Impact of Age and Hearing Impairment on Work Performance during Long Working Hours
Abstract
:1. Introduction
1.1. Hearing Impairment
1.2. Long Working Hours
2. Materials and Methods
2.1. Participants
2.2. Study Design and Materials
2.2.1. Long-Term Selective Attention and Concentration
2.2.2. Reaction Time
2.2.3. Attention and Concentration
2.2.4. Pure-Tone Audiometry
2.3. Statistical Analyses
3. Results
3.1. Long-Term Selective Attention and Concentration
3.2. Reaction Time
3.3. Attention and Concentration
4. Discussion
Study Limitations and Directions for Future Research
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization (WHO). Aging and Working Capacity; WHO: Geneva, Switzerland, 1993. [Google Scholar]
- Age Structure of the Employees in 2012, 2030 and 2050 by Federal Government (According to the Main Scenario. Available online: http://www.statistik.at/web_de/statistiken/index.html (accessed on 8 January 2018).
- O’Neill, G.; Summer, L.; Shirey, L. Hearing Loss: A Growing Problem That Affects Quality of Life; National Academy on an Aging Society: Washington, DC, USA, 1999. [Google Scholar]
- Backenroth-Ohsako, G.A.M.; Wennberg, P.; Klinteberg, B. Personality and work life: A comparison between hearing impaired persons and a normal-hearing population. Soc. Behav. Personal. 2003, 31, 191–204. [Google Scholar] [CrossRef]
- Hannula, S.; Bloigu, R.; Majamaa, K.; Sorri, M.; Mäki-Torkko, E. Self-reported hearing problems among older adults: Prevalence and comparison to measured hearing impairment. J. Am. Acad. Audiol. 2011, 22, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Cruickshanks, K.J.; Wiley, T.L.; Tweed, T.S.; Klein, B.E.K.; Klein, R.; Mares-Perlman, J.A.; Nondahl, D.M. Prevalence of hearing loss in older adults in Beaver Dam, Wisconsin. The epidemiology of hearing loss study. Am. J. Epidemiol. 1998, 148, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Hearing Loss Due to Recreational Exposure to Loud Sounds. A Review. Available online: http://apps.who.int/iris/bitstream/10665/154589/1/9789241508513_eng.pdf (accessed on 8 January 2018).
- Dalton, D.S.; Cruickshanks, K.J.; Klein, B.E.K.; Klein, R.; Wiley, T.L.; Nondahl, D.M. The impact of hearing loss on quality of life in older adults. Gerontologist 2003, 43, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, K.; Bottone, F.G.; Ozminkowski, R.J.; Musich, S.; Bai, M.; Migliori, R.J.; Yeh, C.S. The prevalence of hearing impairment and its burden on the quality of life among adults with Medicare Supplement Insurance. Qual. Life Res. 2012, 21, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, E.; Coulson, N.S.; Henshaw, H.; Barry, J.G.; Ferguson, M.A. Understanding the psychosocial experiences of adults with mild-moderate hearing loss: An application of Leventhal’s self-regulatory model. Int. J. Audiol. 2016, 55, S3–S12. [Google Scholar] [CrossRef] [PubMed]
- Mathers, C.; Smith, A.; Concha, M. Global Burden of Hearing Loss in the Year 2000; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Stephan, Y.; Sutin, A.R.; Bosselut, G.; Terracciano, A. Sensory functioning and personality development among older adults. Psychol. Aging 2017, 32, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Strawbridge, W.J.; Wallhagen, M.I.; Shema, S.J.; Kaplan, G.A. Negative consequences of hearing impairment in old age: A longitudinal analysis. Gerontologist 2000, 40, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.B.; Shaw, L. Impact of hearing loss in the workplace: Raising questions about partnerships with professionals. Work 2008, 30, 289–295. [Google Scholar] [PubMed]
- Lemke, U.; Scherpiet, S. Oral communication in individuals with hearing impairment—Considerations regarding attentional, cognitive and social resources. Front. Psychol. 2015, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Scarincia, N.; Worralla, L.; Hicksona, L. The effect of hearing impairment in older people on the spouse: Development and psychometric testing of the Significant Other Scale for Hearing Disability (SOS-HEAR). Int. J. Audiol. 2009, 48, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Nachtegaal, J.; Kuik, D.J.; Anema, J.R.; Goverts, S.T.; Festen, J.M.; Kramer, S.E. Hearing status, need for recovery after work, and psychosocial work characteristics: Results from an internet-based national survey on hearing. Int. J. Audiol. 2009, 48, 648–691. [Google Scholar] [CrossRef] [PubMed]
- Kramer, S.E.; Kapteyn, T.S.; Houtgast, T. Occupational performance: Comparing normally-hearing and hearing-impaired employees using the Amsterdam Checklist for Hearing and Work. Int. J. Audiol. 2006, 45, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Arlinger, S.; Lunner, T.; Lyxell, B.; Pichora-Fuller, M.K. The emergence of cognitive hearing science. Scand. J. Psychol. 2009, 50, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Baskent, D.; Eiler, C.L.; Edwards, B. Phonemic restoration by hearing-impaired listeners with mild to moderate sensorineural hearing loss. Hear. Res. 2010, 260, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Boxtel, M.P.J.; van Beijsterveldt, C.E.M.; Houx, P.J.L.; Anteunis, J.C.; Metsemakers, J.F.M.; Jolles, J. Mild hearing impairment can reduce verbal memory performance in a healthy adult population. J. Clin. Exp. Neuropsychol. 2000, 22, 147–154. [Google Scholar] [CrossRef]
- Frank, R.L.; Ferrucci, L.; Metter, E.J.; Yang, A.; Zondermann, A.B.; Resnick, S.M. hearing loss and cognition in the baltimore longitudinal study of aging. Neuropsychology 2011, 25, 763–770. [Google Scholar]
- Frtusova, J.B.; Phillips, N.A. The auditory-visual speech benefit on working memory in older adults with hearing impairment. Front. Psychol. 2016, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.R.; Yaffe, K.; Xia, J.; Xue, Q.-L.; Harris, T.B.; Purchase-Helzner, E.; Satterfield, S.; Ayonayon, H.N.; Ferrucci, L.; Simonsick, E.M. Hearing loss and cognitive decline among older adults. JAMA Intern. Med. 2013, 173, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mudar, R.A.; Husain, F.T. Neural alterations in acquired age-related hearing loss. Front. Psychol. 2016, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nachtegaal, J.; Festen, J.M.; Kramer, S.E. Hearing ability in working life and its relationship with sick leave and self-reported work productivity. Ear Hear. 2012, 33, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Neijenhuis, K.; Tschur, H.; Snik, A. The effect of mild hearing impairment on auditory processing tests. J. Am. Acad. Audiol. 2004, 15, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Pearman, A.; Friedman, L.; Brooks, J.O.; Yesavage, J.A. Hearing impairment and serial word recall in older adults. Exp. Aging Res. 2000, 26, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Rönnberg, J.; Danielsson, H.; Rudner, M.; Arlinger, S.; Sternäng, O.; Wahlin, A.; Nilsson, L.G. Hearing loss is negatively related to episodic and semantic long-term memory but not to short-term memory. J. Speech Lang. Hear. Res. 2011, 54, 705–726. [Google Scholar] [CrossRef]
- Rudner, M.; Rönnberg, J.; Lunner, T. Working memory supports listening in noise for persons with hearing impairment. J. Am. Acad. Audiol. 2011, 22, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.A.; Daneman, M.; Murphy, D.R. Speech comprehension difficulties in older adults: Cognitive slowing or age-related changes in hearing? Psychol. Aging 2005, 20, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Shinn-Cunningham, B.G.; Best, V. Selective attention in normal and impaired hearing. Trends Amplif. 2008, 12, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Wingfield, A.; Tun, P.A.; McCoy, S.L. Hearing loss in older adulthood: What it is and how it interacts with cognitive performance. Curr. Dir. Psychol. Sci. 2005, 14, 144–148. [Google Scholar] [CrossRef]
- Zekveld, A.A.; George, E.L.J.; Houtgast, T.; Kramer, S.E. Cognitive abilities relate to self-reported hearing disability. J. Speech Lang. Hear. Res. 2013, 56, 1364–1372. [Google Scholar] [CrossRef]
- Zekveld, A.A.; Deijen, J.B.; Goverts, S.T.; Kramer, S.E. The relationship between nonverbal cognitive functions and hearing loss. J. Speech Lang. Hear. Res. 2007, 50, 74–82. [Google Scholar] [CrossRef]
- Zekveld, A.A.; Kramer, S.E.; Festen, J.M. Cognitive load during speech perception in noise: The influence of age, hearing loss, and cognition on the pupil response. Ear Hear. 2011, 32, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Kahneman, D. Attention and Effort; Prentice-Hall Inc.: Englewood Cliffs, NJ, USA, 1973. [Google Scholar]
- McCoy, S.L.; Tun, P.A.; Cox, L.C.; Colangelo, M.; Stewart, R.A.; Wingfield, A. Hearing loss and perceptual effort: Downstream effects on older adults’ memory for speech. Q. J. Exp. Psychol. A 2005, 58, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Rabbitt, P.M.A. Channel-capacity, intelligibility and immediate memory. Q. J. Exp. Psychol. 1968, 20, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Wagner, V.; Kallus, K.W. Aging working population: Hearing impairment a growing challenge for the working environment. In Human Aspects of IT for the Aged Population. Design for Everyday Life; Zhou, J., Salvendy, G., Eds.; Springer: Cham, Switzerland, 2015; pp. 354–364. [Google Scholar]
- ISO 7029. Acoustics—Statistical Distribution of Hearing Thresholds as a Function of Age; ISO: Geneva, Switzerland, 2000. [Google Scholar]
- Baltes, P.B.; Lindenberger, U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: A new window to the study of cognitive aging? Psychol. Aging 1997, 12, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Salant, S.; Fitzgibbons, P.J. Sources of age-related recognition difficulty for time compressed speech. J. Speech Lang. Hear. Res. 2001, 44, 709–719. [Google Scholar] [CrossRef]
- Tun, P.; McCoy, S.; Wingfield, A. Aging, hearing acuity, and the attentional costs of effortful listening. Psychol. Aging 2009, 24, 761–766. [Google Scholar] [CrossRef] [PubMed]
- European Parliament, Council of the European Union. Directive 2003/88/EC of the European Parliament and of the Council of 4 November 2003 Concerning Certain Aspects of the Organisation of Working Time; European Parliament, Council of the European Union: Luxembourg, 2003. [Google Scholar]
- Caruso, C.; Hitchcock, E.; Dick, R.; Russo, J.; Schmit, J. Overtime and Extended Work Shifts: Recent Findings on Illnesses, Injuries, and Health Behaviors; NIOSH: Cincinnati, OH, USA, 2004.
- Dex, S.; Clark, A.; Taylor, M. Household Labour Supply; ESCR, University of Essex: Essex, UK, 1995. [Google Scholar]
- Beswick, J.; White, J. Working Long Hours; Crown: Sheffield, UK, 2003. [Google Scholar]
- Kodz, J.; Davis, S.; Sheppard, E.; Rick, J.; Strebler, M.; Bates, P.; Cummings, J.; Meager, N.; Anxo, D.; Gineste, S.; et al. Working Long Hours in the UK: A Review of the Research Literature, Analysis of Survey Data and Cross-National Organizational Case Studies; Employment Relations Research Series Nr. 16; DTI: London, UK, 2003.
- Dahlgren, A.; Kecklund, G.; Åkerstedt, T. Overtime work and its effects on sleep, sleepiness, cortisol and blood pressure in an experimental field study. Scand. J. Work Environ. Health 2006, 32, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Boisard, P.; Cartron, D.; Gollac, M.; Valeyre, A. Time and work: Duration of work. In European Foundation for the Improvement of Living and Working Conditions; Office for Official Publications of the European Communities: Luxembourg, Luxembourg, 2003. [Google Scholar]
- Akerstedt, T.; Fredlund, P.; Gillberg, M.; Jansson, B. Work load and work hours in relation to disturbed sleep and fatigue in a large representative sample. J. Psychosom. Res. 2002, 53, 585–588. [Google Scholar] [CrossRef]
- Bannai, A.; Tamakoshi, A. The association between long working hours and health: A systematic review of epidemiological evidence. Scand. J. Work Environ. Health 2014, 40, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Buell, P.; Breslow, L. Mortality from coronary heart disease in California men who work long hours. J. Chronic Dis. 1960, 11, 615–626. [Google Scholar] [CrossRef]
- Caruso, C.C. Possible broad impacts of long work hours: A review article. Ind. Health 2006, 44, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Dembe, A.E.; Erickson, J.B.; Delbos, R.G.; Banks, S.M. The impact of overtime and long work hours on occupational injuries and illnesses: New evidence from the United States. Occup. Environ. Med. 2005, 62, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Folkard, S.; Lombardi, D.A. Modeling the impact of the components of long work hours on injuries and “Accidents”. Am. J. Ind. Med. 2006, 49, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Geurts, S.A.E.; Beckers, D.G.J.; Taris, T.W.; Kompier, M.A.J.; Smulders, P.G.W. Worktime demands and work-family interference: Does worktime control buffer the adverse effects of high demands? J. Bus. Ethics 2009, 84, 229–241. [Google Scholar] [CrossRef]
- Knauth, P. Extended work periods. Ind. Health 2007, 45, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Krenn, M.; Hermann, C. Long Working Hours in Austria. Available online: http://www.eurofound.europa.eu/ewco/2004/12/AT0412NU01.htm (accessed on 8 January 2018).
- Liu, Y.; Tanaka, H. Overtime work, insufficient sleep, and risk of non-fatal acute myocardial infarction in Japanese men. J. Occup. Environ. Med. 2002, 59, 447–451. [Google Scholar] [CrossRef]
- Nachreiner, F. Working time and accident risk. In Psychology of Occupational Safety and Health—New Worlds—Ancient Worlds; Trimpop, R., Zimolong, B., Kalveram, A., Eds.; Asanger: Heidelberg, Germany, 2002. [Google Scholar]
- Nakamura, K.; Shimai, S.; Kikuchi, S.; Takahashi, H.; Tanaka, M.; Nakano, S.; Motohashi, Y.; Nakadaira, H.; Yamamoto, M. Increases in body mass index and waist circumference as outcomes of working overtime. J. Occup. Med. 1998, 48, 189–193. [Google Scholar] [CrossRef]
- Park, J.; Kim, Y.; Cho, Y.; Woo, K.; Chung, K.; Iwasaki, K.; Oka, T.; Sasaki, T.; Hisanaga, N. Regular overtime and cardiovascular function. Ind. Health 2001, 39, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Raediker, B.; Janßen, D.; Schomann, C.; Nachreiner, F. Extended working hours and health. Chronobiol. Int. 2006, 23, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Rau, R.; Triemer, A. Overtime in relation to blood pressure and mood during work, leisure and night time. Soc. Indic. Res. 2004, 67, 51–73. [Google Scholar] [CrossRef]
- Shepard, E.; Clifton, T. Are longer hours reducing productivity in manufacturing? Int. J. Manpower 2000, 21, 540–553. [Google Scholar] [CrossRef]
- Sparks, K.; Cooper, C.; Freid, Y.; Shiroam, A. The effects of hours of work on health: A meta-analytic review. J. Occup. Organ. Psychol. 1997, 70, 391–408. [Google Scholar] [CrossRef]
- Spurgeon, A.; Harrington, J.M.; Cooper, C.L. Health and safety problems associated with long working hours: A review of the current position. Occup. Environ. Med. 1997, 54, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Su, T.-C.; Lin, L.-Y.; Baker, D.; Schnall, P.L.; Chen, M.-F.; Hwang, W.-C.; Chen, C.F.; Wang, J.D. Elevated blood pressure, decreased heart rate variability and incomplete blood pressure recovery after a 12-hour night shift work. J. Occup. Health 2008, 50, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Trimpop, R.; Kirkcaldy, B.; Athanasou, J.; Cooper, C. Individual differences in working hours, work perceptions and accident rates in veterinary surgeries. Work Stress 2000, 14, 181–188. [Google Scholar] [CrossRef]
- Trinkoff, A.M.; Le, R.; Geiger-Brown, J.; Lipscomb, J.; Lang, G. Longitudinal relationship of work hours, mandatory overtime, and on-call to musculoskeletal problems in nurses. Am. J. Ind. Med. 2006, 49, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Uehata, T. Long working hours and occupational stress-related cardiovascular attacks among middle-aged workers in Japan. J. Hum. Ergol. 1991, 20, 147–153. [Google Scholar]
- Van der Hulst, M. Long workhours and health. Scand. J. Work Environ. Health 2003, 29, 171–188. [Google Scholar] [CrossRef] [PubMed]
- Violanti, J.M.; Burchfield, C.M.; Hartley, T.A.; Andrew, M.E.; Vila, B.J. A typical work hours and metabolic syndrome among police officers. Arch. Environ. Occup. Health 2009, 64, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Proctor, S.P.; White, R.F.; Robins, T.G.; Echeverria, D.; Rocskay, A.Z. Effect of overtime work on cognitive function in automotive workers. Scand. J. Work Environ. Health 1996, 22, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Kallus, K.W.; Boucsein, W.; Spanner, N. Eight- and twelve-hour shifts in Austrian rail traffic controllers: A psychophysiological comparison. Psychol. Sci. 2009, 51, 283–297. [Google Scholar]
- Persson, R.; Ørbæk, P.; Ursin, H.; Kecklund, G.; Österberg, K.; Åkerstedt, T. Effects of the implementation of an 84-hour workweek on neurobehavioral test performance and cortisol responsiveness during testing. Scand. J. Work Environ. Health 2003, 29, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Deixelberger-Fritz, D.; Tischler, M.; Kallus, K. Changes in Performace, Mood State and Workload due to Energy Drinks in Pilots. Int. J. Appl. Stud. 2003, 3, 195–205. [Google Scholar]
- Healy, A.F.; Kole, J.A.; Buck-Gengler, C.J.; Bourne, L.E. Effects of prolonged work on data entry speed and accuracy. J. Exp. Psychol. Appl. 2004, 10, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Wagner-Hartl, V.; Kallus, K.W. Investigation of psychophysiological and subjective effects of long working hours—Do age and hearing impairment matter? Front. Psychol. 2018, 8. [Google Scholar] [CrossRef]
- European Working Group on Genetics of Hearing Impairment (EUWG). Available online: http://audiology.unife.it/www.gendeaf.org/hear/infoletters/Info_02.PDF (accessed on 8 January 2018).
- Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Kanfer, R.; Ackerman, P.L. Aging, adult development, and work motivation. Acad. Manag. Rev. 2004, 29, 440–458. [Google Scholar]
- Kallus, K.W.; Schmitt, J.A.; Benton, D. Attention, psychomotor functions and age. Eur. J. Nutr. 2005, 44, 465–484. [Google Scholar] [CrossRef] [PubMed]
- Salthouse, T. The processing-speed theory of adult age differences in cognition. Psychol. Rev. 1996, 103, 403–428. [Google Scholar] [CrossRef] [PubMed]
| Age | Hearing Impairment | MTR (s) | SUMR | ||||||
|---|---|---|---|---|---|---|---|---|---|
| t1 | t2 | t1 | t2 | ||||||
| M | SD | M | SD | M | SD | M | SD | ||
| Younger employees | Normal hearing | 0.70 | 0.07 | 0.67 | 0.07 | 255.67 | 15.77 | 262.24 | 8.34 |
| Hearing impaired | 0.71 | 0.06 | 0.68 | 0.06 | 255.89 | 19.54 | 261.00 | 13.96 | |
| Total | 0.70 | 0.07 | 0.68 | 0.07 | 255.73 | 16.64 | 261.87 | 10.10 | |
| Older employees | Normal hearing | 0.78 | 0.05 | 0.75 | 0.06 | 250.75 | 12.45 | 252.50 | 9.77 |
| Hearing impaired | 0.81 | 0.10 | 0.77 | 0.10 | 227.86 | 51.22 | 236.50 | 45.61 | |
| Total | 0.80 | 0.09 | 0.77 | 0.08 | 236.18 | 42.46 | 242.32 | 37.17 | |
| Total | normal hearing | 0.72 | 0.08 | 0.70 | 0.08 | 254.31 | 14.88 | 259.55 | 9.65 |
| Hearing impaired | 0.77 | 0.10 | 0.74 | 0.09 | 238.83 | 43.41 | 246.09 | 38.07 | |
| Total | 0.74 | 0.09 | 0.71 | 0.09 | 247.46 | 31.54 | 253.60 | 26.87 | |
| Age | Hearing Impairment | MTF (s) | SUMF | ||||||
|---|---|---|---|---|---|---|---|---|---|
| t1 | t2 | t1 | t2 | ||||||
| M | SD | M | SD | M | SD | M | SD | ||
| Younger employees | Normal hearing | 0.70 | 0.08 | 0.70 | 0.11 | 13.81 | 9.75 | 8.86 | 5.67 |
| Hearing impaired | 0.76 | 0.09 | 0.68 | 0.08 | 13.00 | 6.93 | 8.44 | 5.46 | |
| Total | 0.72 | 0.09 | 0.69 | 0.10 | 13.57 | 8.89 | 8.73 | 5.51 | |
| Older employees | Normal hearing | 0.79 | 0.10 | 0.82 | 0.05 | 16.13 | 7.61 | 14.62 | 7.63 |
| Hearing impaired | 0.82 | 0.11 | 0.76 | 0.16 | 34.00 | 26.77 | 17.36 | 12.33 | |
| Total | 0.81 | 0.10 | 0.78 | 0.13 | 27.50 | 23.25 | 16.36 | 10.74 | |
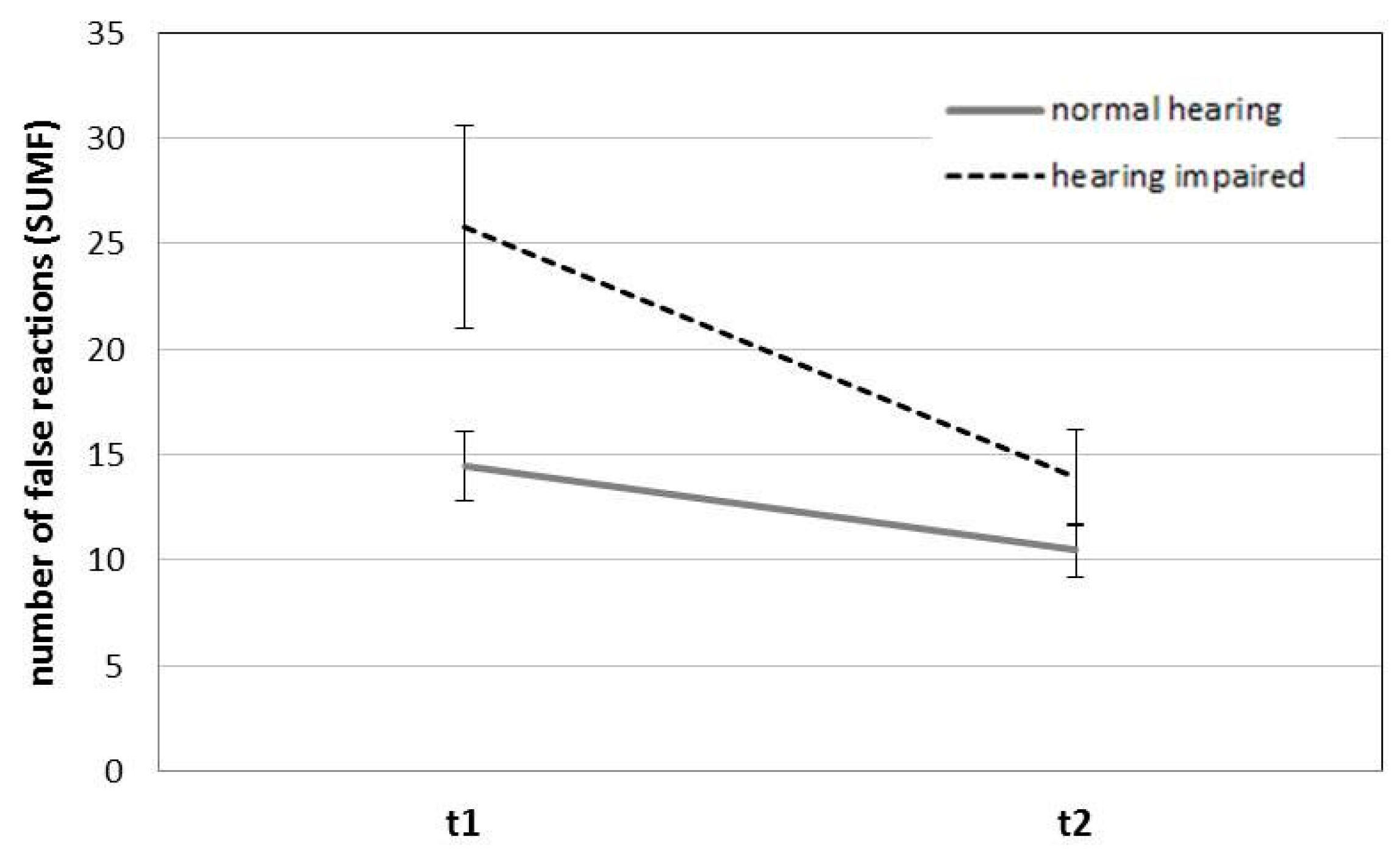
| Total | Normal hearing | 0.73 | 0.10 | 0.73 | 0.11 | 14.45 | 9.14 | 10.45 | 6.66 |
| Hearing impaired | 0.79 | 0.10 | 0.73 | 0.14 | 25.78 | 23.47 | 13.87 | 10.98 | |
| Total | 0.76 | 0.10 | 0.73 | 0.12 | 19.46 | 17.77 | 11.96 | 8.91 | |
| Age | Hearing Impairment | M-RZ (s) | M-MZ (s) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| t1 | t2 | t1 | t2 | ||||||
| M | SD | M | SD | M | SD | M | SD | ||
| Younger employees | Normal hearing | 544.68 | 70.16 | 506.18 | 67.20 | 140.59 | 35.03 | 132.14 | 48.74 |
| Hearing impaired | 527.80 | 72.79 | 521.60 | 72.28 | 175.90 | 82.21 | 146.80 | 52.99 | |
| Total | 539.41 | 70.26 | 511.00 | 68.03 | 151.63 | 55.41 | 136.72 | 49.72 | |
| Older employees | Normal hearing | 570.00 | 77.87 | 542.33 | 57.79 | 183.22 | 68.96 | 154.56 | 71.67 |
| Hearing impaired | 593.07 | 84.63 | 575.36 | 99.11 | 217.57 | 73.24 | 195.50 | 64.46 | |
| Total | 584.04 | 81.06 | 562.43 | 85.38 | 204.13 | 72.06 | 179.48 | 68.85 | |
| Total | Normal hearing | 552.03 | 72.11 | 516.68 | 65.80 | 152.97 | 50.14 | 138.65 | 56.03 |
| Hearing impaired | 565.88 | 84.86 | 552.96 | 91.26 | 200.21 | 78.21 | 175.21 | 63.63 | |
| Total | 558.07 | 77.49 | 532.51 | 79.26 | 173.58 | 67.54 | 154.60 | 61.67 | |
| Age | Hearing Impairment | MTRN (s) | |||
|---|---|---|---|---|---|
| t1 | t2 | ||||
| M | SD | M | SD | ||
| Younger employees | Normal hearing | 2.29 | 0.39 | 2.16 | 0.41 |
| Hearing impaired | 2.28 | 0.37 | 2.17 | 0.32 | |
| Total | 2.29 | 0.38 | 2.16 | 0.38 | |
| Older employees | Normal hearing | 2.43 | 0.25 | 2.38 | 0.30 |
| Hearing impaired | 3.01 | 0.98 | 2.72 | 0.55 | |
| Total | 2.79 | 0.82 | 2.59 | 0.49 | |
| Total | Normal hearing | 2.33 | 0.36 | 2.22 | 0.39 |
| Hearing impaired | 2.71 | 0.86 | 2.49 | 0.54 | |
| Total | 2.49 | 0.65 | 2.34 | 0.47 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner-Hartl, V.; Grossi, N.R.; Kallus, K.W. Impact of Age and Hearing Impairment on Work Performance during Long Working Hours. Int. J. Environ. Res. Public Health 2018, 15, 98. https://doi.org/10.3390/ijerph15010098
Wagner-Hartl V, Grossi NR, Kallus KW. Impact of Age and Hearing Impairment on Work Performance during Long Working Hours. International Journal of Environmental Research and Public Health. 2018; 15(1):98. https://doi.org/10.3390/ijerph15010098
Chicago/Turabian StyleWagner-Hartl, Verena, Nina R. Grossi, and K. Wolfgang Kallus. 2018. "Impact of Age and Hearing Impairment on Work Performance during Long Working Hours" International Journal of Environmental Research and Public Health 15, no. 1: 98. https://doi.org/10.3390/ijerph15010098





