Inhibiting the Physiological Stress Effects of a Sustained Attention Task on Shoulder Muscle Activity
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Study Design
2.2.1. Dependent Variable
2.2.2. Moderator Variable
2.2.3. Independent Variable
Sustained Attention to Response Test
2.3. Experimental Measurements
2.3.1. Upper Trapezius Muscle Activity
2.3.2. Hyperventilation (PetCO2)
2.3.3. Breathing Rate
2.4. Procedure
2.4.1. Study Protocol
2.4.2. Breathing Protocol
2.5. Study Hypotheses
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Implications for Industry
4.2. Limitations
4.3. Study Strengths
4.4. Opportunities for Future Research
5. Conclusions
- It is evident from this study that sustained attention work does initiate a physiological stress response including increased muscular activity and is therefore likely to be a risk factor in the development of WRMSDs.
- The study also provided evidence for hyperventilation being a moderator of the relationship between stress and muscle activity, rather than a mediator of the relationship, as previously proposed in the literature.
- A simple breathing intervention was partially successful in preventing hyperventilation, thereby weakening the relationship between stress and muscle activity.
- Attempts to reduce chronic hyperventilation in a workplace should help to alleviate the effects of sustained attention and other psychosocial stressors on musculoskeletal health.
Author Contributions
Conflicts of Interest
References
- IFR. World Robotics Report. Available online: http://www.ifr.org/news/ifr-press-release/world-robotics-report-2016-832/ (accessed on 29 September 2016).
- Hancock, P.A. Task partitioning effects in semi-automated human-machine system performance. Ergonomics 2013, 56, 1387–1399. [Google Scholar] [CrossRef] [PubMed]
- Berka, C.; Levendowski, D.J.; Lumicao, M.N.; Yau, A.; Davis, G.; Zivkovic, V.T.; Craven, P.L. EEG correlates of task engagement and mental workload in vigilance, learning, and memory tasks. Aviation Space Environ. Med. 2007, 78 (Suppl. 1), B231–B244. [Google Scholar]
- Romeijn, N.; Van Someren, E.J. Correlated fluctuations of daytime skin temperature and vigilance. J. Biol. Rhythms 2011, 26, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Warm, J.; Parasuraman, R.; Matthews, G. Vigilance Requires Hard Mental Work and Is Stressful. Hum. Factors 2008, 50, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Warm, J.; Matthews, G.; Finomore, V. Vigilance, workload, and stress. In Performance under Stress; Ashgate Publishing: Burlington, NJ, USA, 2008; pp. 115–141. [Google Scholar]
- Manly, T.; Robertson, I.H.; Galloway, M.; Hawkins, K. The absent mind: Further investigations of sustained attention to response. Neuropsychologia 1999, 37, 661–670. [Google Scholar] [CrossRef]
- Smallwood, J.; McSpadden, M.; Schooler, J.W. The lights are on but no one’s home: Meta-awareness and the decoupling of attention when the mind wanders. Psychon. Bull. Rev. 2007, 14, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Collet, C.; Petit, C.; Priez, A.; Dittmar, A. Stroop color-word test, arousal, electrodermal activity and performance in a critical driving situation. Biol. Psychol. 2005, 69, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Kaida, K.; Åkerstedt, T.; Kecklund, G.; Nilsson, J.P.; Axelsson, J. Use of subjective and physiological indicators of sleepiness to predict performance during a vigilance task. Ind. Health 2007, 45, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Deeney, C.; O’Sullivan, L.W. Effects of cognitive loading and force on upper trapezius fatigue. Occup. Med. 2018, 67, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Eurostat. Health and Safety at Work in Europe, 1997–2007: A Statistical Potrait; Publications Office of the European Union: Brussels, Belgium, 2010. [Google Scholar]
- Van Stolk, C.; Staetsky, L.; Hassan, E.; Woo, C. Management of Psychosocial Risks at Work: An Analysis of the Findings of the European Survey of Enterprises on New and Emerging Risks; Publications Office of the European Union: Brussels, Belgium, 2012. [Google Scholar]
- Eatough, E.M.; Way, J.D.; Chang, C.-H. Understanding the link between psychosocial work stressors and work-related musculoskeletal complaints. Appl. Ergon. 2012, 43, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.; Ochsmann, E.; Kraus, T.; Lang, J.W.B. Psychosocial work stressors as antecedents of musculoskeletal problems: A systematic review and meta-analysis of stability-adjusted longitudinal studies. Soc. Sci. Med. 2012, 75, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Punnett, L.; Wegman, D.H. Work-related musculoskeletal disorders: The epidemiologic evidence and the debate. J. Electromyogr. Kinesiol. 2004, 14, 13–23. [Google Scholar] [CrossRef] [PubMed]
- EUOSHA. OSH in Figures: Work-Related Musculoskeletal Disorders in the EU-Facts and Figures; Office for Official Publications of the European Communities: Brussels, Belgium, 2010. [Google Scholar]
- Eurofound. Fifth European Working Conditions Survey; Publications Office of the European Union: Brussels, Belgium, 2010. [Google Scholar]
- Lundberg, U.; Forsman, M.; Zachau, G.; Eklöf, M.; Palmerud, G.; Melin, B.; Kadefors, R. Effects of experimentally induced mental and physical stress on motor unit recruitment in the trapezius muscle. Work Stress 2002, 16, 166–178. [Google Scholar] [CrossRef]
- Eijckelhof, B.; Huysmans, M.; Garza, J.B.; Blatter, B.; van Dieën, J.; Dennerlein, J.; van der Beek, A. The effects of workplace stressors on muscle activity in the neck-shoulder and forearm muscles during computer work: A systematic review and meta-analysis. Eur. J. Appl. Physiol. 2013, 113, 2897–2912. [Google Scholar] [CrossRef] [PubMed]
- Larsman, P.; Kadefors, R.; Sandsjö, L. Psychosocial work conditions, perceived stress, perceived muscular tension, and neck/shoulder symptoms among medical secretaries. Int. Arch. Occup. Environ. Health 2013, 86, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Schleifer, L.M.; Ley, R.; Spalding, T.W. A hyperventilation theory of job stress and musculoskeletal disorders. Am. J. Ind. Med. 2002, 41, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Schleifer, L.; Spalding, T.; Hatfield, B.; Kerick, S.; Ley, R. Effects of mental workload stress on end-tidal CO2 in computer work. Biol. Psychol. 2003, 62, 234–235. [Google Scholar]
- Gravenstein, J.S.; Paulus, D.A.; Hayes, T.J. Gas Monitoring in Clinical Practice; Butterworth-Heinemann: Oxford, UK, 1995. [Google Scholar]
- LaValle, T.L.; Perry, A.G. Capnography: Assessing end-tidal CO2 levels. Dimens. Crit. Care Nurs. 1995, 14, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Oakes, D.F. Clinical Practitioner’s Pocket Guide to Respiratory Care; Health Educator Publications: Washington, DC, USA, 2008. [Google Scholar]
- Chaitow, L.; Bradley, D.; Gilbert, C. The Structure and Function of Breathing; Multidisciplinary Approaches to Breathing Pattern Disorders; Churchill Livingstone: Edinburgh, UK, 2002. [Google Scholar]
- Wixted, F.; O’Sullivan, L. The moderating role of end-tidal CO2 on upper trapezius muscle activity in response to sustained attention. Int. J. Ind. Ergon. 2017, 61, 1–12. [Google Scholar] [CrossRef]
- Ley, R.; Yelich, G. Fractional end-tidal CO2 as an index of the effects of stress on math performance and verbal memory of test-anxious adolescents. Biol. Psychol. 1998, 49, 83–94. [Google Scholar] [CrossRef]
- Schleifer, L.; Ley, R. End-tidal CO2 as an Index of psychophysiological activity during VDT data-entry work and relaxation. Ergonomics 1994, 37, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Schleifer, L.M.; Spalding, T.W.; Kerick, S.E.; Cram, J.R.; Ley, R.; Hatfield, B.D. Mental stress and trapezius muscle activation under psychomotor challenge: A focus on EMG gaps during computer work. Psychophysiology 2008, 45, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Courtney, R. The functions of breathing and its dysfunctions and their relationship to breathing therapy. Int. J. Osteopath. Med. 2009, 12, 78–85. [Google Scholar] [CrossRef]
- Hazlett-Stevens, H.; Craske, M.G. 14 Breathing Retraining and Diaphragmatic Breathing. In General Principles Empirically Supported Techniques Cognitive Behavior Therapy; John Wiley & Sons: Hoboken, NJ, USA, 2009; Volume 166. [Google Scholar]
- Criswell, E. Cram’s Introduction to Surface Electromyography; Jones & Bartlett Publishers: Burlington, MA, USA, 2010. [Google Scholar]
- Lucini, D.; Malacarne, M.; Solaro, N.; Busin, S.; Pagani, M. Complementary medicine for the management of chronic stress: Superiority of active versus passive techniques. J. Hypertens. 2009, 27, 2421–2428. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.E.; Hirschman, R. Effects of paced respiration on anxiety reduction in a clinical population. Biofeedback Self-Regul. 1990, 15, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, P.; Vaschillo, E.; Vaschillo, B.; Lu, S.-E.; Scardella, A.; Siddique, M.; Habib, R.H. Biofeedback treatment for asthma. Chest J. 2004, 126, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Grossman, P.; De Swart, J.; Defares, P. A controlled study of a breathing therapy for treatment of hyperventilation syndrome. J. Psychosom. Res. 1985, 29, 49–58. [Google Scholar] [CrossRef]
- Parati, G.; Malfatto, G.; Boarin, S.; Branzi, G.; Caldara, G.; Giglio, A.; Gavish, B. Device-guided paced breathing in the home setting effects on exercise capacity, pulmonary and ventricular function in patients with chronic heart failure: A pilot study. Circ. Heart Fail. 2008, 1, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Limberg, J.K.; Morgan, B.J.; Schrage, W.G.; Dempsey, J.A. Respiratory influences on muscle sympathetic nerve activity and vascular conductance in the steady state. Am. J. Physiol.-Heart Circ. Physiol. 2013, 304, H1615–H1623. [Google Scholar] [CrossRef] [PubMed]
- Raupach, T.; Bahr, F.; Herrmann, P.; Luethje, L.; Heusser, K.; Hasenfuß, G.; Andreas, S. Slow breathing reduces sympathoexcitation in COPD. Eur. Respir. J. 2008, 32, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Pal, G.K.; Agarwal, A.; Karthik, S.; Pal, P.; Nanda, N. Slow yogic breathing through right and left nostril influences sympathovagal balance, heart rate variability, and cardiovascular risks in young adults. N. Am. J. Med. Sci. 2014, 6, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Bott, J.; Blumenthal, S.; Buxton, M.; Ellum, S.; Falconer, C.; Garrod, R.; Mikelsons, C. Guidelines for the Physiotherapy Management of the Adult, Medical, Spontaneously Breathing Patient; BMJ Publ. Group: London, UK, 2009. [Google Scholar]
- Pal, G.K.; Velkumary, S. Effect of short-term practice of breathing exercises on autonomic functions in normal human volunteers. Indian J. Med. Res. 2004, 120, 115. [Google Scholar] [PubMed]
- Prinsloo, G.E.; Derman, W.E.; Lambert, M.I.; Rauch, H.L. The Effect of a Single Session of Short Duration Biofeedback-Induced Deep Breathing on Measures of Heart Rate Variability during Laboratory-Induced Cognitive Stress: A Pilot Study. Appl. Psychophysiol. Biofeedback 2013, 38, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Spicuzza, L.; Gabutti, A.; Porta, C.; Montano, N.; Bernardi, L. Yoga and chemoreflex response to hypoxia and hypercapnia. Lancet 2000, 356, 1495–1496. [Google Scholar] [CrossRef]
- Wijsman, J.; Grundlehner, B.; Penders, J.; Hermens, H. Trapezius muscle EMG as predictor of mental stress. ACM Trans. Embed. Comput. Syst. 2013, 12, 99. [Google Scholar] [CrossRef]
- Waersted, M.; Westgaard, R. Attention-related muscle activity in different body regions during VDU work with minimal physical activity. Ergonomics 1996, 39, 661–676. [Google Scholar] [CrossRef] [PubMed]
- Cacioppo, J.T.; Tassinary, L.G. Inferring psychological significance from physiological signals. Am. Psychol. 1990, 45, 16. [Google Scholar] [CrossRef] [PubMed]
- Helton, W.S. Validation of a short stress state questionnaire. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 2004, 48, 1238–1242. [Google Scholar] [CrossRef]
- Matthews, G.; Joyner, L.; Gilliland, K.; Campbell, S.; Falconer, S.; Huggins, J. Validation of a comprehensive stress state questionnaire: Towards a state big three. Personal. Psychol. Eur. 1999, 7, 335–350. [Google Scholar]
- Helton, W.S.; Fields, D.; Thoreson, J.A. Assessing Daily Stress with the Short Stress State Questionnaire (SSSQ): Relationships with Cognitive Slips-Failures. In Proceedings of the Human Factors and Ergonomics Society Annual Meeting; SAGE Publications: Los Angeles, CA, USA, 2005. [Google Scholar]
- Pirzadeh, A.; Pfaff, M.S. Emotion Expression under Stress in Instant Messaging. In Proceedings of the Human Factors and Ergonomics Society Annual Meeting; SAGE Publications: Los Angeles, CA, USA, 2012. [Google Scholar]
- Schaufeli, W.B.; Bakker, A.B.; Salanova, M. The measurement of work engagement with a short questionnaire a cross-national study. Educ. Psychol. Meas. 2006, 66, 701–716. [Google Scholar] [CrossRef]
- Robertson, I.H.; Manly, T.; Andrade, J.; Baddeley, B.T.; Yiend, J. Oops!’: Performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia 1997, 35, 747–758. [Google Scholar] [CrossRef]
- Dillard, M.B.; Warm, J.S.; Funke, G.J.; Funke, M.E.; Finomore, V.S., Jr.; Matthews, G.; Parasuraman, R. The sustained attention to response task (SART) does not promote mindlessness during vigilance performance. Hum. Factors 2014, 56, 1364–1379. [Google Scholar] [CrossRef] [PubMed]
- Linden, D.V.D.; Keijsers, G.P.; Eling, P.; Schaijk, R.V. Work stress and attentional difficulties: An initial study on burnout and cognitive failures. Work Stress 2005, 19, 23–36. [Google Scholar] [CrossRef]
- Helton, W.S. Impulsive responding and the sustained attention to response task. J. Clin. Exp. Neuropsychol. 2009, 31, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Helton, W.; Russell, P. Feature absence–presence and two theories of lapses of sustained attention. Psychol. Res. 2011, 75, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Seli, P.; Cheyne, J.A.; Barton, K.R.; Smilek, D. Consistency of sustained attention across modalities: Comparing visual and auditory versions of the SART. Can. J. Exp. Psychol. 2012, 66, 44. [Google Scholar] [CrossRef] [PubMed]
- Seli, P.; Jonker, T.R.; Solman, G.J.; Cheyne, J.A.; Smilek, D. A methodological note on evaluating performance in a sustained-attention-to-response task. Behav. Res. Methods 2013, 45, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Hollinger, C.; Hoyt, J. Capnography and respiratory monitoring. In Textbook of Critical Care; WB Saunders: Philadelphia, PA, USA, 1995; pp. 305–310. [Google Scholar]
- Lehrer, P.; Vaschillo, B.; Zucker, T.; Graves, J.; Katsamanis, M.; Aviles, M.; Wamboldt, F. Protocol for heart rate variability biofeedback training. Biofeedback 2013, 41, 98–109. [Google Scholar] [CrossRef]
- Wang, S.-Z.; Li, S.; Xu, X.-Y.; Lin, G.-P.; Shao, L.; Zhao, Y.; Wang, T.H. Effect of slow abdominal breathing combined with biofeedback on blood pressure and heart rate variability in prehypertension. J. Altern. Complement. Med. 2010, 16, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach; Guilford Press: New York, NY, USA, 2013. [Google Scholar]
- Preacher, K.J.; Curran, P.J.; Bauer, D.J. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. J. Educ. Behave. Stat. 2006, 31, 437–448. [Google Scholar] [CrossRef]
- Brown, R.P.; Gerbarg, P.L.; Muench, F. Breathing practices for treatment of psychiatric and stress-related medical conditions. Psychiatr. Clin. N. Am. 2013, 36, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, P.; Carr, R.E.; Smetankine, A.; Vaschillo, E.; Peper, E.; Porges, S.; Hochron, S. Respiratory sinus arrhythmia versus neck/trapezius EMG and incentive inspirometry biofeedback for asthma: A pilot study. Appl. Psychophysiol. Biofeedback 1997, 22, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Vaschillo, E.; Lehrer, P.; Rishe, N.; Konstantinov, M. Heart rate variability biofeedback as a method for assessing baroreflex function: A preliminary study of resonance in the cardiovascular system. Appl. Psychophysiol. Biofeedback 2002, 27, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Cheyne, J.A.; Solman, G.J.; Carriere, J.S.; Smilek, D. Anatomy of an error: A bidirectional state model of task engagement/disengagement and attention-related errors. Cognition 2009, 111, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Christoff, K.; Gordon, A.M.; Smallwood, J.; Smith, R.; Schooler, J.W. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc. Natl. Acad. Sci. USA 2009, 106, 8719–8724. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.G.; Bellgrove, M.A.; Dockree, P.M.; Lau, A.; Fitzgerald, M.; Robertson, I.H. Self-alert training: Volitional modulation of autonomic arousal improves sustained attention. Neuropsychologia 2008, 46, 1379–1390. [Google Scholar] [CrossRef] [PubMed]
| Variable | C.I. of Mean | |||||||
|---|---|---|---|---|---|---|---|---|
| Condition | Mean (SD) | L. | U. | Homogeneity 2 | Test Statistic | df | Sig. | |
| UTMA (μV) | Baseline | 12.24 (5.30) | 10.00 | 14.47 | ||||
| N = 24 | SART1 | 22.46 (16.97) | 15.29 | 29.63 | ||||
| SART2 | 15.17 (9.52) | 11.15 | 19.18 | 0.03 | χ2 = 7.07 1 | 2 | 0.03 * | |
| Breathing Rate (bpm) | Baseline | 15.18 (3.34) | 13.77 | 16.59 | ||||
| N = 24 | SART1 | 17.61(2.61) | 16.51 | 18.72 | ||||
| SART2 | 15.60 (3.84) | 13.98 | 17.22 | 0.37 | F = 0.03 | 2 | 0.03 * | |
| PetCO2 (mmHg) | Baseline | 36.67 (3.64) | 35.12 | 38.20 | ||||
| N = 24 | SART1 | 34.38 (3.09) | 33.07 | 35.68 | ||||
| SART2 | 33.89 (4.53) | 31.98 | 35.81 | 0.20 | F = 0.03 | 2 | 0.03 * | |
| Stress (SSSQ score) | Baseline | NA | ||||||
| N = 24 | SART1 | 0.08 (0.54) | −0.15 | 0.31 | ||||
| SART2 | 0.04 (0.87) | −0.33 | 0.40 | T = 0.22 | 23 | 0.83 | ||
| Variable | SART1 | SART2 Group A (N = 12) with Increased PetCO2 | SART2 Group B (N = 12) with Decreased PetCO2 |
|---|---|---|---|
| BR | 17.61 (2.61) | 15.50 (1.38) | 15.71 (2.83) |
| EMG | 22.46 (16.97) | 15.40 (10.72) | 15.05 (8.62) |
| PetCO2 | 34.38 (3.09) | 36.71 (2.55) | 30.74 (4.36) |
| Stress | 0.08 (0.54) | −0.21 (0.80) | 0.28 (0.90) |
| SART1 (N = 24) | SART2 (N = 24) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect (B) | SEB | T | C.I. L. | U. | p | Effect (B) | SEB | t | C.I. L. | U. | p | ||
| Constant | 96.60 | 32.39 | 2.98 | 29.02 | 164.16 | 0.05 * | −3.88 | 11.00 | −0.23 | −39.33 | 31.57 | 0.82 | |
| PetCO2 | −2.20 | 0.94 | −2.34 | −4.17 | −0.25 | 0.03 * | 0.49 | 0.49 | 1.00 | −0.53 | 1.51 | 0.33 | |
| Stress | −132.13 | 47.05 | −2.81 | −230.29 | −33.96 | 0.01 ** | 45.65 | 17.33 | 2.63 | 9.50 | 81.80 | 0.02 * | |
| PetCO2 X Stress | 3.85 | 1.41 | 2.71 | −0.89 | 6.80 | 0.01 ** | −1.33 | 0.50 | −2.69 | −2.37 | −0.29 | 0.01 ** | |
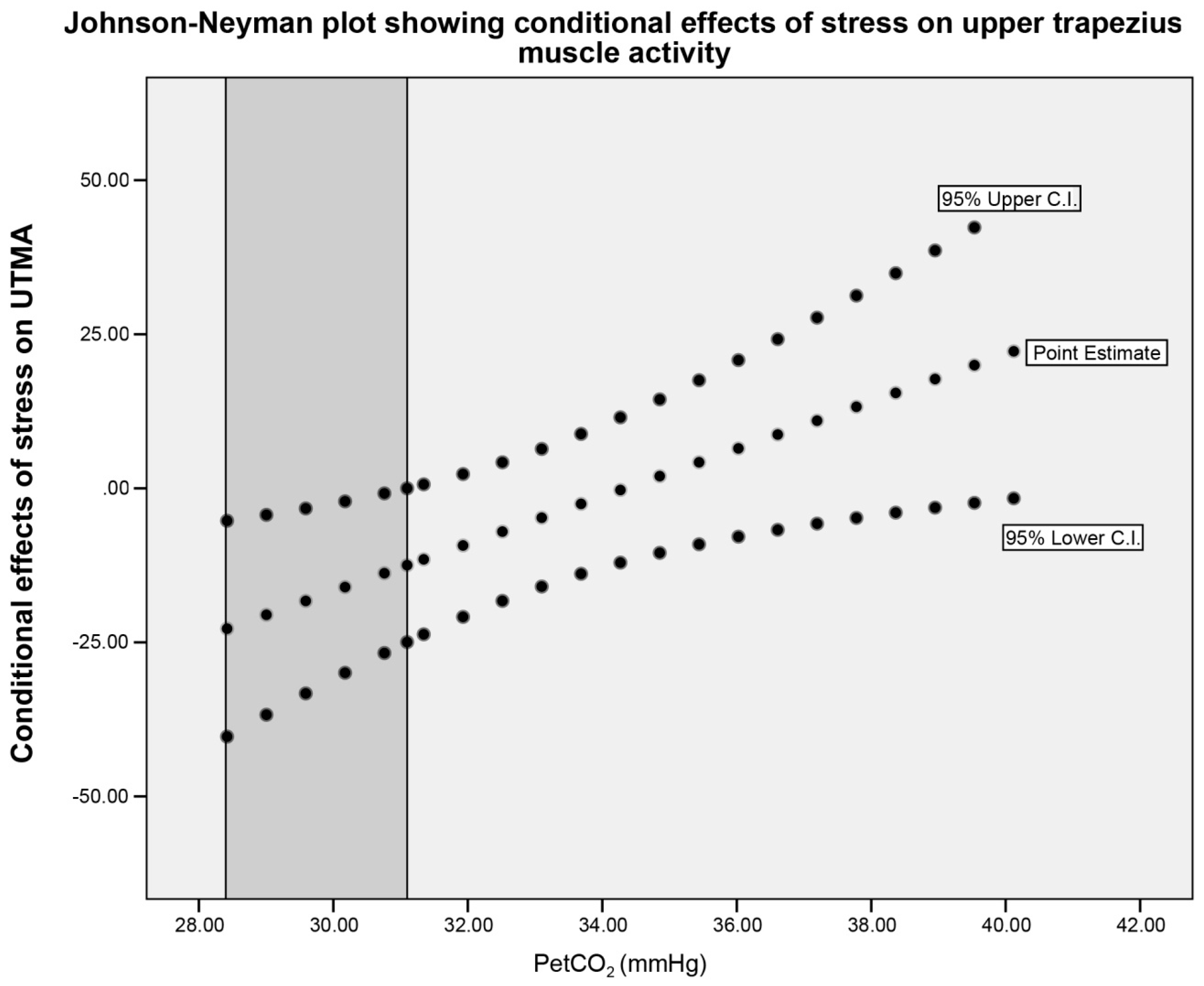
| Conditional effects of stress on UTMA at values of PetCO2 | |||||||||||||
| PetCO2 (mmHg) SART1 | Effect (B) | SEB | T | C.I. L. | U. | p | PetCO2 (mmHg) SART2 | Effect (B) | SEB | t | C.I. L. | U. | p |
| 31.30 | 7.35 | 5.46 | 1.34 | −23.97 | 0.50 | 0.05 * | 29.09 | 6.83 | 3.66 | 1.87 | −0.80 | 14.47 | 0.08 |
| 34.38 | 1.63 | 4.44 | 0.37 | −11.75 | 12.04 | 0.98 | 33.73 | 0.64 | 2.48 | 0.26 | −4.53 | 5.81 | 0.79 |
| 37.47 | −4.08 | 6.37 | −0.64 | −5.29 | 29.35 | 0.16 | 38.36 | −5.54 | 3.08 | −1.80 | −11.97 | 0.88 | 0.08 |
| Model R2 | F-Statistic | p-Value | Interaction (Stress and PetCO2 R2) | F-Statistic | p-Value | |
|---|---|---|---|---|---|---|
| SART1 Model (N = 24) | 0.46 | F(3,20) = 5.73 | 0.01 | 0.19 | F(1,20) = 7.34 | 0.01 ** |
| SART2 Model (N = 24) | 0.27 | F(3,20) = 2.43 | 0.09 | 0.26 | F(1,20) = 7.24 | 0.01 ** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wixted, F.; O’Riordan, C.; O’Sullivan, L. Inhibiting the Physiological Stress Effects of a Sustained Attention Task on Shoulder Muscle Activity. Int. J. Environ. Res. Public Health 2018, 15, 115. https://doi.org/10.3390/ijerph15010115
Wixted F, O’Riordan C, O’Sullivan L. Inhibiting the Physiological Stress Effects of a Sustained Attention Task on Shoulder Muscle Activity. International Journal of Environmental Research and Public Health. 2018; 15(1):115. https://doi.org/10.3390/ijerph15010115
Chicago/Turabian StyleWixted, Fiona, Cliona O’Riordan, and Leonard O’Sullivan. 2018. "Inhibiting the Physiological Stress Effects of a Sustained Attention Task on Shoulder Muscle Activity" International Journal of Environmental Research and Public Health 15, no. 1: 115. https://doi.org/10.3390/ijerph15010115





