Selection of a Very Active Microbial Community for the Coupled Treatment of Tetramethylammonium Hydroxide and Photoresist in Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reactor Set Up and Batches
- -
- Batch T: the reactor was filled with 2 L inoculum and 2 L of feeding solution.
- -
- Batch R (days 7 to 12), batch A (days 13 to 18), batch M (days 19 to 24): at the beginning of each batch, 1 L of activated sludge coming from the previous batch was kept in the reactor and fed with 2 L of feeding solution. Each batch was 6 days long.
- -
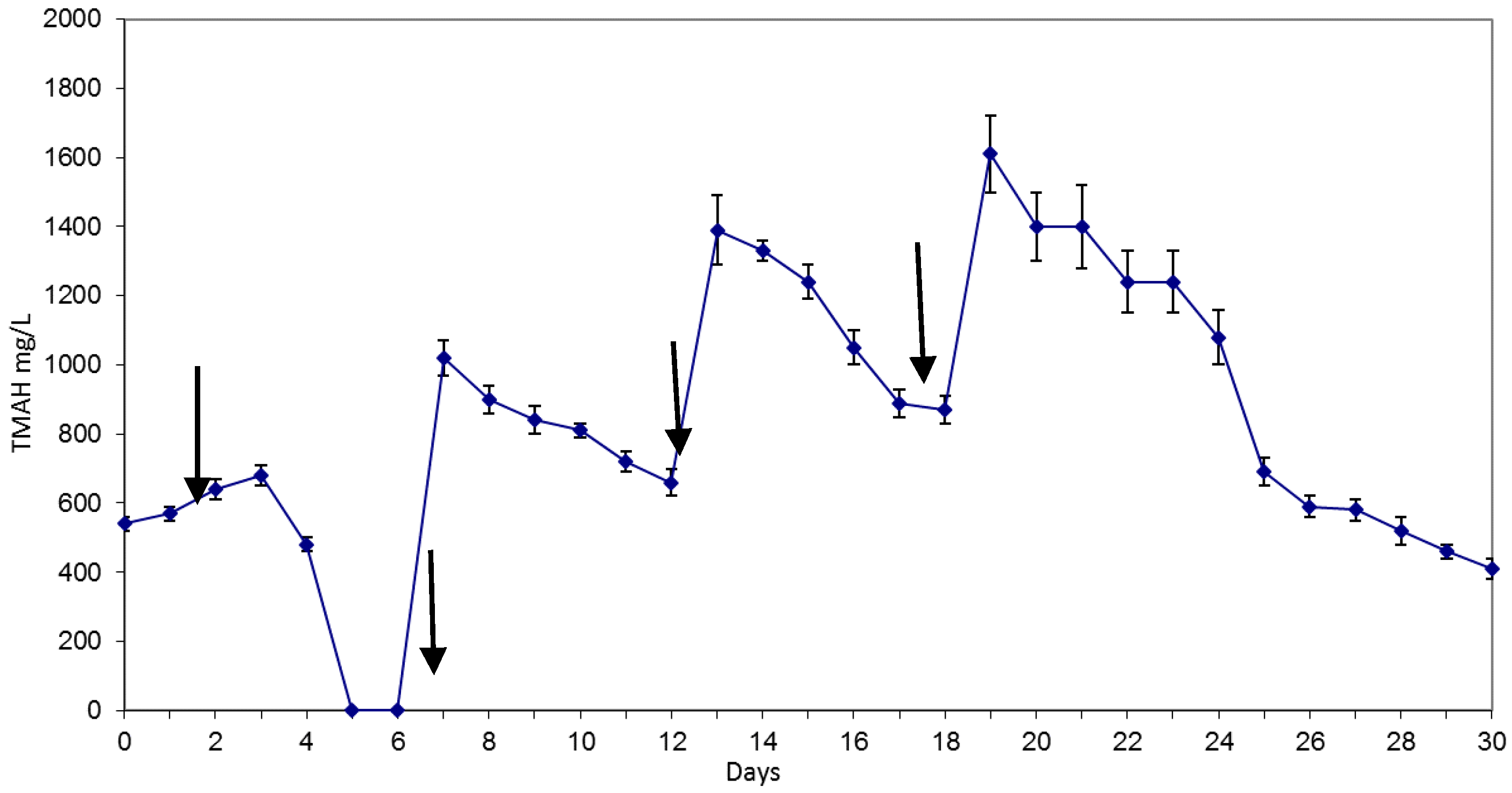
- Batch M0 (since day 25): in this batch, 1 L of activated sludge coming from batch M was fed with 2 L of distilled water and 50 mL of mineral culture medium. Batch’s timeline and TMAH concentration at the different times are outlined in Figure 1.
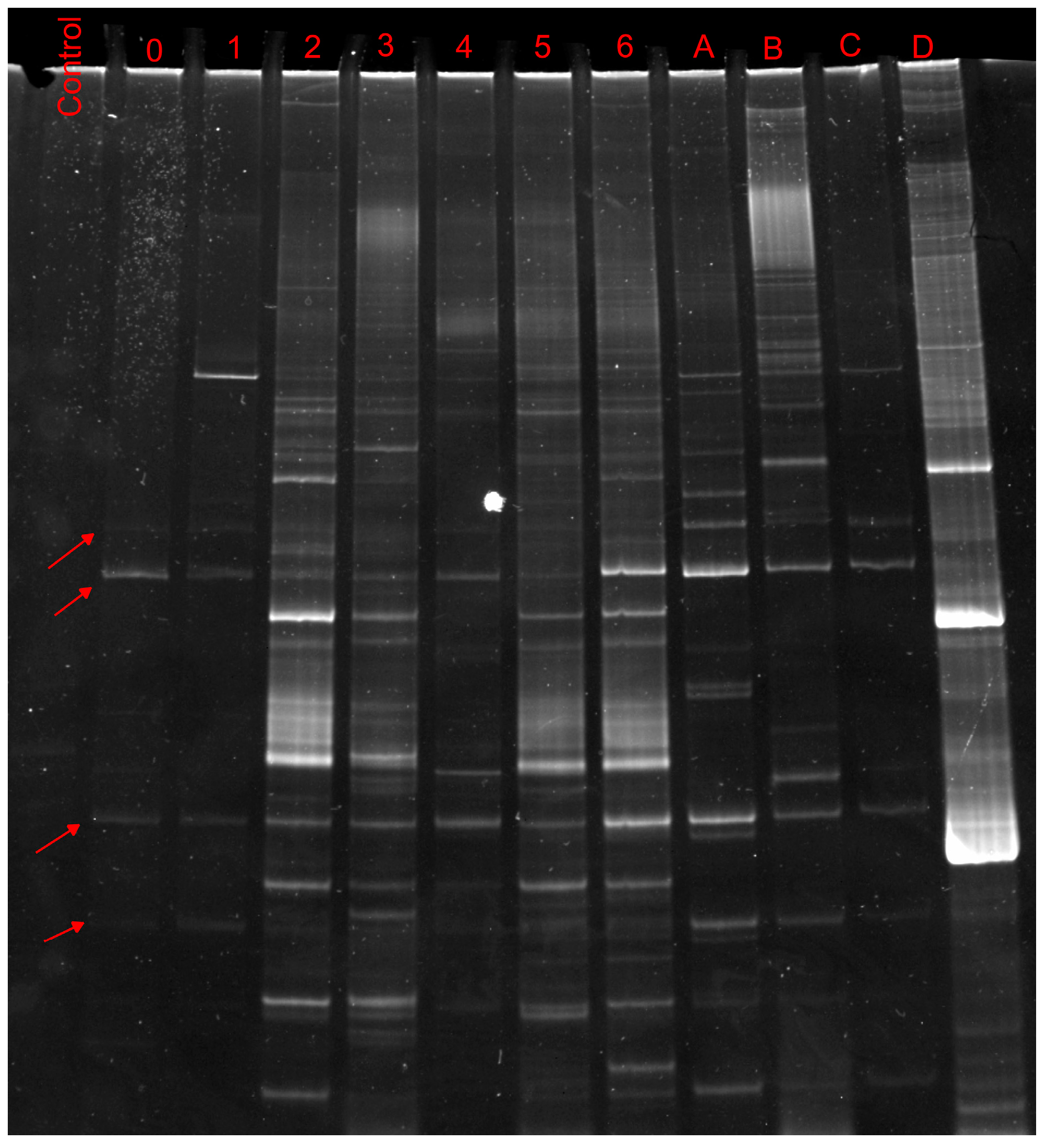
2.2. Biological Analyses
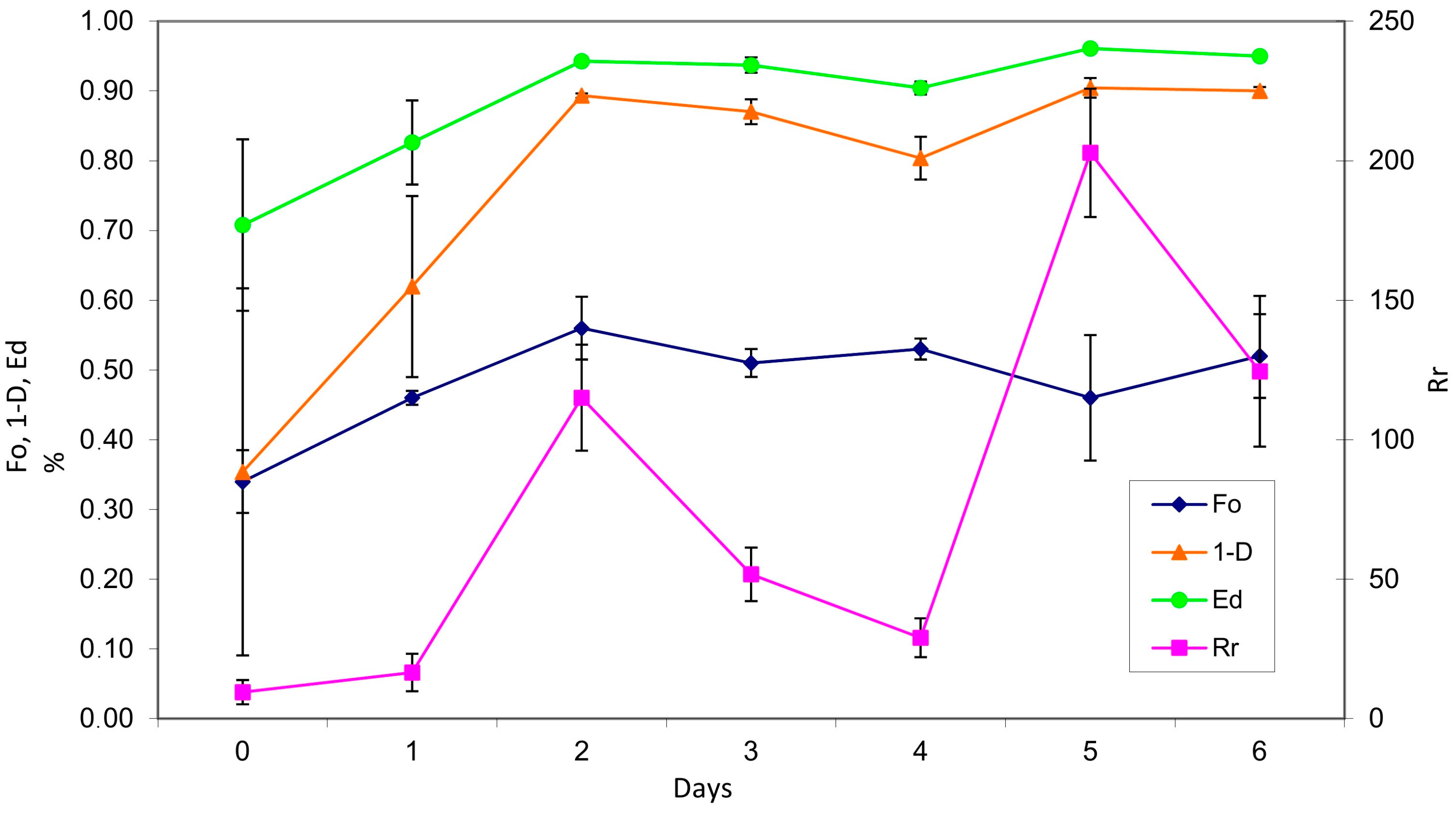
- Range-weighted Richness: ;
- Functional Organization: Fo = y value for x = 20% on Pareto Lorentz distribution of species; Simpson Diversity Index: 2;
- Simpson Evenness Index: ;
3. Results and Discussion
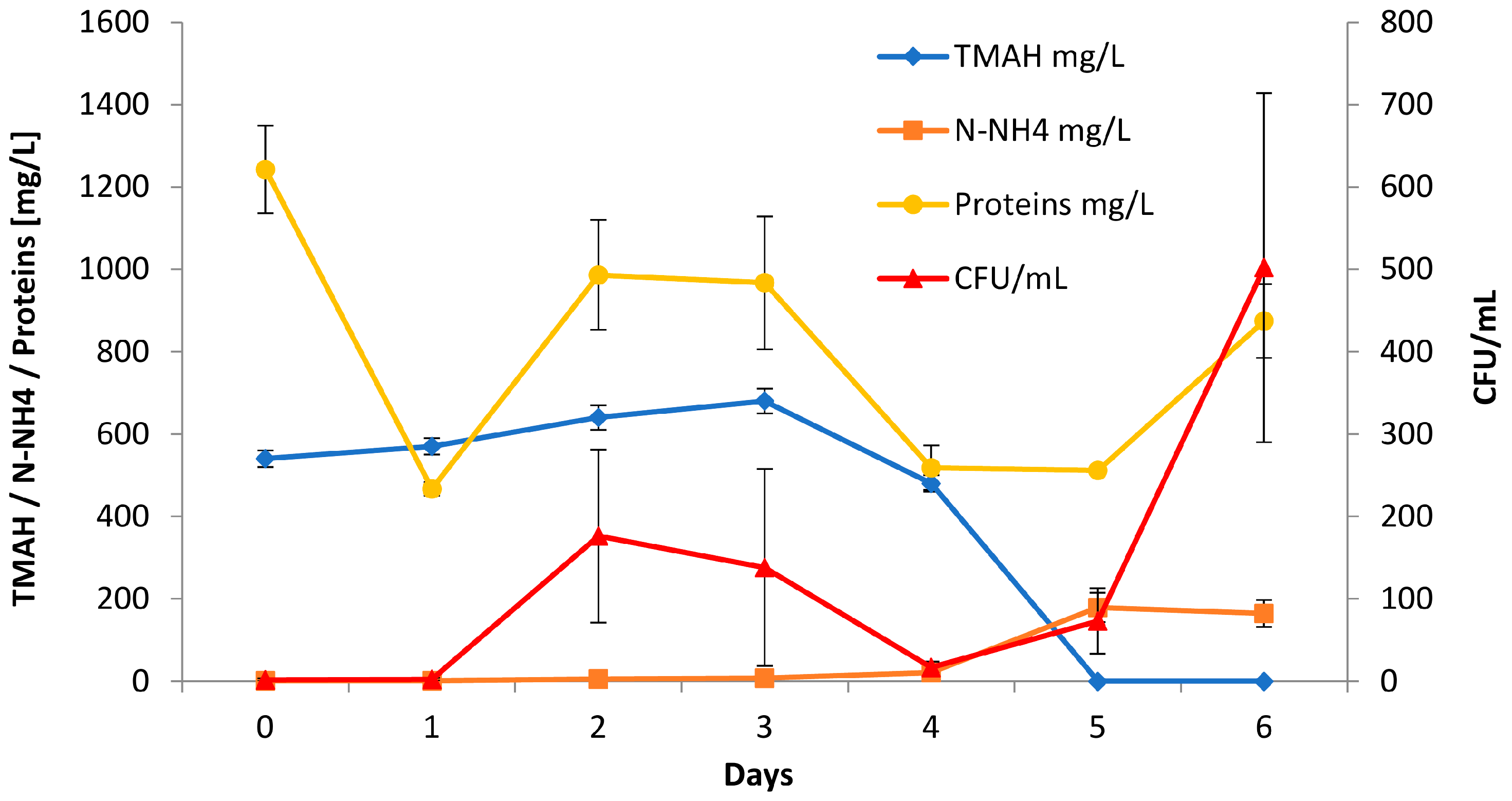
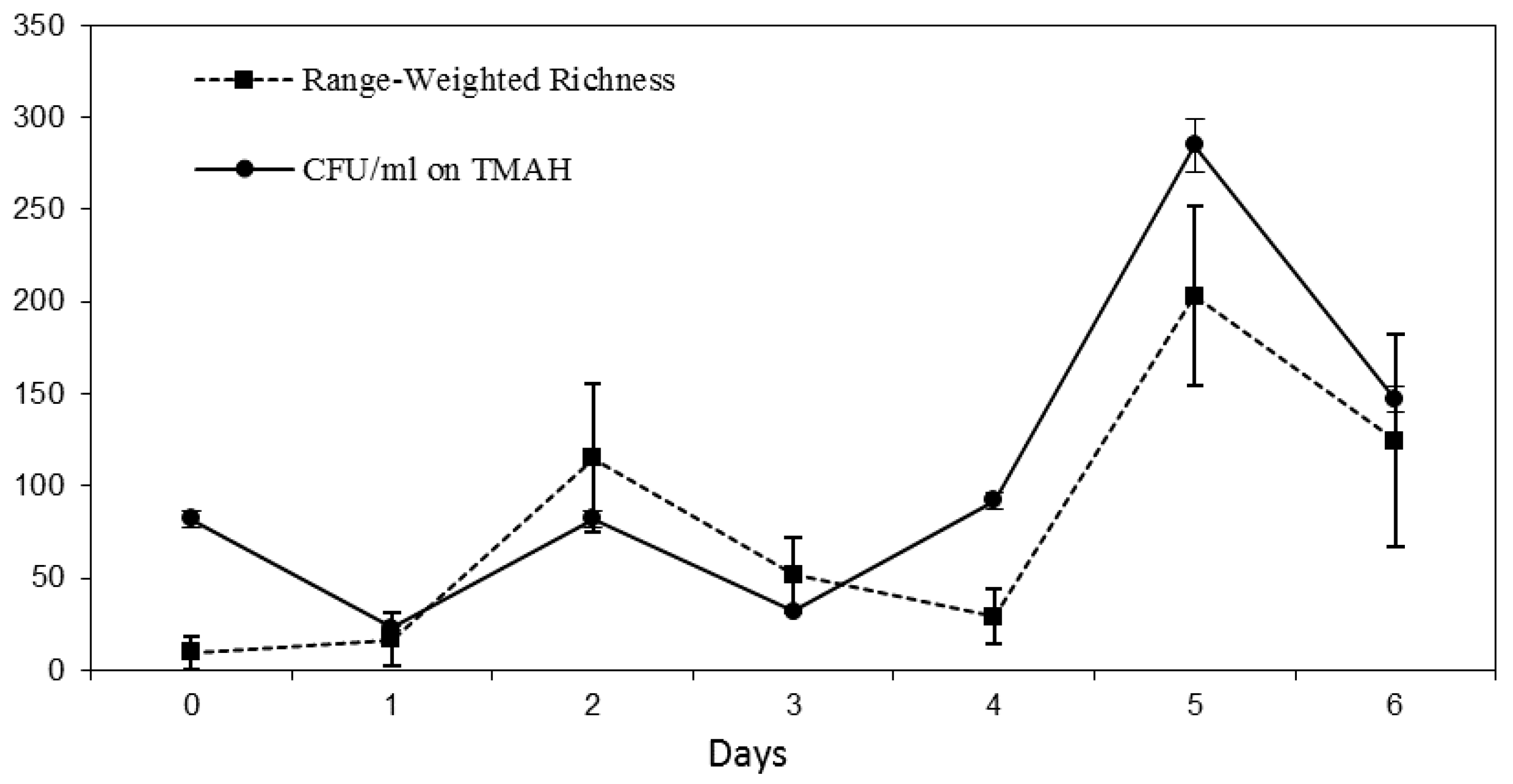
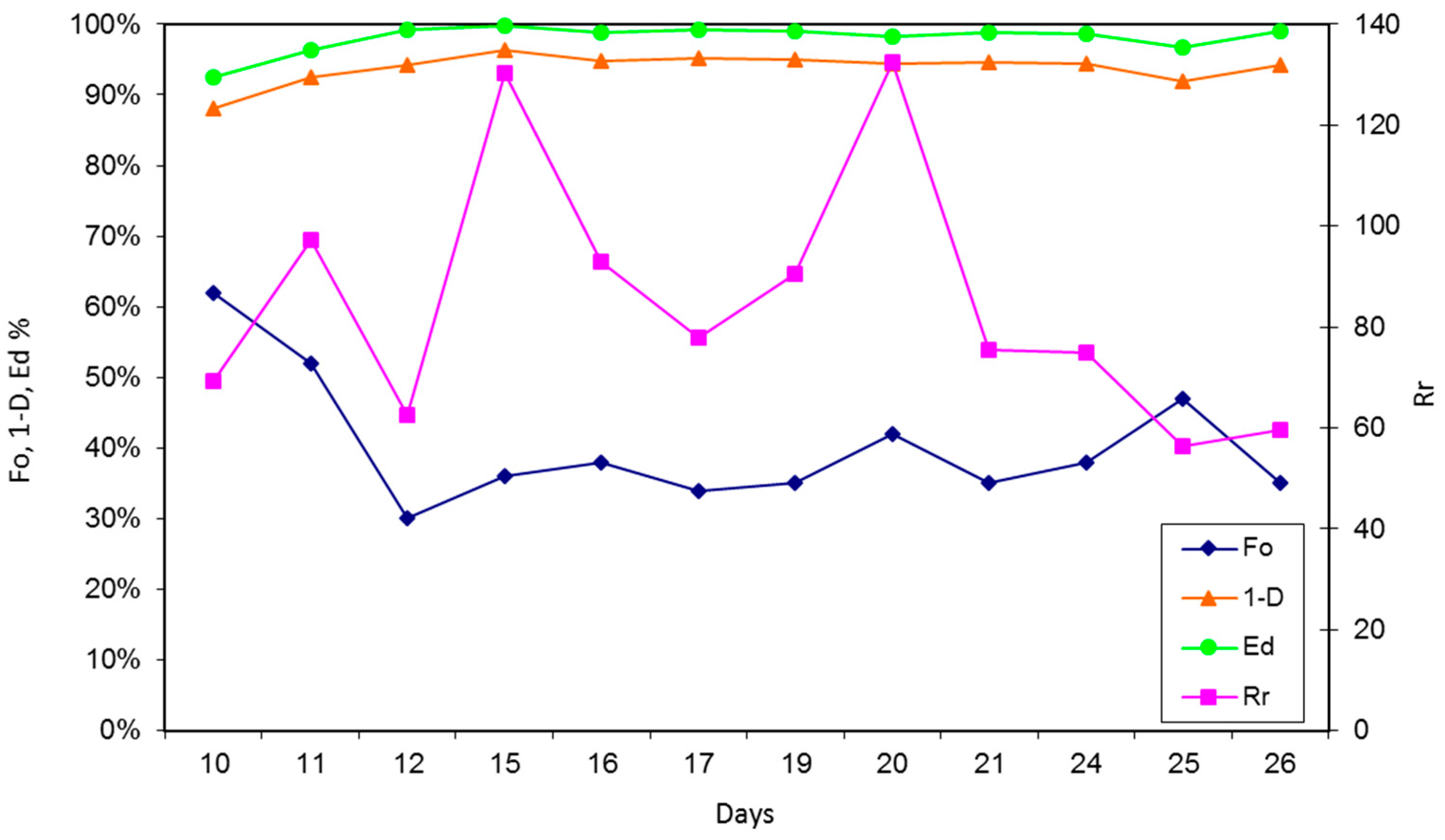
3.1. Reactor Monitoring, Early Phase
3.2. Reactor Monitoring, Late Phase
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ohara, M.; Katayama, Y.; Tsuzaky, M.; Nakamoto, S.; Kuraishi, H. Paracoccus kockurii sp. nov., a Tetramethylammonium-Assimilating Bacterium. Int. J. Syst. Microbiol. 1990, 40, 292–296. [Google Scholar] [CrossRef]
- Lin, H.L.; Chen, B.K.; Hsia, H.P.; Yang, G.H.; Yang, Y.F.; Chao, Y.C.; Cheng, S.S. Use of two-stage biological process in treating thin film transistor liquid crystal display wastewater of tetramethylammonium hydroxide. Sustain. Environ. Res. 2011, 21, 155–160. [Google Scholar]
- Whang, L.M.; Hu, T.H.; Liu, P.W.; Hung, Y.C.; Fukushima, T.; Wu, Y.J.; Chang, S.H. Molecular analysis of methanogens involved in methanogenic degradation of tetramethylammonium hydroxide in full-scale bioreactors. Appl. Microbiol. Biotechnol. 2015, 99, 1485–1497. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.N.; Whang, L.M.; Chen, P.C. Biological treatment of thin-film transistor liquid crystal display (TFT-LCD) wastewater using aerobic and anoxic/oxic sequencing batch reactors. Chemosphere 2010, 81, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, S.; Sauer, K.; Liesack, W.; Thauer, R.K. Tetramethylammonium: Coenzyme M methyltransferase system from Methanococcoides sp. Arch. Microbiol. 1998, 170, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.F.; Yang, S.Y.; You, H.S.; Pan, J.R. Anaerobic treatment of tetramethylammonium hydroxide (TMAH) containing wastewater. IEEE Trans. Semicond. Manuf. 2008, 21, 486–491. [Google Scholar] [CrossRef]
- Hirano, K.; Okamura, J.; Taira, T.; Sano, K.; Toyoda, A.; Ikeda, M. An efficient treatment technique for TMAH wastewater by catalytic oxidation. IEEE Trans. Semicond. Manuf. 2001, 14, 202–206. [Google Scholar] [CrossRef]
- Prahas, D.; Liu, J.C.; Ismadji, S.; Wang, M.J. Adsorption of Tetramethylammonium Hydroxide on Activated Carbon. J. Environ. Eng. 2012, 138, 232–238. [Google Scholar] [CrossRef]
- Nishihama, S.; Murakami, M.; Igarashi, N.Y.; Yamamoto, K.; Yoshizuka, K. Separation and Recovery of Tetramethyl Ammonium Hydroxide with Mesoporous Silica Having a Hexagonal Structure (MCM-41). Solvent Extr. Ion Exch. 2012, 30, 724–734. [Google Scholar] [CrossRef]
- Kelleher, B.K.; Doyle, A.M.; O’Dwyer, T.F.; Hodnett, B.K. The Preparation and Use of a Mesoporous Silicate Material for the Removal of Tetramethyl Ammonium Hydroxide (TMAH) from Aqueous Solution. J. Chem. Technol. Biotechnol. 2001, 76, 1216–1222. [Google Scholar] [CrossRef]
- Citraningrum, H.M.; Liu, J.C.; Chern, J.M. Removal of Tetramethylammonium Hydroxide from Solution Using Ion Exchange. IEEE Trans. Semicond. Manuf. 2013, 26, 214–220. [Google Scholar] [CrossRef]
- Den, W.; Ko, F.H.; Huang, T.Y. Treatment of organic wastewater discharged from semiconductor manufacturing process by ultraviolet/hydrogen peroxide and biodegradation. IEEE Trans. Semicond. Manuf. 2002, 15, 540–551. [Google Scholar] [CrossRef]
- Jennings, M.C.; Minbiole, K.P.C.; Wuest, W.M. Quaternary Ammonium Compounds: An Antimicrobial Mainstay and Platform for Innovation to Address Bacterial Resistance. ACS Infect. Dis. 2015, 1, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Moretti, G.; Matteucci, F.; Ercole, C.; Vegliò, F.; Del Gallo, M. Microbial community distribution and genetic analysis in a sludge active treatment for a complex industrial wastewater: A study using microbiological and molecular analysis and principal component analysis. Ann. Microbiol. 2016, 66, 397–405. [Google Scholar] [CrossRef]
- Torresi, E.; Fowler, S.J.; Polesel, F.; Bester, K.; Andersen, H.R.; Smets, B.F.; Plósz, B.G.; Christensson, M. Biofilm Thickness Influences Biodiversity in Nitrifying MBBRs—Implications on Micropollutant Removal. Environ. Sci. Technol. 2016, 50, 9279–9288. [Google Scholar] [CrossRef] [PubMed]
- Stolz, A.; Busse, H.; Kämpfer, P. Pseudomonas knackmussii sp. nov. Int. J. Syst. Evol. Microbiol. 2007, 57, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Wiegel, J.; Wilke, D.; Baumgartner, J.; Opitz, R.; Schegel, H. Transfer of the Nitrogen-Fixing Hydrogen Bacterium Corynebacterium autotrophicum Baumgarten et al. to Xanthobacter gen. nov. Int. J. Syst. Evol. Microbiol. 1978, 28, 573–581. [Google Scholar] [CrossRef]
- Goodall, J.L.; Thomas, S.M.; Spain, J.C.; Peretti, S.W. Operation of mixed-culture immobilized cell reactors for the metabolism of meta- and para-nitrobenzoate by Comamonas sp. JS46 and Comamonas sp. JS47. Biotechnol. Bioeng. 1998, 59, 21–27. [Google Scholar] [CrossRef]
- Heylen, K.; Vanparys, B.; Peirsegaele, F.; Lebbe, L.; De Vos, P. Stenotrophomonas terrae sp. nov. and Stenotrophomonas humi sp. nov., two nitrate-reducing bacteria isolated from soil. Int. J. Syst. Evol. Microbiol. 2007, 57, 2056–2061. [Google Scholar] [CrossRef] [PubMed]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An overview of sphingolipid metabolism: From synthesis to breakdown. Adv. Exp. Med. Biol. 2010, 688, 1–23. [Google Scholar] [PubMed]
- Wang, Y.N.; He, W.H.; He, H.; Du, X.; Jia, B.; Zeng, Z.P.; An, M.L.; Chen, G.C. Pseudomonas nitritireducens sp. nov., a nitrite reduction bacterium isolated from wheat soil. Arch. Microbiol. 2012, 194, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Tsubouchi, T.; Shimane, Y.; Usui, K.; Shimamura, S.; Mori, K.; Hiraki, T.; Tame, A.; Uematsu, K.; Maruyama, T.; Hatada, Y. Brevundimonas abyssalis sp. nov., a dimorphic prosthecate bacterium isolated from deep-subsea floor sediment. Int. J. Syst. Evol. Microbiol. 2013, 63, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- Poroshina, M.N.; Trotsenko, Y.A.; Doronina, N.V. Methylobrevis pamukkalensis gen. nov., sp. nov., a halotolerant restricted facultative methylotroph isolated from saline water. Int. J. Syst. Evol. Microbiol. 2015, 65, 1321–1327. [Google Scholar] [CrossRef] [PubMed]

| Culture Medium Composition | |
|---|---|
| Component | g/L |
| CuCl2 | 0.05 |
| Na2MoO4 | 0.05 |
| NaHCO3 | 0.7 |
| K2HPO4 | 0.1 |
| MgSO4 | 0.1 |
| FeCl3 | 0.1 |
| Yeast extract | 0.01 |
| Genus/Family | G1 | G2 | Average | Error | Representative OTU | Features | References |
|---|---|---|---|---|---|---|---|
| Comamonas | 22.3 | 14.2 | 18.25 | 5.728 | Comamonas granuli | grows on solvents | [18] |
| Comamonas nitrativorans | Denitrifier | ||||||
| Pseudomonas | 14.3 | 21.8 | 18.05 | 5.303 | Pseudomonas knackmussii strain B13 | grows on polyamines | [16] |
| Pseudomonas nitritireducens | can reduce nitrate | [21] | |||||
| Stenotrophomonas | 14.5 | 10.1 | 12.3 | 3.111 | Stenotrophomonas humii | can reduce nitrate/nitrite | [19] |
| Brevundimonas | 14.1 | 8.7 | 11.4 | 3.818 | Brevimonas abyssalis/B. viscosa | nitrate reduction—grow on tween | [22] |
| Sphingomonadaceae | 8.8 | 7.4 | 8.1 | 0.990 | N.A | ||
| Sphingobacteriaceae | 2.5 | 5.6 | 4.05 | 2.192 | N.A | ||
| Methylophilaceae | 1.8 | 6.3 | 4.05 | 3.182 | Methylobacillus glycogenes | grows on methanol and methylamines | |
| Xanthobacteraceae | 3.4 | 3.2 | 3.3 | 0.141 | Methylobrevis pamukkalensis | grows on methanol and methylamines | [23] |
| Xanthobacter | 2.5 | 2.6 | 2.55 | 0.071 | Xantobacter autotrophicus | Methilotroph, Bioremediation of clorinated compounds | [17] |
| Rhodococcus | 1.5 | 2.3 | 1.9 | 0.566 | N.A |
| Fo | Rr | Diversity | Evenness | |
|---|---|---|---|---|
| Average (adaptation) | 0.48 | 34.8796 | 0.76365 | 0.88993 |
| Average (developed community) | 0.40 | 84.9199 | 0.93809 | 0.98028 |
| Std. Deviation (adaptation) | 0.072736 | 30.8343 | 0.20693 | 0.09237 |
| Std. Dev. (developed community) | 0.091187 | 25.2664 | 0.02128 | 0.01995 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moretti, G.; Matteucci, F.; Saraullo, M.; Vegliò, F.; Del Gallo, M. Selection of a Very Active Microbial Community for the Coupled Treatment of Tetramethylammonium Hydroxide and Photoresist in Aqueous Solutions. Int. J. Environ. Res. Public Health 2018, 15, 41. https://doi.org/10.3390/ijerph15010041
Moretti G, Matteucci F, Saraullo M, Vegliò F, Del Gallo M. Selection of a Very Active Microbial Community for the Coupled Treatment of Tetramethylammonium Hydroxide and Photoresist in Aqueous Solutions. International Journal of Environmental Research and Public Health. 2018; 15(1):41. https://doi.org/10.3390/ijerph15010041
Chicago/Turabian StyleMoretti, Giulio, Federica Matteucci, Matteo Saraullo, Francesco Vegliò, and Maddalena Del Gallo. 2018. "Selection of a Very Active Microbial Community for the Coupled Treatment of Tetramethylammonium Hydroxide and Photoresist in Aqueous Solutions" International Journal of Environmental Research and Public Health 15, no. 1: 41. https://doi.org/10.3390/ijerph15010041





