Aerobic Biodegradation Characteristic of Different Water-Soluble Azo Dyes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup and Bioreactor Operation
2.2. Analytical Methods
2.3. RRF (Relative Removal Factor) Analysis
2.4. EPS (Extracellular Polymeric Substances) Extraction and Analysis
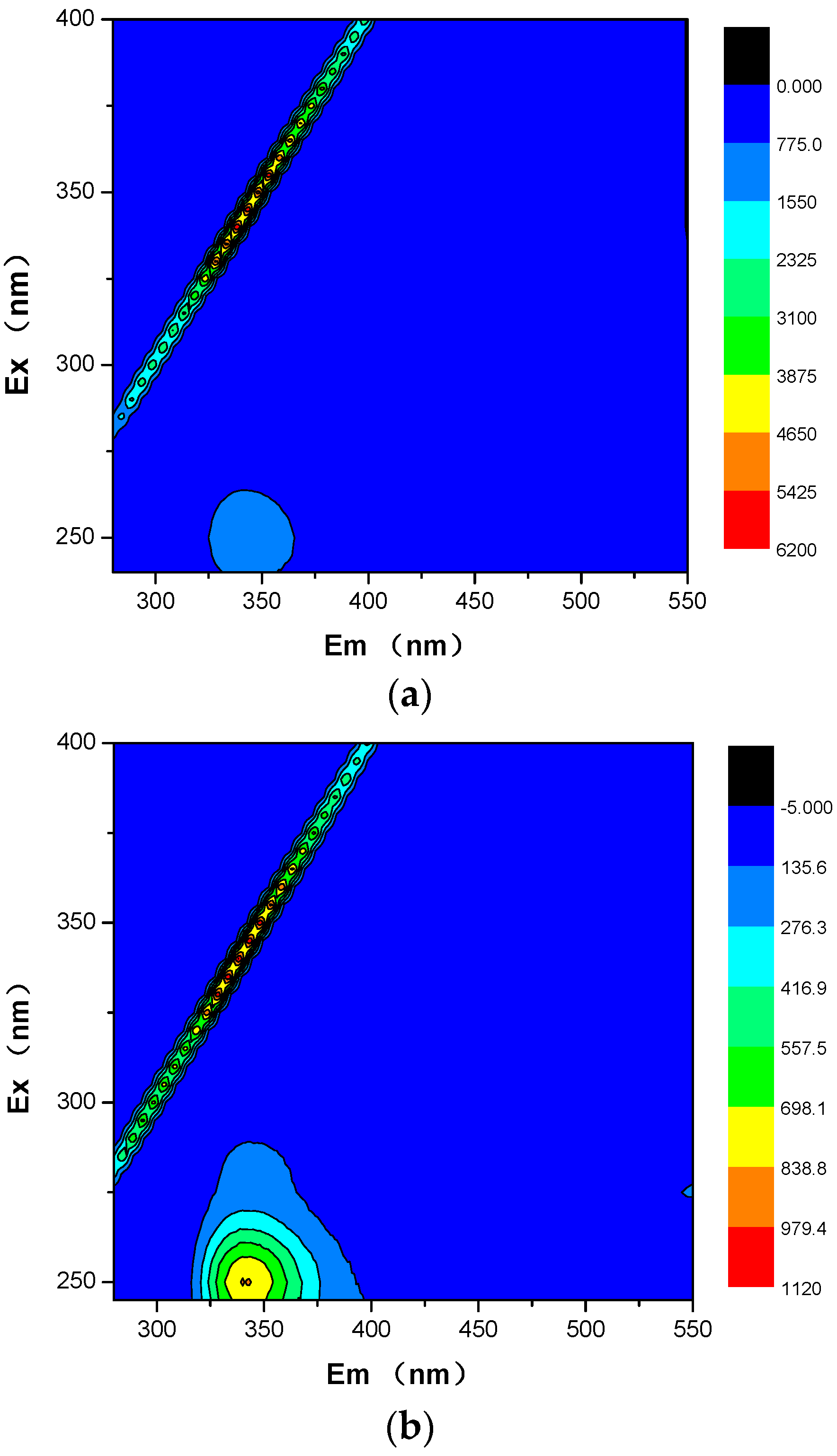
2.5. EEM (Excitation Emission Matrix) Fluorescence Spectra
3. Results
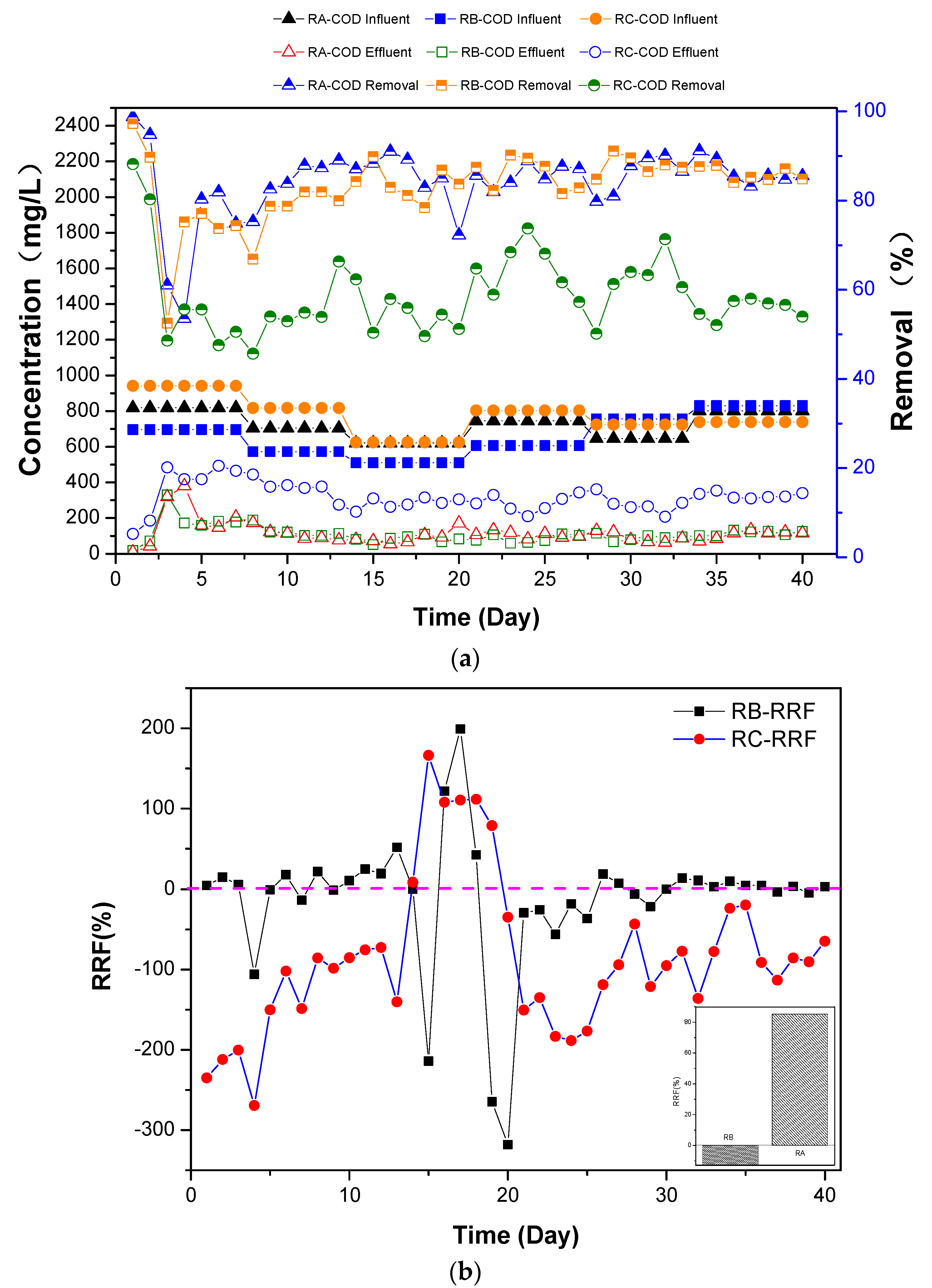
3.1. COD Removal Performance
3.2. Sudan I Removal Performances
3.3. AO7 Degradation
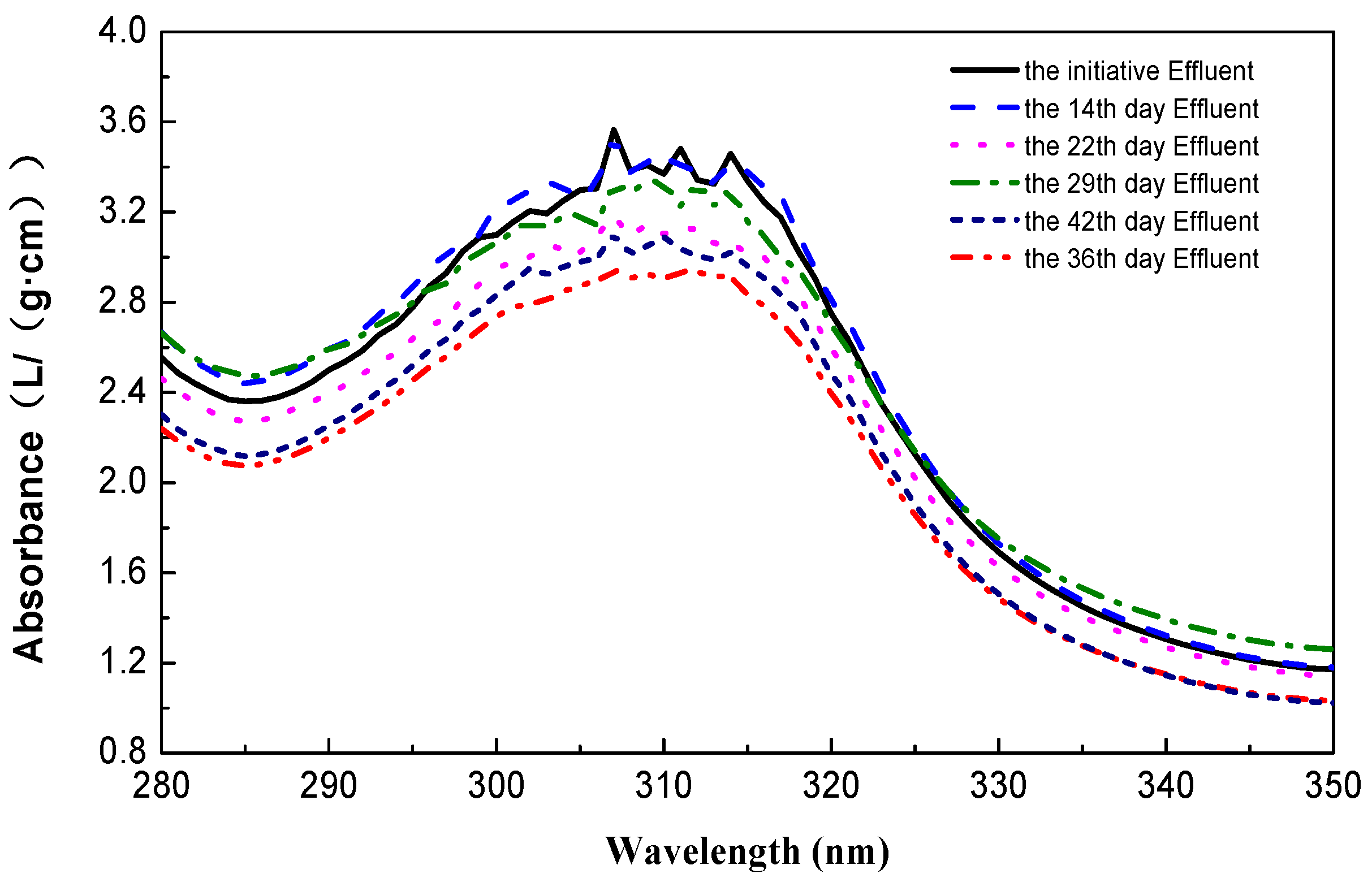
3.4. Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Forgacs, E.; Cserhati, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Ali, H. Biodegradation of synthetic dyes—A review. Water Air Soil Pollut. 2010, 213, 251–273. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, P.; Iyengar, L. Bacterial decolorization and degradation of azo dyes. Int. Biodeterior. Biodegrad. 2007, 59, 73–84. [Google Scholar] [CrossRef]
- Singh, S.N. Microbial Degradation of Synthetic Dyes in Wastewaters; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Banat, I.M.; Nigam, P.; Singh, D.; Marchant, R. Microbial decolorization of textile-dye-containing effluents: A review. Bioresour. Technol. 1996, 58, 217–227. [Google Scholar] [CrossRef]
- Sarayu, K.; Sandhya, S. Current technologies for biological treatment of textile wastewater—A review. Appl. Biochem. Biotechnol. 2012, 167, 645–661. [Google Scholar] [CrossRef] [PubMed]
- Pearce, C. The removal of colour from textile wastewater using whole bacterial cells: A review. Dyes Pigments 2003, 58, 179–196. [Google Scholar] [CrossRef]
- Fu, Y.Z.; Viraraghavan, T. Fungal decolorization of dye wastewaters: A review. Bioresour. Technol. 2001, 79, 251–262. [Google Scholar] [CrossRef]
- García-Martínez, Y.; Bengoa, C.; Stüber, F.; Fortuny, A.; Font, J.; Fabregat, A. Biodegradation of acid orange 7 in an anaerobic–aerobic sequential treatment system. Chem. Eng. Process. Process Intensif. 2015, 94, 99–104. [Google Scholar] [CrossRef]
- Pinheiro, H.M.; Touraud, E.; Thomas, O. Aromatic amines from azo dye reduction: Status review with emphasis on direct UV spectrophotometric detection in textile industry wastewaters. Dyes Pigments 2004, 61, 121–139. [Google Scholar] [CrossRef]
- Gavazza, S.; Guzman, J.J.L.; Angenent, L.T. Electrolysis within anaerobic bioreactors stimulates breakdown of toxic products from azo dye treatment. Biodegradation 2015, 26, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Popli, S.; Patel, U.D. Destruction of azo dyes by anaerobic–aerobic sequential biological treatment: A review. Int. J. Environ. Sci. Technol. 2015, 12, 405–420. [Google Scholar] [CrossRef]
- Kudlich, M.; Bishop, P.L.; Knackmuss, H.J.; Stolz, A. Simultaneous anaerobic and aerobic degradation of the sulfonated azo dye mordant yellow 3 by immobilized cells from a naphthalenesulfonate-degrading mixed culture. Appl. Microbiol. Biotechnol. 1996, 46, 597–603. [Google Scholar] [CrossRef]
- Li, W.H.; Sheng, G.P.; Liu, X.W.; Yu, H.Q. Characterizing the extracellular and intracellular fluorescent products of activated sludge in a sequencing batch reactor. Water Res. 2008, 42, 3173–3181. [Google Scholar] [CrossRef] [PubMed]
- Association, A.P.H.; Association, A.W.W.; Federation, W.P.C.; Federation, W.E. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1915; Volume 2. [Google Scholar]
- Liao, L.B.; Chen, W.M.; Xiao, X.M. The generation and inactivation mechanism of oxidation–reduction potential of electrolyzed oxidizing water. J. Food Eng. 2007, 78, 1326–1332. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Frølund, B.; Griebe, T.; Nielsen, P.H. Enzymatic activity in the activated-sludge floc matrix. Appl. Microbiol. Biotechnol. 1995, 43, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M. Calorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Murali, V.; Ong, S.A.; Ho, L.N.; Wong, Y.S. Evaluation of integrated anaerobic-aerobic biofilm reactor for degradation of azo dye methyl orange. Bioresour. Technol. 2013, 143, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.C.; Pedro, I.S.; Novais, J.M.; Martins-Dias, S. Aerobic degradation of acid orange 7 in a vertical-flow constructed wetland. Water Res. 2006, 40, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Li, H.; Ning, S.; Hao, J. Aerobic decolorization and degradation of Acid Orange G (AOG) by suspended growing cells and immobilized cells of a yeast strain Candida tropicalis TL-F1. Appl. Biochem. Biotechnol. 2014, 174, 1651–1667. [Google Scholar] [CrossRef] [PubMed]
- Yaşar, S.; Cirik, K.; Cinar, O. The effect of cyclic anaerobic-aerobic conditions on biodegradation of azo dyes. Bioprocess Biosyst. Eng. 2012, 35, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Wuhrmann, K.; Mechsner, K.; Kappeler, T. Investigation on rate—Determining factors in the microbial reduction of azo dyes. Eur. J. Appl. Microbiol. Biotechnol. 1980, 9, 325–338. [Google Scholar] [CrossRef]
- Walker, R. The metabolism of azo compounds: A review of the literature. Food Cosmet. Toxicol. 1970, 8, 659–676. [Google Scholar] [CrossRef]
- Wen, C.; Paul, W.; Leenheer, J.A.; Karl, B. Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar]
- Guo, W.; Xu, J.; Wang, J.; Wen, Y.; Zhuo, J.; Yan, Y. Characterization of dissolved organic matter in urban sewage using excitation emission matrix fluorescence spectroscopy and parallel factor analysis. J. Environ. Sci. 2010, 22, 1728–1734. [Google Scholar] [CrossRef]
- Li, W.T.; Chen, S.Y.; XU, Z.X.; Li, Y.; Shuang, C.D.; Li, A.M. Characterization of dissolved organic matter in municipal wastewater using fluorescence parafac analysis and chromatography multi-excitation/emission scan: A comparative study. Environ. Sci. Technol. 2014, 48, 2603–2609. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.Z.; Chen, Y.T.; Zhang, S.Y.; Xu, Y.Z.; Chen, X.L. Study on electrochemical degradation process for Sudan I based on FTO glass. Anal. Test. Technol. Instrum. 2015, 21, 7–11. (In Chinese) [Google Scholar]
- Ranjusha, V.P.; Pundir, R.; Kumar, K.; Dastidar, M.G.; Sreekrishnan, T.R. Biosorption of Remazol Black B dye (azo dye) by the growing Aspergillus flavus. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2010, 45, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Krupskaya, T.V.; Prylutskyy, Y.I.; Evstigneev, M.P.; Tsapko, M.D.; Turov, V.V. PMR characterization of the water structure in Tibetan milk mushroom zooglea: Influence of medium hydration and hydrophobicity. J. Appl. Spectrosc. 2015, 82, 353–359. [Google Scholar] [CrossRef]
- Asgher, M. Biosorption of reactive dyes: A review. Water Air Soil Pollut. 2011, 223, 2417–2435. [Google Scholar] [CrossRef]
- Van der Zee, F.P.; Villaverde, S. Combined anaerobic–aerobic treatment of azo dyes—A short review of bioreactor studies. Water Res. 2005, 39, 1425–1440. [Google Scholar] [CrossRef] [PubMed]
- Field, J.A.; Stams, A.J.M.; Kato, M.; Schraa, G. Enhanced biodegradation of aromatic pollutants in cocultures of anaerobic and aerobic bacterial consortia. Antonie Van Leeuwenhoek 1995, 67, 47–77. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, S.W.; Bishop, P.L. Two stage anaerobic/aerobic treatment of sulfonated azo dyes. J. Environ. Sci. Health A Environ. Sci. Eng. 1995, 30, 1251–1276. [Google Scholar] [CrossRef]
- Seshadri, S.; Bishop, P.L.; Agha, A.M. Anaerobic/aerobic treatment of selected azo dyes in wastewater. Waste Manag. 1994, 14, 127–137. [Google Scholar] [CrossRef]
- Kudlich, M.; Hetheridge, M.J.; Knackmuss, H.J.; Stolz, A. Autoxidation reactions of different aromatic O-aminohydroxynaphthalenes that are formed during the anaerobic reduction of sulfonated azo dyes. Environ. Sci. Technol. 1999, 33, 896–901. [Google Scholar] [CrossRef]
- Razo-Flores, E.; Luijten, M.; Donlon, B.A.; Lettinga, G.; Field, J.A. Complete biodegradation of the azo dye azodisalicylate under anaerobic conditions. Environ. Sci. Technol. 1997, 31, 2098–2103. [Google Scholar] [CrossRef]
- Feigel, B.J.; Knackmuss, H.J. Syntrophic interactions during degradation of 4-aminobenzenesulfonic acid by a two species bacterial culture. Arch. Microbiol. 1993, 159, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Nörtemann, B.; Baumgarten, J.; Rast, H.; Knackmuss, H. Bacterial communities degrading amino-and hydroxynaphthalene-2-sulfonates. Appl. Environ. Microbiol. 1986, 52, 1195–1202. [Google Scholar] [PubMed]

| Trial | Reactor | Starch (mg L−1) | Sodium Sulfate (mg L−1) | Glucose (mg L−1) | Sudan I (m mol L−1) | Acid Orange 7 (m mol L−1) |
|---|---|---|---|---|---|---|
| 1 | Reactor A (RA) | 500 ± 55 | 1250 ± 11 | 230 ± 50 | -- | -- |
| 2 | Reactor B (RB) | 500 ± 50 | 1250 ± 13 | -- | 0.75 ± 0.6 | -- |
| 3 | Reactor C (RC) | 500 ± 53 | 1250 ± 10 | -- | -- | 0.75 ± 0.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, S.; Liu, B.; Hou, X.; Wu, B.; Yao, F.; Ding, X.; Huang, L. Aerobic Biodegradation Characteristic of Different Water-Soluble Azo Dyes. Int. J. Environ. Res. Public Health 2018, 15, 35. https://doi.org/10.3390/ijerph15010035
Sheng S, Liu B, Hou X, Wu B, Yao F, Ding X, Huang L. Aerobic Biodegradation Characteristic of Different Water-Soluble Azo Dyes. International Journal of Environmental Research and Public Health. 2018; 15(1):35. https://doi.org/10.3390/ijerph15010035
Chicago/Turabian StyleSheng, Shixiong, Bo Liu, Xiangyu Hou, Bing Wu, Fang Yao, Xinchun Ding, and Lin Huang. 2018. "Aerobic Biodegradation Characteristic of Different Water-Soluble Azo Dyes" International Journal of Environmental Research and Public Health 15, no. 1: 35. https://doi.org/10.3390/ijerph15010035




