An Interoperable System toward Cardiac Risk Stratification from ECG Monitoring
Abstract
:1. Introduction
2. Materials and Methods
2.1. ECG Indices for SCD Risk Stratification
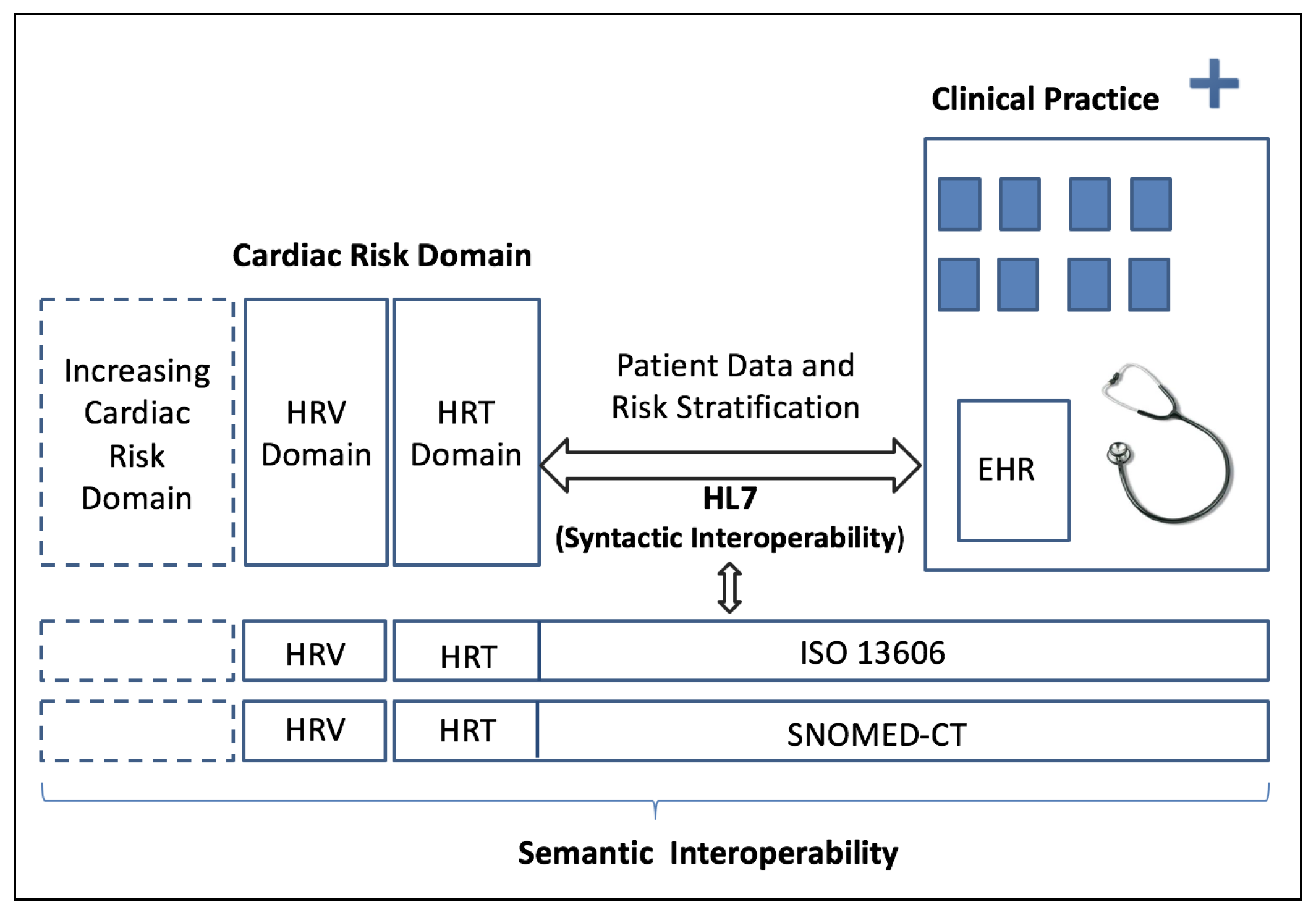
2.2. Clinical Interoperability Standards
3. The Proposed Interoperable System and Results
3.1. System Specifications and Functional Scheme
- to create a system, external to the EHR and HIS, that is interoperable with them, thanks to its design following clinical standards;
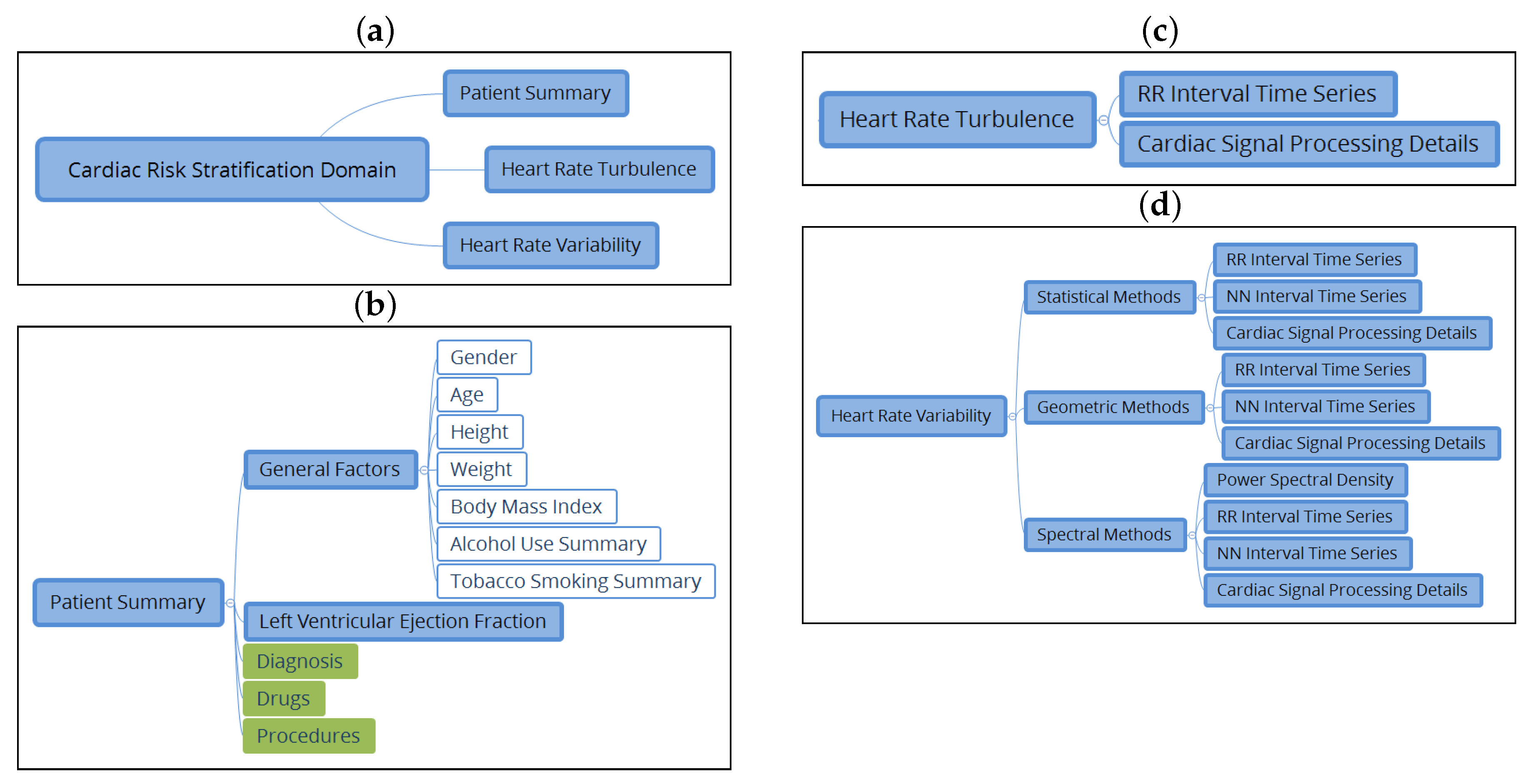
- to cover a significant part of the CRS domain from ECG-derived indices measured in Holter recordings, namely, patient summary data, HRT, and HRV subdomains;
- to be able to grow by including other cardiac risk subdomains;
- to allow us to perform signal processing;
- to generate alerts related to the CRS domain, with different levels, and to feed them back into the EHR.
3.2. Subdomain Definitions for CRS
- An archetype of statistical measures was created, which includes the value in milliseconds of the SDNN, the SDNN index, and the SDANN indices, calculated from the series of NN intervals, the values of indices NN50, SDS, and RMSS in milliseconds, and pNN50 described as a proportion.
- An archetype of geometric measurements that includes the value of the TINN, the triangular index, the differential index, the logarithmic index, and the poincare SD1 and SD2 indices was created.
- An archetype of spectral measurements of HRV was created. For the case of short-term analysis, it includes the value of total power, VLF power, LF power, HF power (power values in ), normalized LF power, normalized HF power, and LF/HF—in the case of an ambulatory analysis, total power, ULF power, VLF power, LF power, HF power, and alpha index.
3.3. Database Description
3.4. System Validation
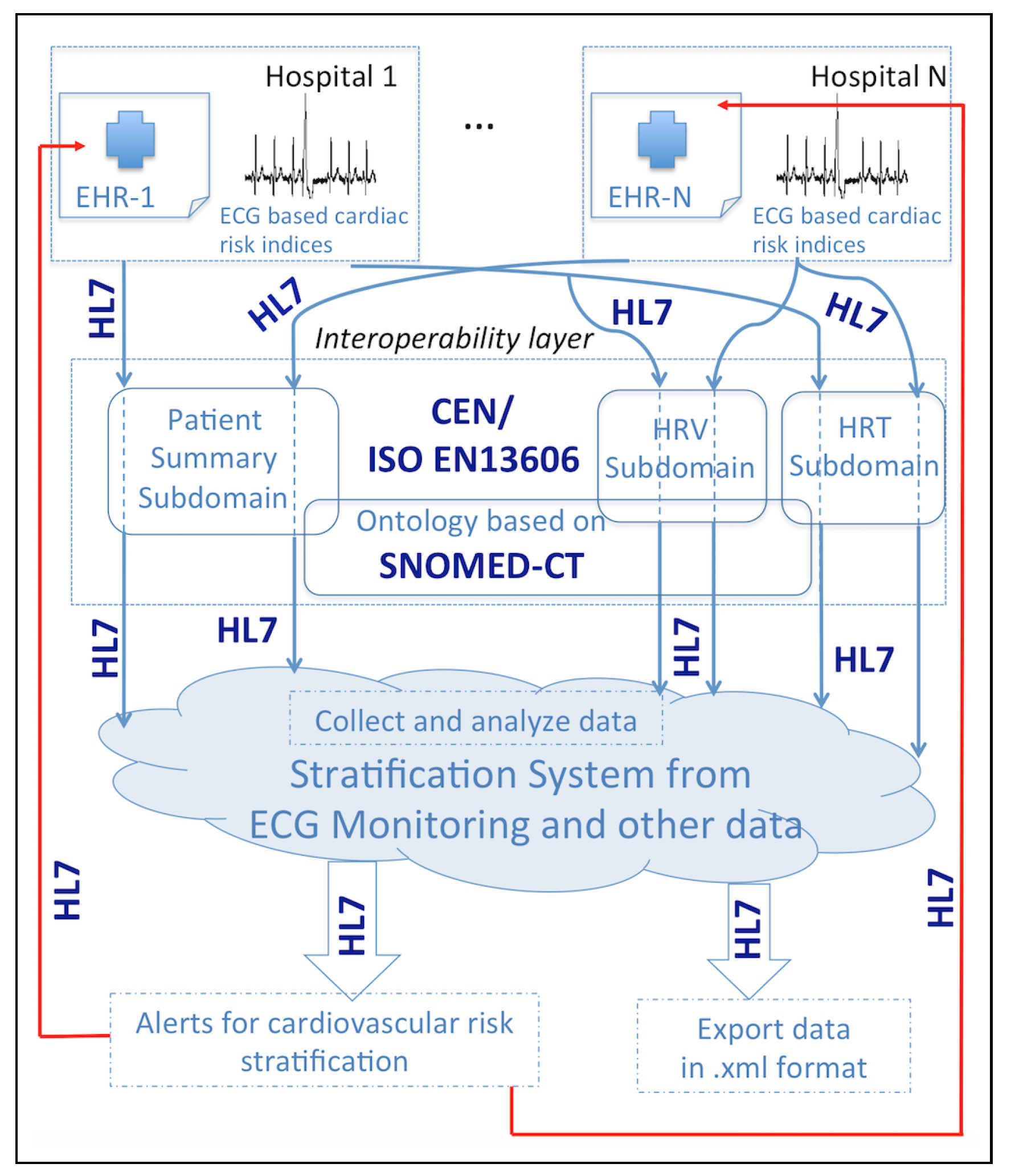
- Demographic and cardiac data from the patient is recorded in the EHR, as shown in Figure 4a.
- A CRS request is made in the EHR by a clinician. This generates an OMG HL7 message (general clinical order message), which is sent from the EHR to the RSEM following the structure of the patient summary and the HRT and HRV archetypes. This message starts with a header identified by the segment MSH, followed by segments PID (patient identification) and PV1 (patient visit). OBR describes the observation request, and OBX is the observation/result. This segment contains the information related to tobacco or alcohol, among others (see Figure 4b for details).
- The RSEM receives in real time both the CRS request and the patient summary and HRT and HRV indices, which are saved. Note that RSEM can work with different EHRs from different hospitals, supporting a potential multicentric study. Toward that end, a field indicating the hospital is included (for example H12O, see Figure 4c). Apart from receiving a request from a specific EHR of a hospital, an instance of a new patient can be created manually through the RSEM as shown in Figure 4c.
- HRT and HRV indices are evaluated, allowing for the classification of patients into the three categories previously described. The evaluation provided by RSEM is sent to the EHR, generating an alert that shows the result of the previous request in Figure 4d. We achieve this by means of two HL7 messages: a PPR message (patient problem message) to send the alert and an ORU message (result message) to finalize the request.
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Organization, W.H. International Classification of Diseases (ICD); Technical Report; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Health Care Financing Administration: Washington, DC, USA, 2005. [Google Scholar]
- Priori, S.; Aliot, E.; Blomstrom-Lundqvist, C.; Bossaert, L.; Breithardt, G.; Brugada, P.; Camm, A.; Cappato, R.; Cobbe, S.; Di Mario, C.; et al. Task Force on Sudden Cardiac Death of the European Society of Cardiology. Eur. Heart J. 2001, 22, 1374–1450. [Google Scholar] [CrossRef] [PubMed]
- Zipes, D.; Wellens, J. Sudden Cardiac Death. Circulation 1998, 98, 2334–2351. [Google Scholar] [CrossRef] [PubMed]
- Gimeno-Blanes, F.; Blanco-Velasco, M.; Barquero-Pérez, O.; García-Alberola, A.; Rojo-Álvarez, J.L. Sudden Cardiac Risk Stratification with Electrocardiographic Indices—A Review on Computational Processing, Technology Transfer, and Scientific Evidence. Front. Physiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Berner, E.S.; La Lande, T.J. Overview of clinical decision support systems. In Clinical Decision Support Systems; Springer: Cham, Switzerland, 2016; pp. 1–17. [Google Scholar]
- Rodriguez-Loya, S.; Kawamoto, K. Newer Architectures for Clinical Decision Support. In Clinical Decision Support Systems; Springer: Cham, Switzerland, 2016; pp. 87–97. [Google Scholar]
- Marcos, M.; Maldonado, J.A.; Martínez-Salvador, B.; Boscá, D.; Robles, M. Interoperability of clinical decision-support systems and electronic health records using archetypes: A case study in clinical trial eligibility. J. Biomed. Inform. 2013, 46, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Soguero-Ruiz, C.; Hindberg, K.; Rojo-Álvarez, J.L.; Skrøvseth, S.O.; Godtliebsen, F.; Mortensen, K.; Revhaug, A.; Lindsetmo, R.O.; Augestad, K.M.; Jenssen, R. Support vector feature selection for early detection of anastomosis leakage from bag-of-words in electronic health records. IEEE J. Biomed. Health Inform. 2016, 20, 1404–1415. [Google Scholar] [CrossRef] [PubMed]
- Middleton, B.; Sittig, D.; Wright, A. Clinical decision support: A 25 year retrospective and a 25 year vision. Yearb. Med. Inform. 2016, S103–S116. [Google Scholar] [CrossRef] [PubMed]
- Anani, N.; Chen, R.; Moreira, T.P.; Koch, S. Retrospective checking of compliance with practice guidelines for acute stroke care: A novel experiment using open EHR’s Guideline Definition Language. BMC Med. Inform. Decis. Mak. 2014, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- Cornet, R. Infrastructure and Capacity Building for Semantic Interoperability in Healthcare in the Netherlands. Build. Capacit. Health Inform. Future 2017, 234, 70. [Google Scholar]
- Fritzsche, D.; Grüninger, M.; Baclawski, K.; Bennett, M.; Berg-Cross, G.; Schneider, T.; Sriram, R.; Underwood, M.; Westerinen, A. Ontology Summit 2016 Communique: Ontologies within semantic interoperability ecosystems. Appl. Ontol. 2017, 12, 91–111. [Google Scholar] [CrossRef]
- Marco-Ruiz, L.; Moner, D.; Maldonado, J.A.; Kolstrup, N.; Bellika, J.G. Archetype-based data warehouse environment to enable the reuse of electronic health record data. Int. J. Med. Inform. 2015, 84, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.H.; Lai, F.; Lai, J.S. A service-oriented healthcare message alerting architecture in an Asia medical center: A case study. Int. J. Environ. Res. Public Health 2009, 6, 1870–1881. [Google Scholar] [CrossRef] [PubMed]
- Corredor, I.; Metola, E.; Bernardos, A.M.; Tarrío, P.; Casar, J.R. A lightweight Web of Things open platform to facilitate context data management and personalized healthcare services creation. Int. J. Environ. Res. Public Health 2014, 11, 4676–4713. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Malik, M.; Schmidt, G.; Barthel, P.; Bonnemeier, H.; Cygankiewicz, I.; Guzik, P.; Lombardi, F.; Müller, A.; Oto, A.; et al. Heart Rate Turbulence: Standards of Measurement, Physiological Interpretation, and Clinical Use: (ISHNE Consensus). J. Am. Coll. Cardiol. 2008, 52, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, J.J.; Cain, M.E.; Hohnloser, S.H.; Kadish, A.H.; Knight, B.P.; Lauer, M.S.; Maron, B.J.; Page, R.L.; Passman, R.S.; Siscovick, D.; et al. American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society Scientific Statement on Noninvasive Risk Stratification Techniques for Identifying Patients at Risk for Sudden Cardiac Death: A Scientific Statement From the American Heart Association Council on Clinical Cardiology Committee on Electrocardiography and Arrhythmias and Council on Epidemiology and Prevention. Circulation 2008, 118, 1497–1518. [Google Scholar] [PubMed]
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC) Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar] [PubMed]
- Antezano, E.; Hong, M. Sudden cardiac death. J. Intensive Care Med. 2003, 18, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Bigger, J.T.; Camm, A.J.; Kleiger, R.E.; Malliani, A.; Moss, A.J.; Schwartz, P.J. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Rajendra Acharya, U.; Paul Joseph, K.; Kannathal, N.; Lim, C.M.; Suri, J.S. Heart rate variability: A review. Med. Biol. Eng. Comput. 2006, 44, 1031–1051. [Google Scholar] [CrossRef] [PubMed]
- Hossena, A.; Ghunaimib, B.A.; Hassanc, M. Subband decomposition soft-decision algorithm for heart rate variability analysis in patients with obstructive sleep apnea and normal controls. Signal Process. 2005, 85, 95–106. [Google Scholar] [CrossRef]
- Colak, O. Preprocessing effects in time–frequency distributions and spectral analysis of heart rate variability. Digital Signal Process. 2009, 19, 731–739. [Google Scholar] [CrossRef]
- Health Level Seven (HL7) International. 2007–2012. Available online: http://www.hl7.org/index.cfm?ref=nav (accessed on 30 September 2017).
- EN 13606 Association. Available online: http://www.en13606.org (accessed on 30 September 2017).
- Dixon, B.E.; Vreeman, D.J.; Grannis, S.J. The long road to semantic interoperability in support of public health: Experiences from two states. J. Biomed. Inform. 2014, 49, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, O.; Alin, C.; Serban, M.; Aurel, V. Integrated Medical System Using DICOM and HL7 Standards; INTECH Open Access Publisher: Bucharest, Romania, 2010. [Google Scholar]
- Health Informatics—Electronic Health Record Communication—Part 1: Reference Model (ISO 13606-1:2008). Available online: https://standards.cen.eu/dyn/www/f?p=204:110:0::::FSP_PROJECT,FSP_ORG_ID:37863,6232&cs=1E54087E682E74AB915B95A3268807640 (accessed on 3 October 2012).
- Health Informatics—Electronic Health Record Communication—Part 2: Archetypes Interchange Specification. Available online: https://standards.cen.eu/dyn/www/f?p=204:110:0::::FSP_PROJECT,FSP_ORG_ID:26090,6232&cs=177706333DE04FAD6E0B8A39A504A6205 (accessed on 1 August 2007).
- SNOMED-CT. International Health Terminology Standards Development Organisation. Available online: http://www.ihtsdo.org/snomed-ct/ (accessed on 31 October 2017).
- Soguero-Ruiz, C.; Lechuga-Suárez, L.; Mora-Jiménez, I.; Ramos-López, J.; Barquero-Pérez, O.; García-Alberola, A.; Rojo-Álvarez, J.L. Ontology for heart rate turbulence domain from the conceptual model of SNOMED-CT. IEEE Trans. Biomed. Eng. 2013, 60, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Caro, A.; Soguero-Ruiz, C.; Lechuga, L.; Mora-Jiménez, I.; Ramos-Lopez, J.; García-Alberola, A.; Serrano-Balazote, P.; Rojo-Álvarez, J.L. Towards semantic interoperability for cardiovascular risk stratification into the electronic health records using archetypes and SNOMED-CT. In Proceedings of the 2014 IEEE Computing in Cardiology Conference (CinC), Cambridge, MA, USA, 7–10 September 2014; pp. 497–500. [Google Scholar]
- Reding, V. Commission Recommendation of 2 July 2008 on Cross-Border Interoperability of Electronic Health Record Systems (Notified Under Document Number (C(2008) 3282). Off. J. Eur. Union. 2008. Available online: http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX (accessed on 7 July 2008).
- Camm, A.; Malik, M.; Bigger, J.; Breithardt, G.; Cerutti, S.; Cohen, R.; Coumel, P.; Fallen, E.; Kennedy, H.; Kleiger, R.; et al. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar]
- Watanabe, M.A. Heart rate turbulence: A review. Indian Pacing Electrophysiol. J. 2003, 3, 10–22. [Google Scholar]
- Su, C.J.; Chiang, C.Y. IAServ: An intelligent home care web services platform in a cloud for aging-in-place. Int. J. Environ. Res. Public Health 2013, 10, 6106–6130. [Google Scholar] [CrossRef] [PubMed]
- Addy, N.A.; Shaban-Nejad, A.; Buckeridge, D.L.; Dubé, L. An innovative approach to addressing childhood obesity: A knowledge-based infrastructure for supporting multi-stakeholder partnership decision-making in Quebec, Canada. Int. J. Environ. Res. Public Health 2015, 12, 1314–1333. [Google Scholar] [CrossRef] [PubMed]
- Nimmagadda, S.L.; Dreher, H.V. On robust methodologies for managing public health care systems. Int. J. Environ. Res. Public Health 2014, 11, 1106–1140. [Google Scholar] [CrossRef] [PubMed]
- Lasierra, N.; Alesanco, A.; Garcia, J. Designing an architecture for monitoring patients at home: Ontologies and web services for clinical and technical management integration. IEEE J. Biomed. Health Inform. 2014, 18, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Somolinos, R.; Muñoz, A.; Hernando, M.E.; Pascual, M.; Cáceres, J.; Sánchez-de Madariaga, R.; Fragua, J.A.; Serrano, P.; Salvador, C.H. Service for the pseudonymization of electronic healthcare records based on ISO/EN 13606 for the secondary use of information. IEEE J. Biomed. Health Inform. 2015, 19, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.L.; Rosenbaum, D.S. Repolarization alternans: Implications for the mechanism and prevention of sudden cardiac death. Cardiovasc. Res. 2003, 57, 599–614. [Google Scholar] [CrossRef]
- Narayan, S.M. T-wave alternans and the susceptibility to ventricular arrhythmias. J. Am. Coll. Cardiol. 2006, 47, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Bakhshi, A.; Bashir, S.; Shah, S.; Maud, M. Non-linear trend estimation of cardiac repolarization using wavelet thresholding for improved T-wave alternans. Digital Signal Process. 2013, 23, 1197–1208. [Google Scholar] [CrossRef]
- Exner, D.V.; Kavanagh, K.M.; Slawnych, M.P.; Mitchell, L.B.; Ramadan, D.; Aggarwal, S.G.; Noullett, C.; Van Schaik, A.; Mitchell, R.T.; Shibata, M.A.; et al. Noninvasive risk assessment early after a myocardial infarction: The REFINE study. J. Am. Coll. Cardiol. 2007, 50, 2275–2284. [Google Scholar] [CrossRef] [PubMed]
- Kreuz, J.; Lickfett, L.; Schwab, J. Modern noninvasive risk stratification in primary prevention of sudden cardiac death. J. Interv. Card. Electrophysiol. 2008, 23, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.; Chugh, S.S. The 12-lead electrocardiogram and risk of sudden death: Current utility and future prospects. EP Europace 2016, 17, ii7–ii13. [Google Scholar] [CrossRef] [PubMed]
- Laleci, G.B.; Yuksel, M.; Dogac, A. Providing semantic interoperability between clinical care and clinical research domains. IEEE J. Biomed. Health Inform. 2013, 17, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Christensen, B.; Ellingsen, G. Evaluating Model-Driven Development for large-scale EHRs through the openEHR approach. Int. J. Med. Inform. 2016, 89, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Wollersheim, D.; Sari, A.; Rahayu, W. Archetype-based electronic health records: A literature review and evaluation of their applicability to health data interoperability and access. Health Inf. Manag. J. 2009, 38, 7–17. [Google Scholar] [CrossRef]
- Tinnakornsrisuphap, T.; Billo, R.E. An Interoperable System for Automated Diagnosis of Cardiac Abnormalities from Electrocardiogram Data. IEEE J. Biomed. Health Inform. 2015, 19, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Pelliccia, A.; Heidbuchel, H.; Sharma, S.; Link, M.; Basso, C.; Biffi, A.; Buja, G.; Delise, P.; Gussac, I.; et al. Recommendations for interpretation of 12-lead electrocardiogram in the athlete. Eur. Heart J. 2009, 31, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Novo, J.; Hermida, A.; Ortega, M.; Barreira, N.; Penedo, M.; López, J.; Calvo, C. Hydra: A web-based system for cardiovascular analysis, diagnosis and treatment. Comput. Methods Programs Biomed. 2017, 139, 61–81. [Google Scholar] [CrossRef] [PubMed]

| General Factors | Diagnosis | Drugs | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pat. | Age | Gender | Height | Weigth | BMI | Tobac. | LVEF | Diab. mell. | Hyper- Tension | Hyper- Cholesterol | Myocar. Infarct | -Blocker |
| 1 | 71 | M | 90 | 160 | 35.16 | yes | 28 | yes | yes | yes | yes | |
| 2 | 45 | M | 95 | 177 | 30.32 | yes | 35 | no | yes | yes | no | low |
| 3 | 75 | M | 81 | 169 | 28.36 | no | 35 | no | yes | no | no | |
| 4 | 60 | M | 75 | 175 | 24.49 | yes | 35 | yes | yes | yes | yes | medium |
| 5 | 56 | M | 90 | 178 | 28.41 | yes | 24 | no | yes | yes | yes | low |
| 6 | 60 | M | 70 | 170 | 24.22 | no | 26 | yes | yes | yes | yes | low |
| 7 | 57 | F | 94 | 156 | 38.63 | yes | 30 | yes | yes | yes | no | |
| 8 | 59 | F | 70 | 150 | 31.11 | yes | 29 | yes | yes | no | no | low |
| 9 | 66 | F | 63 | 168 | 22.32 | no | 35 | no | no | no | no | medium |
| 10 | 67 | F | 68 | 155 | 28.3 | no | 33 | yes | no | no | yes | low |
| 11 | 51 | M | 78 | 180 | 24.07 | yes | 32 | yes | yes | no | no | |
| 12 | 50 | F | 90 | 160 | 35.16 | yes | 19 | no | no | no | no | |
| 13 | 60 | M | 75 | 168 | 26.57 | yes | 26 | yes | yes | no | yes | low |
| 14 | 70 | M | 70 | 165 | 25.71 | no | 27 | no | yes | no | no | |
| 15 | 61 | F | 61 | 157 | 24.75 | no | 21 | no | no | no | no | medium |
| 16 | 78 | M | 85 | 167 | 30.48 | no | 20 | yes | yes | no | no | |
| 17 | 65 | M | 102 | 173 | 34.08 | yes | 23 | yes | no | no | no | low |
| 18 | 71 | M | 78 | 169 | 27.31 | yes | 32 | yes | yes | no | yes | low |
| 19 | 76 | M | 60 | 160 | 23.44 | no | 26 | yes | no | no | no | low |
| 20 | 63 | M | 80 | 161 | 30.86 | no | 14 | no | yes | yes | no | medium |
| 21 | 52 | M | 104 | 170 | 35.99 | yes | 22 | no | no | no | no | |
| 22 | 78 | F | 87 | 163 | 32.74 | no | 26 | yes | yes | yes | no | high |
| 23 | 57 | M | 90 | 170 | 31.14 | yes | 37 | no | no | no | no | |
| 24 | 50 | F | 54 | 167 | 19.36 | yes | 22 | no | no | no | no | |
| HRT | HRV | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Statistical | Geometrical | Spectral | |||||||||||
| Pat. | TS | TO | Cat. | SDNN | SDANN | RMSSD | pNN50 | TINN | SD1 | SD2 | LF | HF | Cat. |
| 2 | 3 | 1.77 | 1 | 70.15 | 64.79 | 15.24 | 1.17 | 242.83 | 10.78 | 98.61 | 113.89 | 22.47 | 1 |
| 4 | 7 | −0.17 | 0 | ||||||||||
| 11 | 0.41 | −0.37 | 1 | 34.61 | 29.37 | 10.21 | 0.22 | 142.24 | 7.22 | 48.41 | 24.25 | 19.15 | 1 |
| 12 | 11.67 | −3.41 | 0 | ||||||||||
| 16 | 1.48 | 5.26 | 2 | 34.74 | 47.28 | 48.68 | 20.13 | 62.03 | 91.96 | ||||
| 18 | 1.03 | −0.37 | 1 | ||||||||||
| 19 | 69.50 | 59.18 | 6.02 | 0.01 | 16.85 | 4.26 | 98.19 | 4.27 | 3.86 | 2 | |||
| 20 | 0.81 | 4.70 | 0 | 13.36 | 5.20 | 13.90 | 1.37 | 28.70 | 9.83 | 16.14 | 21.40 | 43.00 | 2 |
| 21 | 1.75 | −0.22 | 1 | 63.35 | 52.05 | 18.94 | 2.06 | 274.82 | 13.39 | 88.58 | 174.37 | 62.50 | 1 |
| 23 | 0.93 | 0.58 | 2 | ||||||||||
| 24 | 0.06 | 0.86 | 2 | 281.31 | 308.13 | 33.50 | 11.36 | 119.56 | 23.69 | 397.13 | 24.70 | 16.59 | 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soguero-Ruiz, C.; Mora-Jiménez, I.; Ramos-López, J.; Quintanilla Fernández, T.; García-García, A.; Díez-Mazuela, D.; García-Alberola, A.; Rojo-Álvarez, J.L. An Interoperable System toward Cardiac Risk Stratification from ECG Monitoring. Int. J. Environ. Res. Public Health 2018, 15, 428. https://doi.org/10.3390/ijerph15030428
Soguero-Ruiz C, Mora-Jiménez I, Ramos-López J, Quintanilla Fernández T, García-García A, Díez-Mazuela D, García-Alberola A, Rojo-Álvarez JL. An Interoperable System toward Cardiac Risk Stratification from ECG Monitoring. International Journal of Environmental Research and Public Health. 2018; 15(3):428. https://doi.org/10.3390/ijerph15030428
Chicago/Turabian StyleSoguero-Ruiz, Cristina, Inmaculada Mora-Jiménez, Javier Ramos-López, Teresa Quintanilla Fernández, Antonio García-García, Daniel Díez-Mazuela, Arcadi García-Alberola, and José Luis Rojo-Álvarez. 2018. "An Interoperable System toward Cardiac Risk Stratification from ECG Monitoring" International Journal of Environmental Research and Public Health 15, no. 3: 428. https://doi.org/10.3390/ijerph15030428






