdala against fear disorders. Expert. Opin. Biol. Ther. 2010, 10, 1289–1303. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R.; Sapolsky, R.M.; Vyas, A. Toxoplasma gondii infection induces dendritic retraction in basolateral amygdala accompanied by reduced corticosterone secretion. Dis. Model. Mech. 2013, 6, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Berdoy, M.; Webster, J.P.; Macdonald, D.W. Fatal attraction in rats infected with Toxoplasma gondii. Proc. R. Soc. Lond. B Biol. Sci. 2000, 267, 1591–1594. [Google Scholar] [CrossRef] [PubMed]
- House, P.K.; Vyas, A.; Sapolsky, R. Predator cat odors activate sexual arousal pathways in brains of Toxoplasma gondii infected rats. PLoS ONE 2011, 6, e23277. [Google Scholar] [CrossRef] [PubMed]
- Vyas, A.; Kim, S.K.; Giacomini, N.; Boothroyd, J.C.; Sapolsky, R.M. Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc. Natl. Acad. Sci. USA 2007, 104, 6442–6447. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.Y.; Liu, Y.J.; Xu, M.Y.; Zhang, Y.H.; Zhang, J.X.; Wu, Y.J. Inactivation of the bed nucleus of the stria terminalis suppresses the innate fear responses of rats induced by the odor of cat urine. Neuroscience 2012, 221, 21–27. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.J. Neurons of the lateral and basolateral amygdaloid nuclei: A Golgi study in the rat. J. Comp. Neurol. 1982, 212, 293–312. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; Figueiredo, H.; Mueller, N.K.; Ulrich-Lai, Y.; Ostrander, M.M.; Choi, D.C.; Cullinan, W.E. Central mechanisms of stress integration: Hierarchical circuitry controlling hypothalamo–pituitary–adrenocortical responsiveness. Front. Neuroendocrinol. 2003, 24, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Bourne, A.R.; Mohan, G.; Stone, M.F.; Pham, M.Q.; Schultz, C.R.; Meyerhoff, J.L.; Lumley, L.A. Olfactory cues increase avoidance behavior and induce Fos expression in the amygdala, hippocampus and prefrontal cortex of socially defeated mice. Behav. Brain Res. 2013, 256, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.N.; Lorson, N.G.; Sanabria, F.; Olive, M.F.; Conrad, C.D. Chronic stress disrupts fear extinction and enhances amygdala and hippocampal Fos expression in an animal model of post-traumatic stress disorder. Neurobiol. Learn. Mem. 2014, 112, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, M.S.; Grissom, N.; Paul, E.; Bhatnagar, S.; Maier, S.F.; Spencer, R.L. Inescapable but not escapable stress leads to increased struggling behavior and basolateral amygdala c-fos gene expression in response to subsequent novel stress challenge. Neuroscience 2010, 170, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.M.; LeDoux, J.E.; Sapolsky, R.M. The influence of stress hormones on fear circuitry. Annu. Rev. Neurosci. 2009, 32, 289–313. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.M.; Sapolsky, R.M. Disruption of fear memory through dual-hormone gene therapy. Biol. Psychiatry 2009, 65, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Salm, A.K.; Pavelko, M.; Krouse, E.M.; Webster, W.; Kraszpulski, M.; Birkle, D.L. Lateral amygdaloid nucleus expansion in adult rats is associated with exposure to prenatal stress. Brain Res. Dev. Brain Res. 2004, 148, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Ward, H.E.; Johnson, E.A.; Salm, A.K.; Birkle, D.L. Effects of prenatal stress on defensive withdrawal behavior and corticotropin releasing factor systems in rat brain. Physiol. Behav. 2000, 70, 359–366. [Google Scholar] [CrossRef]
- Mitra, R.; Adamec, R.; Sapolsky, R. Resilience against predator stress and dendritic morphology of amygdala neurons. Behav. Brain Res. 2009, 205, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Adamec, R.; Hebert, M.; Blundell, J.; Mervis, R.F. Dendritic morphology of amygdala and hippocampal neurons in more and less predator stress responsive rats and more and less spontaneously anxious handled controls. Behav. Brain Res. 2012, 226, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Dielenberg, R.A.; McGregor, I.S. Defensive behavior in rats towards predatory odors: A review. Neurosci. Biobehav. Rev. 2001, 25, 597–609. [Google Scholar] [PubMed]

| Estimates | Center Time in Open Field | Median Dendritic Length (in Microns) | Median Number of Branch Points | Median Spines on Primary Dendrites (per 60 Microns) | % Open Arm Time in EPM | % Open Arm Entries in EPM | Number of Enclosed Arm Entries in EPM | Corticosterone Post Open Field (pg/mL) | Corticosterone Post EPM (pg/mL) | Corticosterone Post Sacrifice (pg/mL) |
|---|---|---|---|---|---|---|---|---|---|---|
| Median | 3.50 | 1529 | 14.5 | 19.75 | 7.33 | 27.27 | 7 | 14.10 | 35.20 | 48.50 |
| Range | 12 | 499 | 4.5 | 9.50 | 40.33 | 46.67 | 7 | 38.40 | 194.10 | 151.10 |
| Mean | 4.90 | 1581 | 14.5 | 21.13 | 14.52 | 24.86 | 6 | 18.09 | 57.64 | 62.38 |
| SEM | 1.269 | 55 | 0.5 | 1.25 | 4.77 | 4.76 | 1 | 4.17 | 18.56 | 14.81 |
| N | 10 | 10 | 10 | 8 | 9 | 9 | 9 | 10 | 10 | 10 |
| Median Dendritic Length (in Microns) | Median Number of Branch Points | Median Spines on Primary Dendrites (per 60 Microns) | % Open Arm Time in EPM | % Open Arm Entries in EPM | Number of Enclosed Arm Entries in EPM | Corticosterone Post Open Field (pg/mL) | Corticosterone Post EPM (pg/mL) | Corticosterone Post Sacrifice (pg/mL) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Center Time in Open Field | Coefficient | 0.216 | 0.013 | 0.216 | −0.616 | −0.809 | −0.562 | −0.370 | −0.191 | 0.179 |
| Sig. | 0.549 | 0.973 | 0.607 | 0.077 | 0.008 | 0.115 | 0.292 | 0.596 | 0.621 | |
| N | 10 | 10 | 8 | 9 | 9 | 9 | 10 | 10 | 10 | |
| Median Dendritic Length (in microns) | Coefficient | −0.067 | 0.623 | −0.200 | −0.017 | −0.280 | 0.418 | −0.224 | −0.321 | |
| Sig. | 0.853 | 0.099 | 0.606 | 0.966 | 0.466 | 0.229 | 0.533 | 0.365 | ||
| N | 10 | 8 | 9 | 9 | 9 | 10 | 10 | 10 | ||
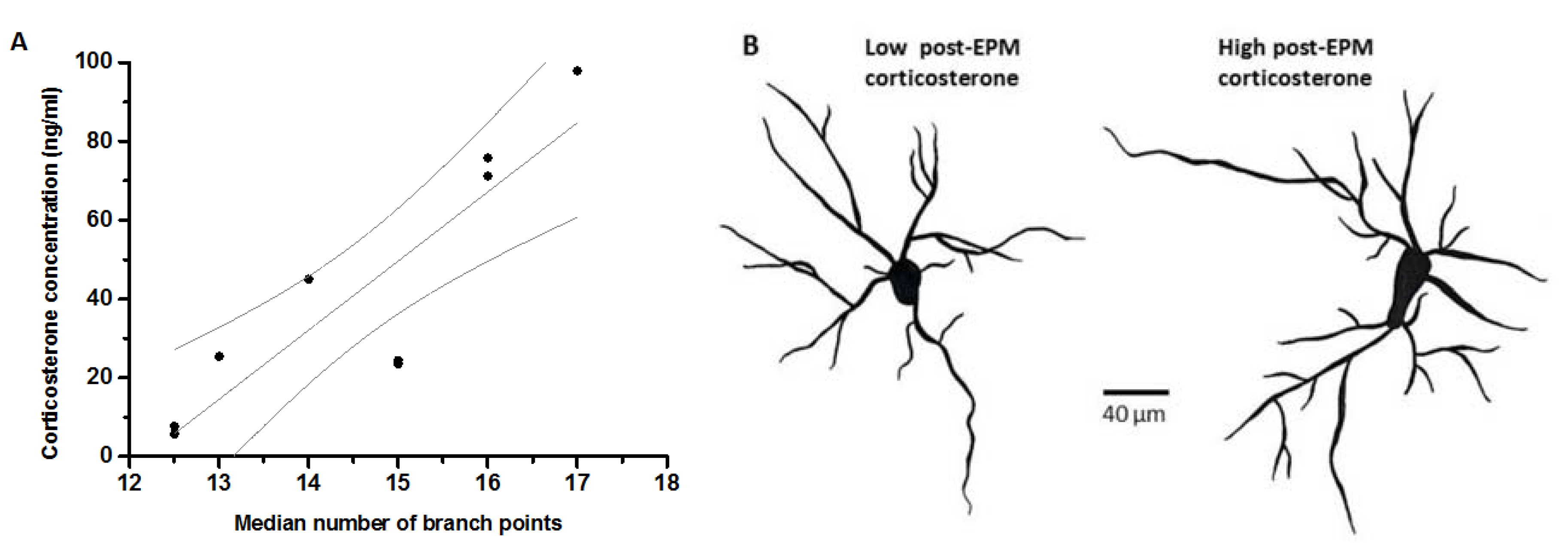
| Median Number of Branch Points | Coefficient | 0.370 | 0.101 | 0.097 | 0.185 | 0.301 | 0.638 | 0.190 | ||
| Sig. | 0.367 | 0.795 | 0.803 | 0.635 | 0.399 | 0.047 | 0.599 | |||
| N | 8 | 9 | 9 | 9 | 10 | 10 | 10 | |||
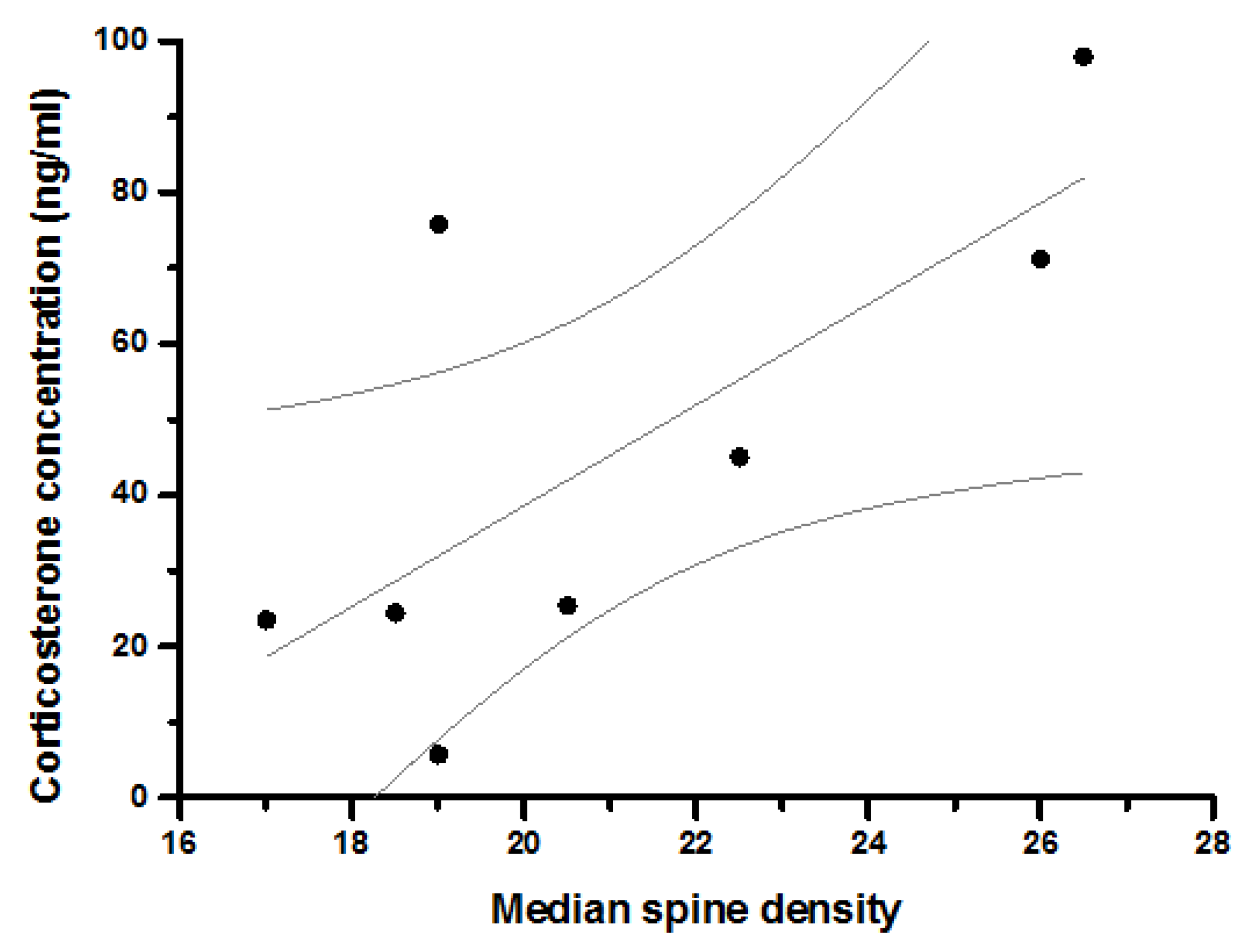
| Median Spines on Primary Dendrites (per 60 microns) | Coefficient | 0.360 | −0.306 | 0.248 | 0.108 | 0.719 | 0.503 | |||
| Sig. | 0.427 | 0.504 | 0.592 | 0.799 | 0.045 | 0.204 | ||||
| N | 7 | 7 | 7 | 8 | 8 | 8 | ||||
| % Open Arm Time in EPM | Coefficient | 0.460 | 0.856 | 0.133 | 0.633 | 0.483 | ||||
| Sig. | 0.213 | 0.003 | 0.732 | 0.067 | 0.187 | |||||
| N | 9 | 9 | 9 | 9 | 9 | |||||
| % Open Arm Entries in EPM | Coefficient | 0.460 | 0.477 | −0.008 | −0.351 | |||||
| Sig. | 0.213 | 0.194 | 0.983 | 0.354 | ||||||
| N | 9 | 9 | 9 | 9 | ||||||
| Number of Enclosed Arm Entries in EPM | Coefficient | −0.068 | 0.525 | 0.475 | ||||||
| Sig. | 0.862 | 0.146 | 0.197 | |||||||
| N | 9 | 9 | 9 | |||||||
| Corticosterone Post Open Field (pg/mL) | Coefficient | −0.079 | −0.418 | |||||||
| Sig. | 0.829 | 0.229 | ||||||||
| N | 10 | 10 | ||||||||
| Corticosterone Post EPM (pg/mL) | Coefficient | 0.685 | ||||||||
| Sig. | 0.029 | |||||||||
| N | 10 | |||||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hegde, A.; Soh Yee, P.; Mitra, R. Dendritic Architecture of Principal Basolateral Amygdala Neurons Changes Congruently with Endocrine Response to Stress. Int. J. Environ. Res. Public Health 2017, 14, 779. https://doi.org/10.3390/ijerph14070779
Hegde A, Soh Yee P, Mitra R. Dendritic Architecture of Principal Basolateral Amygdala Neurons Changes Congruently with Endocrine Response to Stress. International Journal of Environmental Research and Public Health. 2017; 14(7):779. https://doi.org/10.3390/ijerph14070779
Chicago/Turabian StyleHegde, Akshaya, Poh Soh Yee, and Rupshi Mitra. 2017. "Dendritic Architecture of Principal Basolateral Amygdala Neurons Changes Congruently with Endocrine Response to Stress" International Journal of Environmental Research and Public Health 14, no. 7: 779. https://doi.org/10.3390/ijerph14070779




