Rapid Screening of Cadmium in Rice and Identification of Geographical Origins by Spectral Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation, Apparatus and Measurement Conditions
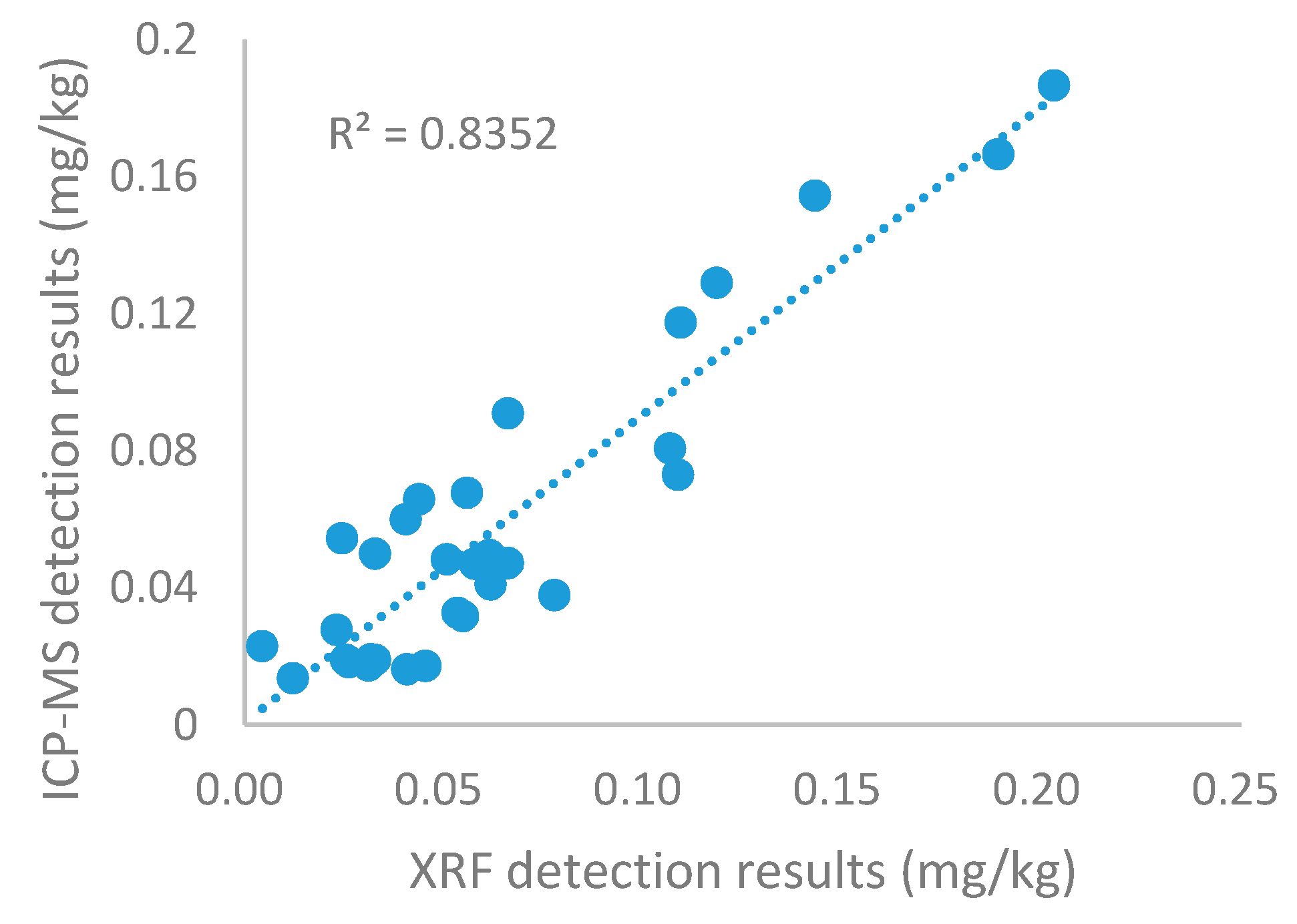
2.2. XRF Detection Performance Verification and Raman Spectral Data Pre-Treatment
2.3. Statistical Analysis of Cadmium Concentrations in Rice Grain Samples by XRF
2.4. Chemometric Methods for Raman Spectra Analysis
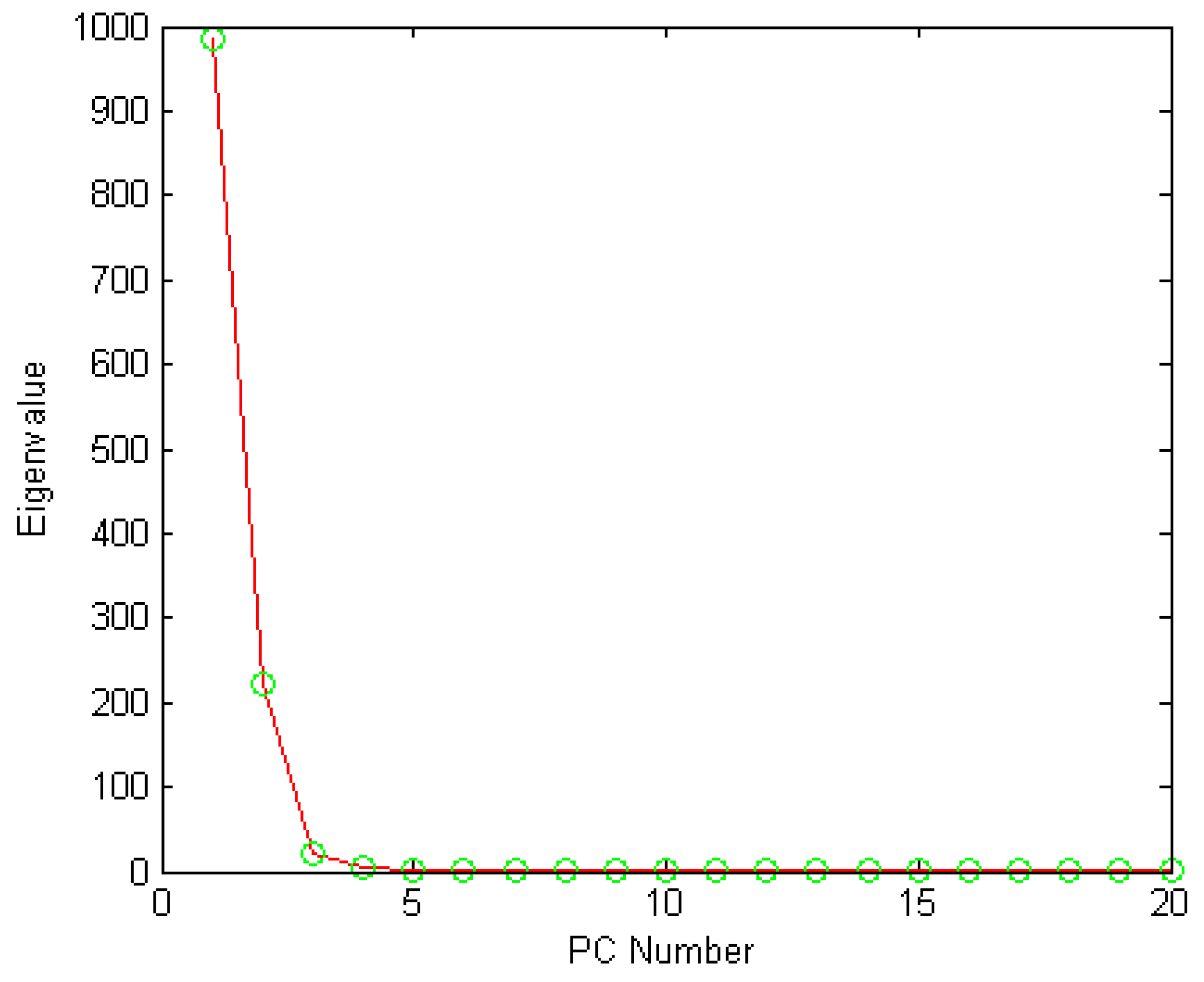
2.4.1. PCA
2.4.2. KMC
2.4.3. HC
2.4.4. SVM
3. Results and Discussion
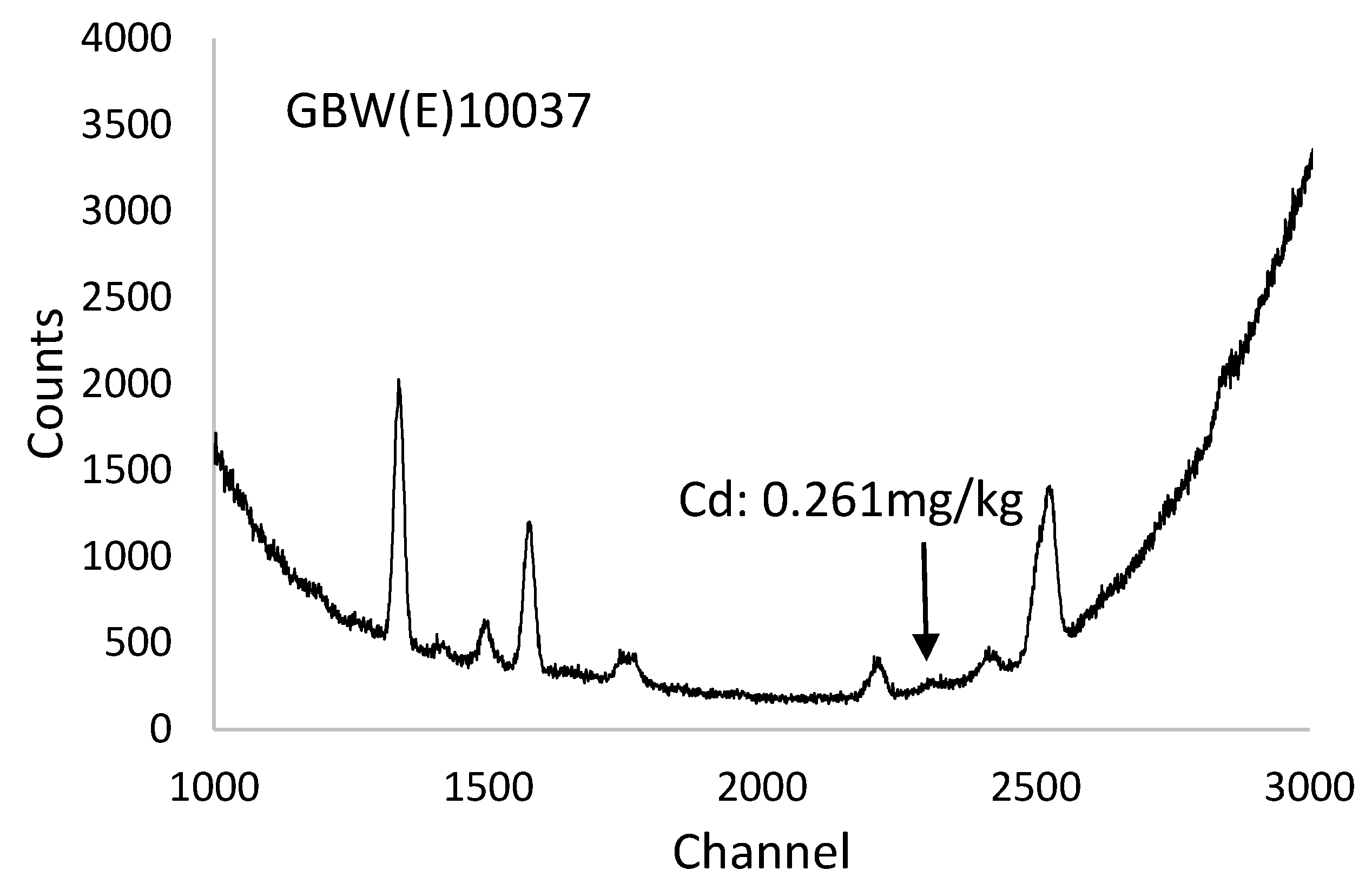
3.1. Accuracy, Repeatability and Detection Limits of PXRF Spectrometer
3.2. Concentration of Cadmium in Rice Grain Samples Detected by XRF
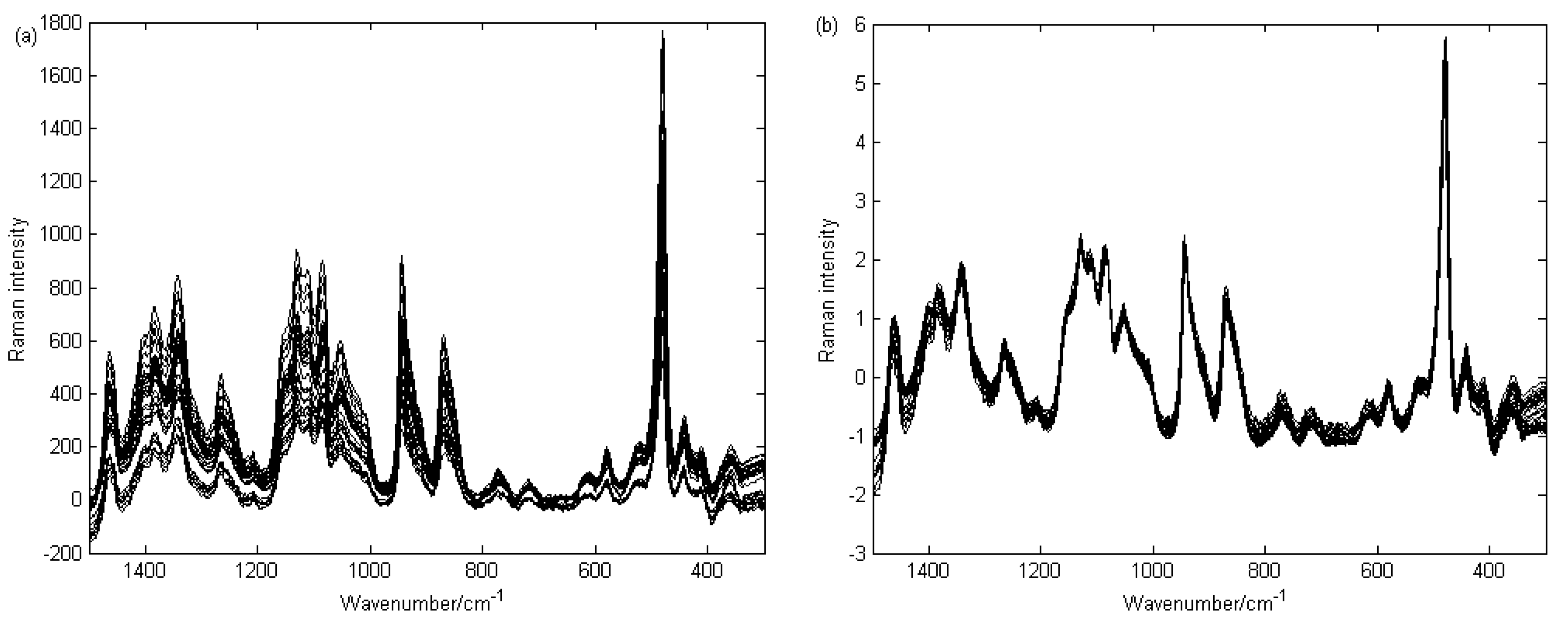
3.3. Raman Spectral Analysis
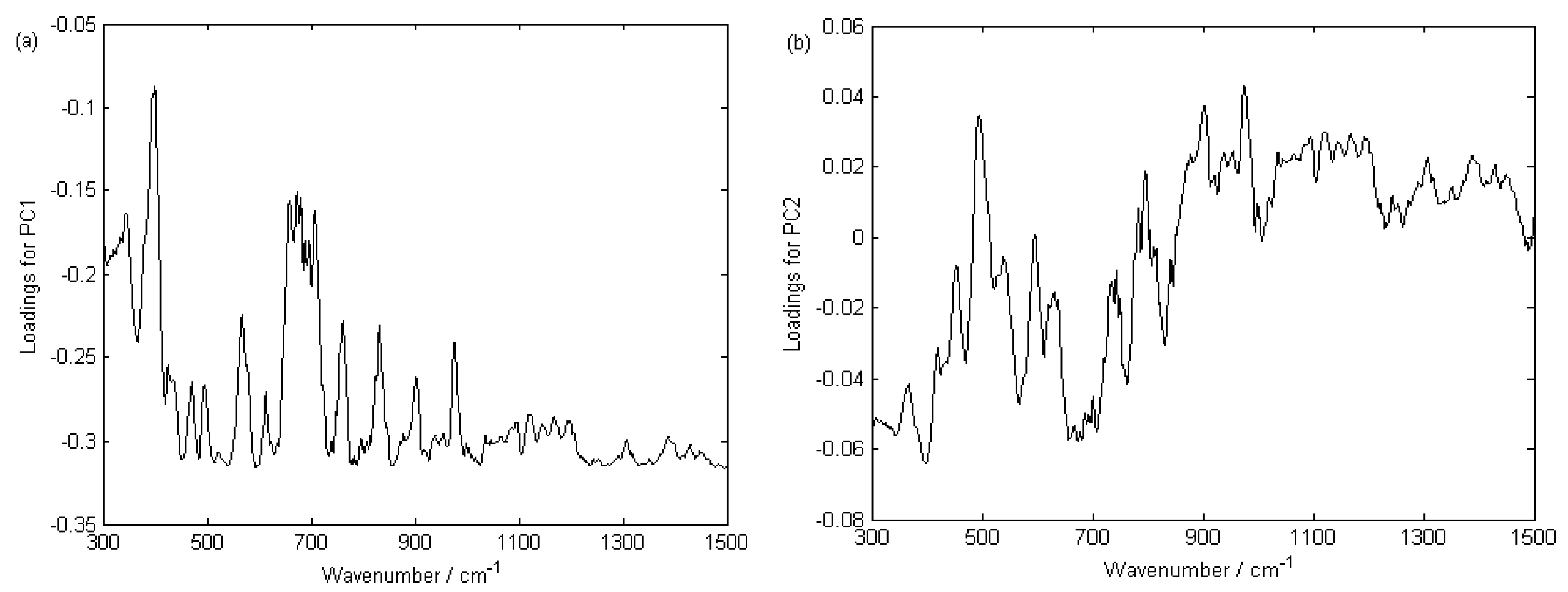
3.3.1. Raman Spectral Pretreatment
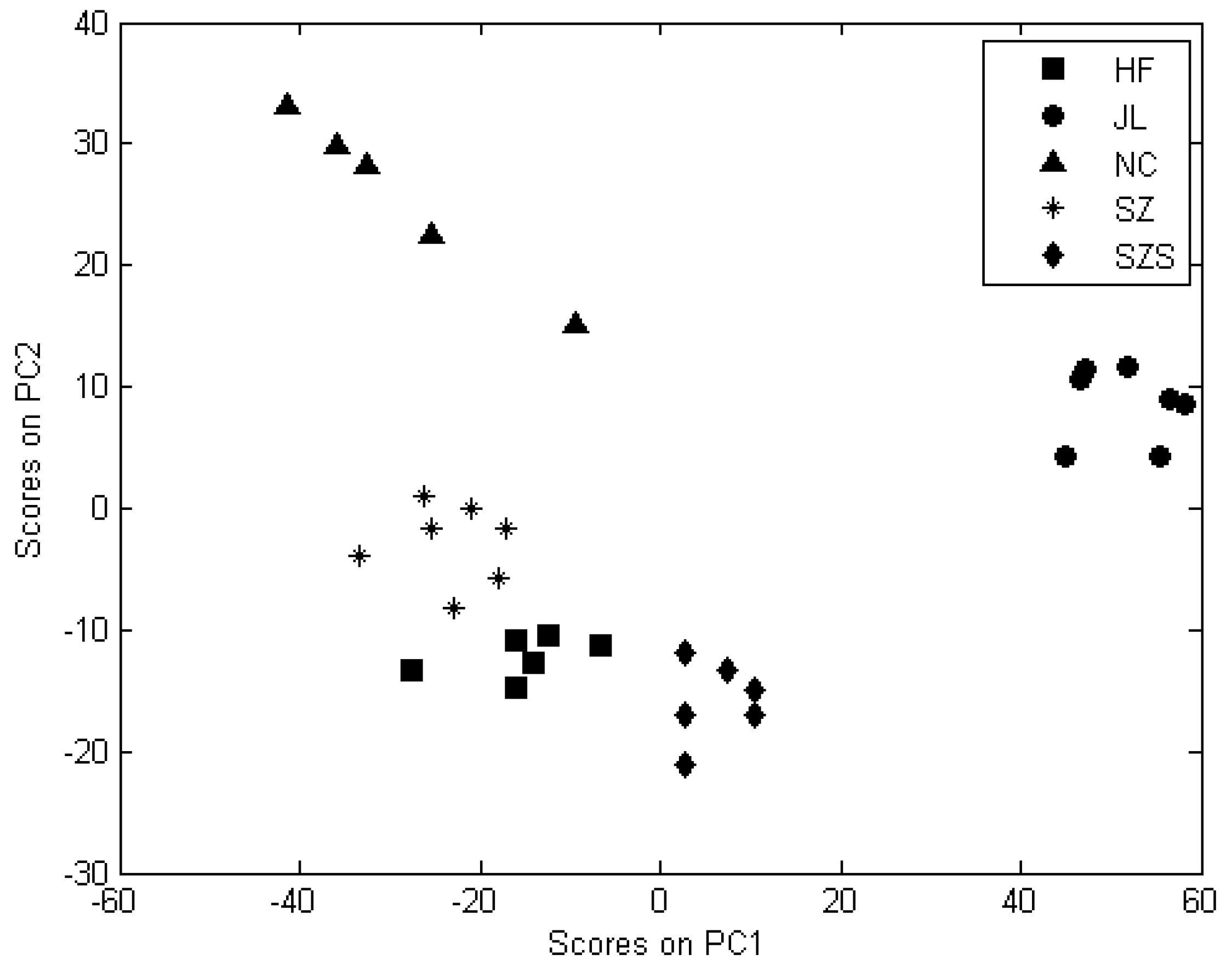
3.3.2. PCA Analysis for Geographical Origin Discrimination
3.3.3. Other Chemometric Methods for Geographical Origin Discrimination
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ellepola, S.W.; Choi, S.M.; Phillips, D.L.; Ma, C.Y. Raman spectroscopic study of rice globulin. J. Cereal Sci. 2006, 43, 85–93. [Google Scholar] [CrossRef]
- Meharg, A.A.; Norton, G.; Deacon, C.; Williams, P.; Adomako, E.E.; Price, A.; Zhu, Y.; Li, G.; Zhao, F.J.; McGrath, S.; et al. Variation in rice cadmium related to human exposure. Environ. Sci. Technol. 2013, 47, 5613–5618. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, H. Current status of cadmium exposure among Japanese, especially regarding the safety standard for cadmium concentration in rice and adverse effects on proximal renal tubular function observed in farmers exposed to cadmium through consumption of self-grown rice. Nihon Eiseigaku Zasshi. Jpn. J. Hyg. 2012, 67, 447–454. [Google Scholar]
- Font, R.; Vélez, D.; Del Río-Celestino, M.; De Haro-Bailón, A.; Montoro, R. Screening inorganic arsenic in rice by visible and near-infrared spectroscopy. Microchim. Acta 2005, 151, 231–239. [Google Scholar] [CrossRef]
- Yu, H.Y.; Xu, H.R.; Ying, Y.B.; Xie, L.J.; Zhou, Y.; Fu, X.P. Prediction of trace metals in Chinese rice wine by Fourier Transform near-infrared spectroscopy. Trans. ASABE 2006, 49, 1463–1467. [Google Scholar] [CrossRef]
- Li, F.; Lu, A.; Wang, J. Modeling of Chromium, Copper, Zinc, Arsenic and Lead Using Portable X-ray Fluorescence Spectrometer Based on Discrete Wavelet Transform. Int. J. Environ. Res. Public Health 2017, 14, 1163. [Google Scholar] [CrossRef] [PubMed]
- Paltridge, N.G.; Palmer, L.J.; Milham, P.J.; Guild, G.E.; Stangoulis, J.C. Energy-dispersive X-ray fluorescence analysis of zinc and iron concentration in rice and pearl millet grain. Plant Soil 2012, 361, 251–260. [Google Scholar] [CrossRef]
- Bull, A.; Brown, M.T.; Turner, A. Novel use of field-portable-XRF for the direct analysis of trace elements in marine macroalgae. Environ. Pollut. 2017, 220, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Galtier, O.; Dupuy, N.; Le Dréau, Y.; Ollivier, D.; Pinatel, C.; Kister, J.; Artaud, J. Geographic origins and compositions of virgin olive oils determinated by chemometric analysis of NIR spectra. Anal. Chim. Acta 2007, 595, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Latorre, C.H.; Crecente, R.P.; Martín, S.G.; García, J.B. A fast chemometric procedure based on NIR data for authentication of honey with protected geographical indication. Food Chem. 2013, 141, 3559–3565. [Google Scholar] [CrossRef] [PubMed]
- Nescatelli, R.; Carradori, S.; Marini, F.; Caponigro, V.; Bucci, R.; De Monte, C.; Mollica, A.; Mannina, L.; Ceruso, M.; Supuran, C.T.; et al. Geographical characterization by MAE-HPLC and NIR methodologies and carbonic anhydrase inhibition of Saffron components. Food Chem. 2017, 221, 855–863. [Google Scholar] [CrossRef] [PubMed]
- De Luca, S.; De Filippis, M.; Bucci, R.; Magrì, A.D.; Magrì, A.L.; Marini, F. Characterization of the effects of different roasting conditions on coffee samples of different geographical origins by HPLC-DAD, NIR and chemometrics. Microchem. J. 2016, 129, 348–361. [Google Scholar] [CrossRef]
- Marquetti, I.; Link, J.V.; Lemes, A.L.G.; dos Santos Scholz, M.B.; Valderrama, P.; Bona, E. Partial least square with discriminant analysis and near infrared spectroscopy for evaluation of geographic and genotypic origin of arabica coffee. Comput. Electron. Agric. 2016, 121, 313–319. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, D.H.; Wang, X.C.; Liu, L.P.; Fan, Y.X.; Cao, J.X. Prediction of chemical composition and geographical origin traceability of Chinese export tilapia fillets products by near infrared reflectance spectroscopy. LWT-Food Sci. Technol. 2015, 60, 1214–1218. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kim, S.H.; Kim, Y.C.; Kim, H.J.; Sung, S.H. Discrimination of Scrophulariae Radix according to geographical origin and determination of active constituents by near infrared spectroscopy (NIRS). Microchem. J. 2011, 99, 213–217. [Google Scholar] [CrossRef]
- Zhao, H.; Guo, B.; Wei, Y.; Zhang, B. Near infrared reflectance spectroscopy for determination of the geographical origin of wheat. Food Chem. 2013, 138, 1902–1907. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.A.; Kim, H.J.; Ze, K.R.; Chung, H. Near-infrared (NIR) spectroscopy for the non-destructive and fast determination of geographical origin of Angelicae gigantis Radix. J. Pharm. Biomed. Anal. 2005, 36, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, Z.; Haughey, S.A.; Galvin-King, P.; Han, L.; Elliott, C.T. Classification the geographical origin of corn distillers dried grains with solubles by near infrared reflectance spectroscopy combined with chemometrics: A feasibility study. Food Chem. 2015, 189, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Wang, S.; Ning, J.; Xu, R.; Wang, Y.; Xing, Z.; Wan, X.; Zhang, Z. Quantitative analysis and geographical traceability of black tea using Fourier transform near-infrared spectroscopy (FT-NIRS). Food Res. Int. 2013, 53, 822–826. [Google Scholar] [CrossRef]
- Costa, M.C.A.; Morgano, M.A.; Ferreira, M.M.C.; Milani, R.F. Analysis of bee pollen constituents from different Brazilian regions: Quantification by NIR spectroscopy and PLS regression. LWT-Food Sci. Technol. 2017, 80, 76–83. [Google Scholar] [CrossRef]
- Schmitt, M.; Popp, J. Raman spectroscopy at the beginning of the twenty-first century. J. Raman Spectrosc. 2006, 37, 20–28. [Google Scholar] [CrossRef]
- Baeten, V.; Dardenne, P. Spectroscopy: Developments in instrumentation and analysis. Grasas Aceites 2002, 53, 45–63. [Google Scholar] [CrossRef]
- Fan, D.; Ma, W.; Wang, L.; Huang, J.; Zhao, J.; Zhang, H.; Chen, W. Determination of structural changes in microwaved rice starch using Fourier transform infrared and Raman spectroscopy. Starch-Stärke 2012, 64, 598–606. [Google Scholar] [CrossRef]
- Woo, Y.A.; Kim, H.J.; Chung, H. Classification of cultivation area of ginseng radix with NIR and Raman spectroscopy. Analyst 1999, 124, 1223–1226. [Google Scholar] [CrossRef]
- Rubayiza, A.B.; Meurens, M. Chemical discrimination of arabica and robusta coffees by Fourier transform Raman spectroscopy. J. Agric. Food Chem. 2005, 53, 4654–4659. [Google Scholar] [CrossRef] [PubMed]
- Muik, B.; Lendl, B.; Molina-Díaz, A.; Ortega-Calderón, D.; Ayora-Cañada, M.J. Discrimination of olives according to fruit quality using Fourier transform Raman spectroscopy and pattern recognition techniques. J. Agric. Food Chem. 2004, 52, 6055–6060. [Google Scholar] [CrossRef] [PubMed]
- Morháč, M. An algorithm for determination of peak regions and baseline elimination in spectroscopic data. Nucl. Instrum. Methods Phys. Res. Sect. A 2009, 600, 478–487. [Google Scholar] [CrossRef]
- Fearn, T.; Riccioli, C.; Garrido-Varo, A.; Guerrero-Ginel, J.E. On the geometry of SNV and MSC. Chemom. Intell. Lab. Syst. 2009, 96, 22–26. [Google Scholar] [CrossRef]
- Matousek, P. Deep non-invasive Raman spectroscopy of living tissue and powders. Chem. Soc. Rev. 2007, 36, 1292–1304. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K. Data clustering: 50 Years beyond K-means. Pattern Recognit. Lett. 2010, 31, 651–666. [Google Scholar] [CrossRef]
- Fouedjio, F. A hierarchical clustering method for multivariate geostatistical data. Spat. Stat. 2016, 18, 333–351. [Google Scholar] [CrossRef]
- Hao, M.; Qiao, Z. The Discrimination of Fluorescence Spectra of Mineral Oil Based on the FASTICA and SVM. In Proceedings of the 2015 Fifth International Conference on Instrumentation and Measurement, Computer, Communication and Control (IMCCC), Qinhuangdao, China, 18–20 September 2015; pp. 1721–1724. [Google Scholar]

| Area/Label | Province | No. of Samples | |
|---|---|---|---|
| Hefei | HF | Anhui | 6 |
| Jilin | JL | Jilin | 7 |
| Nanchang | NC | Jiangxi | 5 |
| Shizuishan | SZS | Ningxia | 6 |
| Suzhou | SZ | Jiangsu | 7 |
| No. of Tests | C Sample (mg/kg) | GBW(E)100378 (mg/kg) |
|---|---|---|
| 1 | 2.205 | 0.184 |
| 2 | 2.612 | 0.169 |
| 3 | 2.073 | 0.157 |
| 4 | 2.220 | 0.170 |
| 5 | 2.212 | 0.175 |
| 6 | 2.432 | 0.144 |
| 7 | 2.109 | 0.176 |
| 8 | 2.210 | 0.148 |
| 9 | 2.096 | 0.187 |
| 10 | 2.372 | 0.172 |
| 11 | 2.011 | 0.165 |
| Arithmetic mean (mg/kg) | 2.232 | 0.168 |
| Standard value (mg/kg) | 2.155 | 0.169 |
| Relative standard deviation/% | 7.932 | 7.738 |
| t | 1.442 | 0.266 |
| t0.05,10 | 2.228 | |
| No. of Test | Test Results (mg/kg) | No. of Test | Test Results (mg/kg) |
|---|---|---|---|
| 1 | 0.076 | 9 | 0.086 |
| 2 | 0.043 | 10 | 0.073 |
| 3 | 0.081 | 11 | 0.049 |
| 4 | 0.073 | 12 | 0.047 |
| 5 | 0.061 | 13 | 0.083 |
| 6 | 0.084 | 14 | 0.074 |
| 7 | 0.068 | 15 | 0.059 |
| 8 | 0.052 | ||
| Arithmetic mean (mg/kg) | 0.0673 | ||
| Standard deviation | 0.014 | ||
| Qualitative detection limit (mg/kg) | 0.0419 | ||
| Quantitative detection limit (mg/kg) | 0.1397 | ||
| Sample Number | Min. (mg/kg) | Max. (mg/kg) | Mean (mg/kg) | SD | RSD/% |
|---|---|---|---|---|---|
| 1 | 0.065 | 0.067 | 0.066 | 0.001 | 1.515 |
| 2 | 0.014 | 0.040 | 0.024 | 0.014 | 56.697 |
| 3 | 0.031 | 0.065 | 0.044 | 0.029 | 65.790 |
| 4 | 0.024 | 0.051 | 0.040 | 0.014 | 35.614 |
| 5 | 0.080 | 0.132 | 0.109 | 0.026 | 24.301 |
| 6 | 0.083 | 0.124 | 0.109 | 0.023 | 20.904 |
| 7 | 0.000 | 0.013 | 0.004 | 0.008 | 173.219 |
| 8 | 0.040 | 0.077 | 0.052 | 0.040 | 76.923 |
| 9 | 0.036 | 0.086 | 0.053 | 0.028 | 53.077 |
| 10 | 0.021 | 0.044 | 0.033 | 0.012 | 35.215 |
| 11 | 0.047 | 0.069 | 0.053 | 0.036 | 67.924 |
| 12 | 0.199 | 0.210 | 0.203 | 0.006 | 2.996 |
| 13 | 0.056 | 0.072 | 0.065 | 0.019 | 29.231 |
| 14 | 0.051 | 0.063 | 0.056 | 0.006 | 11.549 |
| 15 | 0.109 | 0.131 | 0.118 | 0.011 | 9.611 |
| 16 | 0.049 | 0.076 | 0.061 | 0.024 | 39.344 |
| 17 | 0.172 | 0.205 | 0.189 | 0.017 | 8.742 |
| 18 | 0.158 | 0.178 | 0.163 | 0.016 | 9.816 |
| 19 | 0.042 | 0.070 | 0.055 | 0.014 | 25.956 |
| 20 | 0.016 | 0.088 | 0.062 | 0.040 | 64.383 |
| 21 | 0.052 | 0.071 | 0.061 | 0.016 | 26.230 |
| 22 | 0.007 | 0.040 | 0.023 | 0.017 | 71.838 |
| 23 | 0.039 | 0.093 | 0.061 | 0.028 | 45.952 |
| 24 | 0.001 | 0.050 | 0.026 | 0.025 | 94.290 |
| 25 | 0.000 | 0.054 | 0.033 | 0.029 | 87.944 |
| 26 | 0.064 | 0.068 | 0.066 | 0.002 | 3.030 |
| 27 | 0.096 | 0.121 | 0.107 | 0.013 | 12.091 |
| 28 | 0.068 | 0.083 | 0.078 | 0.008 | 10.798 |
| 29 | 0.000 | 0.039 | 0.028 | 0.051 | 182.142 |
| 30 | 0.012 | 0.049 | 0.025 | 0.021 | 81.123 |
| 31 | 0.000 | 0.036 | 0.012 | 0.021 | 173.205 |
| Area | Min. (mg/kg) | Max. (mg/kg) | Range (xmax. − xmin.) | Mean (mg/kg) | SD | RSD/% |
|---|---|---|---|---|---|---|
| HF | 0.011 | 0.132 | 0.121 | 0.065 | 0.038 | 58.240 |
| JL | 0.000 | 0.210 | 0.210 | 0.059 | 0.066 | 113.091 |
| NC | 0.000 | 0.205 | 0.205 | 0.111 | 0.059 | 52.745 |
| SZ | 0.000 | 0.093 | 0.093 | 0.041 | 0.029 | 69.077 |
| SZS | 0.000 | 0.121 | 0.121 | 0.058 | 0.039 | 67.284 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Wang, J.; Xu, L.; Wang, S.; Zhou, M.; Yin, J.; Lu, A. Rapid Screening of Cadmium in Rice and Identification of Geographical Origins by Spectral Method. Int. J. Environ. Res. Public Health 2018, 15, 312. https://doi.org/10.3390/ijerph15020312
Li F, Wang J, Xu L, Wang S, Zhou M, Yin J, Lu A. Rapid Screening of Cadmium in Rice and Identification of Geographical Origins by Spectral Method. International Journal of Environmental Research and Public Health. 2018; 15(2):312. https://doi.org/10.3390/ijerph15020312
Chicago/Turabian StyleLi, Fang, Jihua Wang, Li Xu, Songxue Wang, Minghui Zhou, Jingwei Yin, and Anxiang Lu. 2018. "Rapid Screening of Cadmium in Rice and Identification of Geographical Origins by Spectral Method" International Journal of Environmental Research and Public Health 15, no. 2: 312. https://doi.org/10.3390/ijerph15020312




